Abstract
Background
The ginseng berry has various bioactivities, including antidiabetic, anticancer, antiinflammatory, and antioxidative properties. Moreover, we have revealed that the active antiaging component of the ginseng berry, syringaresinol, has the ability to stimulate longevity via gene activation. Despite the many known beneficial effects of ginseng, its effects on skin aging are poorly understood. In this study, we investigated the effects of ginseng and the ginseng berry on one of the skin aging processes, melanogenesis, and age-related pigment lipofuscin accumulation, to elucidate the mechanism of action with respect to antiaging.
Methods
The human melanoma MNT1 cell line was treated with ginseng root extract, ginseng berry extract, or syringaresinol. Then, the cells were analyzed using a melanin assay, and the tyrosinase activity was estimated. The Caenorhabditis elegans wild type N2 strain was used for the life span assay to analyze the antiaging effects of the samples. A lipofuscin fluorescence assay was performed during 10 passages with the syringaresinol treatment.
Results
A 7-d treatment with ginseng berry extract reduced melanin accumulation and tyrosinase activity more than ginseng root extract. These results may be due to the active compound of the ginseng berry, syringaresinol. The antimelanogenic activity was strongly coordinated with the activation of the longevity gene foxo3a. Moreover, the ginseng berry extract had more potent antiaging effects, caused a life span extension, and reduced lipofuscin accumulation.
Conclusion
Taken together, our results suggest that these antimelanogenic effects and antiaging effects of ginseng berry mediate the activation of antioxidation–FoxO3a signaling.
Keywords: antiaging, Caenorhabditis elegans, Ginseng berry, lipofuscin, skin pigmentation
1. Introduction
A recent trend in the development of new medications and antiaging agents is to find candidates among natural products because they have relatively low toxicities in clinical applications. As a traditional herb, red ginseng is an adaptogen that restores and improves normal well-aging. The use of this herbal plant has been widely because of its therapeutic effects. Its well-known biochemical and pharmacological effects include anticancer [1], antifatigue [2], and antidiabetic effects [3], although it also promotes the synthesis of DNA, RNA, and proteins [4]. Recently, many health reports have recommended an increase in fruit intake as part of a healthy diet [5], [6]. Unlike the widely used ginseng root, the ginseng berry is reserved for use in planting and has not been utilized by the general population. A recent study reported that the ginseng leaf and berry have higher levels of certain ginsenosides than the ginseng root, and their pharmacological activities have also been reported [7]. Currently, ginseng berry extract is being evaluated in clinical and preclinical trials because it has high levels of active compounds, especially ginsenoside Re and vitamin E [8].
The class O forkhead/winged helix transcription factor (FoxO) is a critical regulation factor of various biological events, including metabolism, growth, development, and longevity [9], [10]. FoxO factors are involved in cellular signaling and are activated by various environmental stimuli, such as insulin, insulin growth factors (IGFs), oxidative stress, cytokines, and nutrients. In particular, oxidative stress inhibits or activates FoxO factors in a context-dependent manner. For instance, hydrogen peroxide inhibits FoxO activity by activating the phosphoinositide 3 kinase protein B pathway by affecting insulin-mimetic effects [11], whereas reactive oxygen species (ROS)-activated macrophage-stimulating 1, mitogen-activated protein kinase, and c-Jun N-terminal kinase activate the FoxO protein via phosphorylation and breakage of binding to the 14-3-3 protein [12], [13]. In the skin, FoxO3a downregulation promotes cellular senescence in human dermal fibroblasts [14]. In addition, FoxO3a regulates protection against UV-B radiation, and its nuclear translocation is also regulated by UV irradiation [15], [16]. These studies suggest that FoxO3a has an important role in cellular responses in the skin induced by external stimulation. As a photo-protector for skin, melanin is produced by serial oxidation processes in which tyrosine is converted to eumelanin and pheomelanin. Melanogenesis is one of the processes in skin aging and involves a series of tightly regulated oxidation steps. Recently, we showed that FoxO3a is an antimelanogenic factor that mediates antioxidant-induced depigmentation [17]. Antioxidants, such as vitamin C, N-acetylcysteine, and trolox, decreased melanin levels in parallel with FoxO3a nuclear translocation, and this effect disappeared in the FoxO3a null condition. Because FoxO3a orchestrates the expression of numerous genes in order to regulate cellular phenotypes in a variety of environmental states and it is a regulator involved in melanogenesis regulation, FoxO3a also modulates an antiaging signaling pathway.
The purpose of this study is to discover FoxO3a-activating compounds originating from oriental medicinal plants and to provide scientific evidence of the antimelanogenic and antiaging effects of the activators. We hypothesized that ginseng berry extract and its component syringaresinol could be novel antimelanogenic and antiaging agents, and we reveal a possible molecular mechanism for their effects. We showed that ginseng berry extract decreases melanin accumulation as well as ginseng root extract, and this effect is mainly derived from its active component syringaresinol. As ginseng root extract, ginseng berry extract, and syringaresinol exhibited antioxidative activities, we next investigated their relationship with antiaging gene protein FoxO3a. These ginseng components induced the nuclear translocation of FoxO3a, which resulted in the activation of antiaging genes. To confirm the antiaging effects of ginseng components, we determined the survival curves of the aging model organism Caenorhabditis elegans when treated with the components. We showed that these ginseng components extended the life span of the wild type N2 strain. Furthermore, syringaresinol treatment decreased the accumulation of the age-related pigment lipofuscin in human dermal fibroblasts.
2. Materials and methods
2.1. Cell culture and C. elegans strain
Highly pigmented human melanoma, MNT1 cells were maintained and passaged in Minimum Essential Media (Gibco, Carlsbad, CA, USA) containing 10% Dulbecco's modified Eagle's medium (DMEM), 20% fetal bovine serum, 20mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma Aldrich, St. Louis, USA), 100 U/mL penicillin G, and 100 μg/mL streptomycin sulfate. MNT1 cells were incubated at 37°C with 5% carbon dioxide (CO2) and regularly passaged at a density of 80% (1:8 ratio).
Dermal fibroblasts from adult skin were purchased from Lonza (Walkersville, MD, USA) and maintained in DMEM supplemented with 1% penicillin/streptomycin and 10% heat-inactivated fetal bovine serum (FBS). The cells were cultured and regularly passaged at a density of 90% confluent.
The C. elegans N2 wild-type strain was obtained by the Caenorhabditis Genetic Center (University of Minnesota, Minneapolis, MN, USA). The C. elegans strains were maintained at 20°C. Age-synchronous populations were prepared as previously mentioned [18]. Hatched worms (L1-stage larvae) were seeded to fresh agar plates and cultured at 20°C with Escherichia coli OP50 as a food source until they reached the L4 larval stage.
2.2. Panax ginseng and ginseng berry extract preparation
After fresh P. ginseng and ginseng berry was water-washed, the seeds were removed and the remainder (pulp and rinds) was collected. After 70% ethanol extraction under reflux condition for 10 h, extract was filtered and evaporated. Then, the extract solution was lyophilized to acquire a powder state of ginseng berry extract and stored at −20°C until use. For standardization and comparison of ginsenoside composition, the chromatographic separation was performed using ACQUITY UPLC system (Waters Co., Milford, MA, USA). The column was ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 mm × 50 mm). The column temperature and autosampler tray temperature were maintained at 40°C and 10°C, respectively. The data were obtained and analyzed with MassLynx version 4.1 (Waters Co.).
2.3. Determination of melanin levels and tyrosinase enzymatic activity assay
To measure cellular tyrosinase activity, the same amounts of cell lysates (10 μg) were incubated with 10mM L-dihydroxyphenylalanine (L-DOPA; pH 6.8; Sigma) at 37°C for 1 h. The amount of melanin calculated from L-DOPA via the tyrosinase activity in the cell extracts was measured using a UV–Vis spectrometer (Molecular Devices, Sunnyvale, CA, USA) at 490 nm. To determine the cellular melanin levels, the cell pellets were dissolved in 50 μL of 1N sodium hydroxide, and the melanin levels were determined by measuring the absorbance at 490 nm. The melanin levels were normalized to the protein input of samples.
2.4. Antioxidant activity assay
The catalase activity was determined from the amount of the residual H2O2 as measured by a spectrophotometric method [19]. Briefly, 800 μL of 25μM H2O2 and 200 μL of a sample solution in water that contained ginseng root extract, ginseng berry extract, or syringaresinol were added to initiate the quenching reaction. After 5 min incubation, the concentration of H2O2 was measured at 240 nm. Water was used as a vehicle control, and the residual H2O2 is represented as a percentage of the control.
The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity was calculated from the residual DPPH concentration, as estimated by a spectrophotometric method [20], [21]. In brief, 500 μL of 60μM DPPH was placed in a quartz cuvette, and same volume of a sample solution in ethanol that contained ginseng root extract, ginseng berry extract, or syringaresinol were added to initiate the DPPH quenching. The quenching reaction was performed for 30 min incubation at room temperature (RT) and absorbance at 520 nm was measured using a spectrometer. The residual DPPH is expressed as a percentage of the control and ethanol was used as a vehicle control.
2.5. FoxO3a localization assay using immunofluorescence
MNT1 cells were treated with each reagent [ginseng root extract (GR), ginseng berry extract (GB), and syringaresinol (SYR)] for 4 d. The cells were washed with PBS, fixed in 4% paraformaldehyde for 30 min, and incubated in 0.1% Triton X-100 (Sigma Aldrich, St. Louis, USA) for 10 min. Then, the cells were incubated with antiFoxO3a (1:200) antibody diluted in Hank's solution (0.44mM KH2PO4, 5.37mM KCl, 0.34mM Na2HPO4, 136.89mM NaCl, and 5.55mM D-glucose) overnight at 4°C. Secondary antibodies (Alexa Fluor 555-conjugated goat antirabbit, Invitrogen, Carlsbad, CA) were added for 1 h at room temperature. After washing process, the coverslips were mounted onto glass slides and visualized with a confocal laser scanning microscope (LSM7, Carl Zeiss, NY, USA; with an excitation wavelength at 555 nm and an emission wavelength at 565 nm with DAPI fluorescence). Cells with FoxO3a localization were counted and represented by the means of a confocal laser scanning microscope with the ZEN software (Carl Zeiss).
2.6. Lifespan analysis
L4 larvae were moved to S-medium [S-basal medium supplemented with 3mM CaCl2, 3mM MgSO4, 50μM EDTA, 25μM FeSO4, 10μM MnCl2, 1μM CuSO4, 10μM ZnSO4, and 10mM KH2PO4 (pH 6.0)] with OP50. Then each reagent was added to the medium. This transfer day was designated as “age of 0 d.” We transferred the worms to fresh culture medium every 2nd d and their survival were recorded at the time of transfer. The worms that removed from vulval bursting were excluded from the analysis. Life span assays were repeated more than three times.
2.7. Lipofuscin accumulation
Dermal fibroblasts from adult skin were cultured with each reagent (GR, GB, and SYR) for 10 passages (3–4 d per 1 passage), washed with PBS, and fixed in 4% paraformaldehyde for 30 min. The cells were then washed and incubated in 0.1% Triton X-100 for 10 min. After washing, the cover slips were mounted onto glass slides and visualized by a confocal laser scanning microscope (LSM7, Carl Zeiss; with an excitation wavelength at 350 nm and an emission wavelength at 420 nm). The fluorescence intensities of the cells were measured densitometrically with the ZEN Lite software (Carl Zeiss) by calculating the average pixel intensity in each cell. The significance of the differences among the control and experimental groups were analyzed using a one-way analysis of variance (ANOVA).
2.8. Statistical analysis
For the lifespan assay, the mortality data were subjected to a Kaplan–Meier survival analysis to depict survival curves. A statistical comparison of the mean lifespan values among the control and treated worms were analyzed using Peto's log-rank test. Each experiment was performed in triplicate and analyzed by SPSS statistics standard version 21 (IBM Inc., Armonk, NY, USA). All statistical tests were two-sided, and the threshold for statistical significance was 0.05 (* p < 0.05).
3. Results and discussion
3.1. Ginseng berry and its bioactive component syringaresinol have antimelanogenesis activity
Recently, many studies have endorsed an increase in fruit intake as part of a healthy supplementation [22]. Berries are a good source of potassium or fiber, and recent reports have shown that berry fruits contain many phytochemicals that have a broad spectrum of bioactivities and a positive impact on public health [23], [24]. A recent study reported that the ginseng leaf and berry have higher levels of certain ginsenosides than the ginseng root, and their pharmacological activities have also been reported [7].
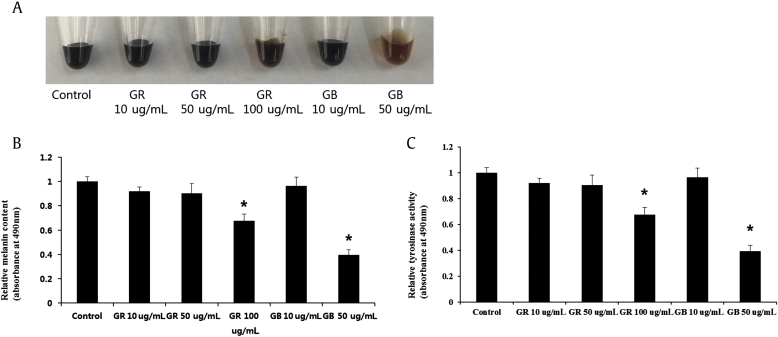
To find natural compounds from ginseng extracts with antimelanogenesis effects, we performed a melanin accumulation assay using human melanoma cells, the MNT1 cell line, and ginseng root and ginseng berry extracts. From the results of this assay, it was found that the ginseng berry extract and the ginseng root extract inhibited melanin accumulation without toxicity (Figs. 1A, 1B). Melanogenesis is initiated by a tyrosinase-catalyzed oxidation process [25]. As a result, the ginseng berry extract inhibited melanin accumulation as well as intracellular tyrosinase activity more than the same concentration of ginseng root extract (Figs. 1A–1C). There are several studies that have indicated that the ginseng berry has higher levels of ginsenoside content than the root [23]. Additionally, the ginseng berry not only exhibits ginseng root-like effects, but also has many other specific biological activities [26]. Due to its numerous potent biological activities, there have been many efforts to discover other useful components in the ginseng berry besides ginsenosides. Recently, a lignan compound, syringaresinol {4,4′-(1S,3aR,4S,6aR)-tettrahydro-1H,3H-furo [3,4-c]furan-a,4-diylbis(2,6-dimethoxyphenol)}, was isolated from Panax ginseng pulp and found to activate SIRT1 gene expression, leading to delayed cellular senescence and improved endothelial cellular function in endothelial cells [27]. As syringaresinol has protective effects against hypoxia/reoxygenation-induced injury [28], which closely correlates with oxidative stress, we speculate that the potent antimelanogenic effects of ginseng berry may be due to its bioactive component syringaresinol.
Fig. 1.
Ginseng root and ginseng berry extracts inhibit melanin accumulation by lowering tyrosinase activity. (A) MNT1 cells were treated with the ginseng root extract or the ginseng berry extract for 48 h, and the melanin levels were visualized after dissolving the cell pellets in 1N NaOH. (B) The melanin levels were quantified in the dissolved cell pellets by measuring the absorbance at 490 nm with a UV-vis spectrometer (Molecular Devices, Sunnyvale, CA, USA) and normalized to the protein input. (C) The tyrosinase activity was measured at 490 nm using a UV-vis spectrometer with melanin synthesized from L-dihydroxyphenylalanine (L-DOPA) by the tyrosinase present in the equal amounts of cell lysates (10 μg). Data are presented as mean ± standard deviation. * p < 0.05 by two–tailed Student t test.
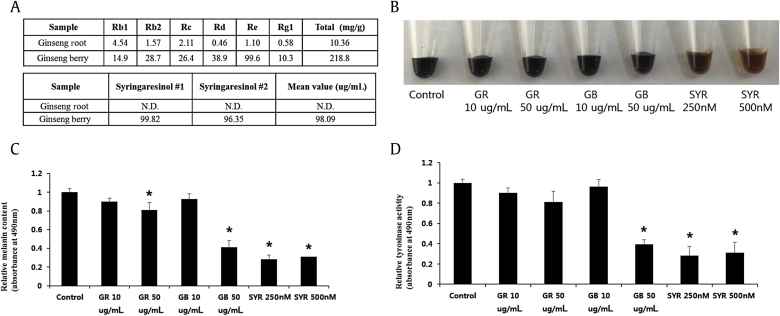
To verify that syringaresinol is the main factor in melanogenesis inhibition, we measured the ginsenoside and syringaresinol content in ginseng root and ginseng berry extracts using an HPLC method [29]. Syringaresinol was only found in the ginseng berry extract (Fig. 2A). Additionally, we performed a melanin assay with ginseng contents, including syringaresinol. As a result, melanin accumulation was strongly inhibited by syringaresinol (Figs. 2B, 2C), and the compound inhibited tyrosinase activity also (Fig. 2D). These results suggest that the antimelanogenic effects of the ginseng berry are mainly derived from its active component syringaresinol.
Fig. 2.
Ginseng berry extract reduces melanogenesis by inhibiting tyrosinase activity, and this effect is mainly derived from its effective component, syringaresinol. (A) The amount of syringaresinol in ginseng root or ginseng berry extract. The mean value (ug/mL) is presented. The melanin levels were visualized after dissolving the cell pellets in 1N NaOH (B) and quantified (C). The tyrosinase activity was also measured (D). Ginseng root, ginseng berry extract, or syringaresinol were used to treat MNT1 cells for 48 h. Data are presented as mean ± standard deviation. * p < 0.05 by two-tailed Student t test.
3.2. Elevated nuclear translocation of FoxO3a by syringaresinol is a key mechanism of melanogenesis inhibition
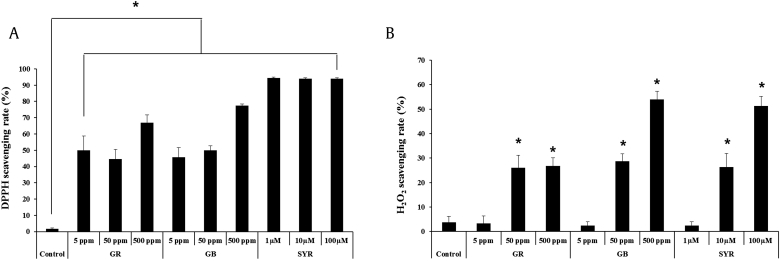
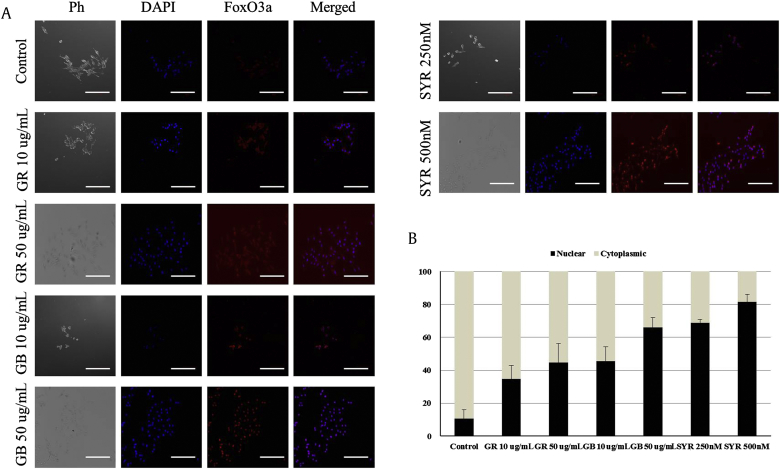
Lignan compounds commonly occur in plants and represent a diverse spectrum of health-promoting effects, such as antioxidative, antitumorigenic, and antiviral properties [30], [31], [32]. As several oxidation steps occur during the melanin synthesis process, we measured the antioxidant effects of syringaresinol by analyzing its DPPH radical and hydrogen peroxide scavenging activities. Although ginseng root and ginseng berry extracts showed reactive oxygen species (ROS) scavenging activities, syringaresinol exhibited a potent antioxidant activity (Figs. 3A, 3B). In our previous report [17], we showed that the antimelanogenic activity of antioxidants is mediated by the activation of the longevity gene, FoxO3a, via its nuclear translocation. Based on our results, the antimelanogenic effect of antioxidants coordinated well with FoxO3a activation. FoxO3a activation is induced by its nuclear translocation; therefore, we performed immunofluorescence assays to analyze its localization. Increasing concentrations of each ginseng derivative (ginseng root extract, ginseng berry extract, and syringaresinol) induced the nuclear translocation of FoxO3a, showing a gradual enrichment of FoxO3a within the nucleus (Figs. 4A, 4B). The nuclear translocation rate of FoxO3a was well coordinated with the reduction in melanin levels (Fig. 2, Fig. 4B). These results suggest that the antimelanogenic effects of the ginseng components were mediated principally by the activation of the antimelanogenic factor FoxO3a.
Fig. 3.
Ginseng components have antioxidative activities. Ginseng root, ginseng berry, and syringaresinol have DPPH radical-scavenging activity (A) and H2O2 scavenging activity (B). Data are presented are the mean ± SD. * p < 0.05 by two-tailed Student t test.
Fig. 4.
Ginseng root, ginseng berry, and syringaresinol induce the nuclear translocation of forkhead box-O3a (FoxO3a). (A) The indicated ginseng components were added to MNT1 cells for 48 h. The cells were stained with an antiFoxO3a antibody (FoxO3a; red) and 4′,6–diamidino–2–phenylindole (DAPI) (nucleus; blue). Representative images were obtained using a confocal laser scanning microscope (LSM7, Carl Zeiss, NY, USA) and merged. (B) Cells with FoxO3a localization were counted and depicted by the means of a confocal laser scanning microscope. Data are presented as mean ± standard deviation. Scale Bar = 100 μm. Ph, phase-contrast image.
3.3. The antiaging effects of ginseng berry in C. elegans and proliferating human fibroblasts
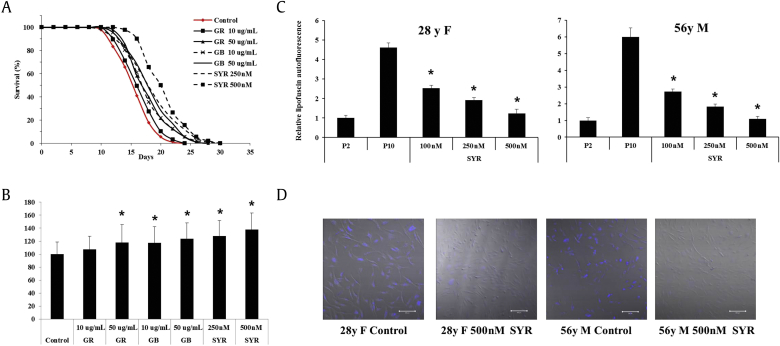
Despite the numerous biological activities reported for lignans [30], [31], [32], [33], [34], there is surprisingly little reports on the antiaging effects of syringaresinol. We used the C. elegans wild-type N2 strain as a model organism of aging to examine the lifespan extension effects of ginseng berry extract and syringaresinol. Syringaresinol extended the N2 lifespan between 27.8% and 38.1% within a dose range of 250–500nM (Figs. 5A, 5B and Table 1). The effects of syringaresinol were compared with those of ginseng root extract (7.3% and 17.8% at 10–50 μg/mL) and ginseng berry extract (17.2% and 23.6% at 10–50 μg/mL). These differences also coordinated well with the FoxO3a nuclear localization rate (Fig. 4, Fig. 5B). We hypothesized that the lifespan extension effect of the ginseng components may be derived from their longevity gene activation activities.
Fig. 5.
Ginseng components have potent antiaging activity. Ginseng root, ginseng berry, and syringaresinol induce a life span extension in the Caenorhabditis elegans wild type N2 strain. The survival curves (A) and the mean life span (B) are presented. The data were analyzed via a Kaplan–Meier survival analysis. The mean adult life span of each condition is (mean ± standard deviation) as follows: N2 control, 16.2 ± 3.0 d; GR 10 μg/mL, 17.4 ± 3.3 d; GR 50 μg/mL, 19.1 ± 4.5 d; GB 10 μg/ml, 19.0 ± 4.1 d; GB 50 μg/mL, 20.1 ± 4.0 d; SYR 250nM, 20.8 ± 3.9 d; and SYR 500nM, 22.4 ± 4.1 d. * p < 0.05 by Peto's log–rank test. The data represent one experiment with two additional repeats. (C, D) Ginseng components decrease lipofuscin accumulation in passaged human dermal fibroblasts. Dermal fibroblasts from a 28-yr-old female and a 56-yr-old male were treated with 100–500nM of syringaresinol for 10 passages, and the accumulated age-related lipofuscin was measured (C) and visualized (D). The accumulated lipofuscin levels were detected using a confocal laser scanning microscope (Ex: 350 nm; Em: 420 nm). The fluorescence intensities of the cells were calculated densitometrically by measuring the average pixel intensity in each cell. The data are presented as the mean ± standard deviation of three independent experiments. * p < 0.05 by two-tailed Student t test. Em, Emission; Ex, Excitation; GB, ginseng berry extract; GR, ginseng root extract; SYR, syringaresinol.
Table 1.
Ginseng components extends lifespan of Caenorhabditis elegans1)
| Strain/treatment | Adult lifespan (Mean ± SD) | N | % vs control strain | p vs control strain | Maximal adult lifespan |
|---|---|---|---|---|---|
| Control | 16.2 ± 3.0 | 90 | – | – | 24 |
| GR 10 μg/mL | 17.4 ± 3.3 | 87 | 7.3 | 0.2374 | 24 |
| GR 50 μg/mL | 19.1 ± 4.5 | 90 | 17.8 | 0.0823 | 26 |
| GB 10 μg/mL | 19.0 ± 4.1 | 88 | 17.2 | 0.0013 | 28 |
| GB 50 μg/mL | 20.1 ± 4.0 | 92 | 23.6 | 8.9E–06– | 30 |
| SYR 250nM | 20.8 ± 3.9 | 93 | 27.8 | 1.4E–07 | 30 |
| SYR 500nM | 22.4 ± 4.1 | 95 | 38.1 | 4.3E–08 | 34 |
GB, ginseng berry extract; GR, ginseng root extract; SYR, syringaresinol.
Data obtained from Kaplan–Meier survival curves. p values calculated from log–rank test. Lifespan analysis was performed by three independent trials. The lifespan graphs can be found in Fig. 5A.
Furthermore, we performed a lipofuscin assay using proliferating human dermal fibroblasts to assess the antiaging activity in skin cells. The pigment lipofuscin, which is detected in replicating cells, accumulates during the aging process [35]. Lipofuscin accumulation is considered one of the well-known biomarkers of cellular senescence. A correlation between the rate of lipofuscin accumulation and the rate of aging in mammals has also been previously reported [36]. Lipofuscin accumulates as the replicative age of human fibroblast strains increases [37]. We used human dermal fibroblasts from adult skin to measure the antiaging effect of syringaresinol by detecting the lipofuscin accumulation level with increased passages. Striking differences were observed, with a marked decrease in lipofuscin fluorescence in passage-aged fibroblasts (Figs. 5C, 5D). A large number of lipofuscin aggregates were present in the control groups, and scarcely any aggregates were evident in the same fibroblast populations identified with nuclei and dendritic cells. Syringaresinol largely reduced lipofuscin accumulation by 53.8–73.4% of the control in the dose range of 100–500nM in the 28-yr-old female fibroblast-aged group and by 72.7–84.3% of the control in the 56-yr-old male fibroblast aged group (Fig. 5C). The representative images (500nM syringaresinol) displayed were compared with the control groups (Fig. 5D). These results suggest that the antiaging effects of ginseng berry extract are also relevant in the skin aging model. However, further studies need to be performed in vivo to confirm these effects.
4. Conclusion
Ginseng berry extract and its natural phytochemical component syringaresinol showed antimelanogenic effects on a human melanoma cell line, extended the lifespan of the aging model organism C. elegans, and reduced the accumulation of the age-related pigment lipofuscin in human dermal fibroblasts. These effects may be due to potent antioxidant activity and the activation of the longevity gene foxo3a. These data suggest that the antipigmentation and antiaging activity of ginseng berry extract induced by the activation of the longevity gene FoxO3a may represent a good target for studying antimelanogenic signaling pathways using ginseng components and for designing pharmacological or antimelanogenic agents that regulate antiaging signaling.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors are grateful to the Caenorhabditis Genetics Center for the C. elegans strains.
Contributor Information
Dae Bang Seo, Email: sdbang@amorepacific.com.
Song Seok Shin, Email: ssshin@amorepacific.com.
References
- 1.Xie J., Wang C., Zhang E., Mehendale S.R., Li X.L., Sun S., Han A.H., Du W., He T.C., Yuan C.S. In vitro and in vivo anticancer effects of American ginseng berry: exploring representive compounds. Biol Pharm Bull. 2009;32:1552–1558. doi: 10.1248/bpb.32.1552. [DOI] [PubMed] [Google Scholar]
- 2.Christensen L.P. Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2008;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 3.Xie J., Mehendale S., Yuan C. Ginseng and diabetes. Am J Chin Me. 2005;33:397–404. doi: 10.1142/S0192415X05003004. [DOI] [PubMed] [Google Scholar]
- 4.King M.L., Murphy L. Role of cyclin inhibitor protein p21 in the inhibition of HCT116 human colon cancer cell proliferation by American ginseng and its constituents. Phytomedicine. 2010;17:261–268. doi: 10.1016/j.phymed.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.USDA; US Department of Health and Human Services . 7th ed. US Government Printing Office; Washington: 2010. Dietary guidelines for Americans, 2010. [Google Scholar]
- 6.Kiefte-de Jong J.C., Mathers J.C., Franco O.H. Nutrition and healthy ageing: the key ingredients. Proc Nutr Soc. 2014;73:249–259. doi: 10.1017/S0029665113003881. [DOI] [PubMed] [Google Scholar]
- 7.Wang C.Z., Zhang B., Song W.X., Wang A., Ni M., Luo X., Aung H.H., Xie J.T., Tong R., He T.C. Steamed American ginseng berry: ginsenoside analysis and anticancer activities. J Agric Food Chem. 2006;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 8.Kim J., Cho S.Y., Kim S.H., Kim S., Park C.W., Park H.W., Seo D.B., Shin S.S. Ginseng berry, a promising anti–aging strategy: recent opinions on the biological effects of a traditional Korean ingredient. SOJ Biotech. 2016;1:8. [Google Scholar]
- 9.Wolkow C.A., Kimura K.D., Lee M.S., Ruvkun G. Regulation of C. elegans life–span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- 10.Libina N., Berman J.R., Kenyon C. Tissue–specific activities of C. elegans DAF–16 in the regulation of lifespan. Cell. 2010;143:299–312. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 11.Heffetz D., Rutter W.J., Zick Y. The insulinomimetic agents H2O2 and vanadate stimulate tyrosine phosphorylation of potential target proteins for the insulin receptor kinase in intact cells. Biochem J. 1992;288:631–635. doi: 10.1042/bj2880631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Essers M.A., Weijzen S., de Vries–Smits A.M., Saarloos I., de Ruiter N.D., Bos J.L., Burgering B.M. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi W., Xiao L., Jia Y., Wu J., Xie Q., Ren J., Ji G., Yuan Z. c-Jun N-terminal kinase enhances MST1–mediated pro–apoptotic signaling through phosphorylation at serine 82. J Biol Chem. 2010;285:6259–6264. doi: 10.1074/jbc.M109.038570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H.K., Kim Y.K., Song I.H., Baek S.H., Lee S.R., Hye Kim J., Kim J.R. Down–regulation of a forkhead transcription factor, FoxO3a, accelerates cellular senescence in human dermal fibroblasts. J Getontol. 2005;60A:4–9. doi: 10.1093/gerona/60.1.4. [DOI] [PubMed] [Google Scholar]
- 15.Mandinova H., Lefort K., di Vignano A.T., Stonely W., Ostano P., Chiorino G., Iwaki H., Nakanishi J., Dotto G.P. The FoxO3a gene is a key negative target of canonical Notch signaling in the keratinocyte UVB response. EMBO J. 2008;27:1243–1254. doi: 10.1038/emboj.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Chen W.R., Xing D. A pathway from JNK through decreased ERK and Akt activities for FoxO3a nuclear translocation in response to UV irradiation. J Cell Physiol. 2012;227:1168–1178. doi: 10.1002/jcp.22839. [DOI] [PubMed] [Google Scholar]
- 17.Kim J., Choi H., Cho E., Lee T.R. FoxO3a is an antimelanogenic factor that mediates antioxidant–induced depigmentation. J Invest Dermatol. 2014;134:1378–1388. doi: 10.1038/jid.2013.510. [DOI] [PubMed] [Google Scholar]
- 18.Kim J., Takahashi M., Shimizu T., Shirasawa T., Kajita M., Kanayama A., Miyamoto Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech Ageing Dev. 2008;129:322–331. doi: 10.1016/j.mad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Ueno S., Iwasaka M. Catalytic activity of catalase under strong magnetic fields of up to 8T. 1996;79:4705–4707. [Google Scholar]
- 20.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 21.Yamazaki K., Hashimoto A., Kokusenya Y., Miyamoto T., Sato T. vol. 42. 1994. pp. 1663–1665. (Chemical pharmaceutical bulletin). [DOI] [PubMed] [Google Scholar]
- 22.Kiefte-de Jong J.C., Mathers J.C., Franco O.H. Nutrition and healthy ageing: the key ingredients. Proc Nutr Soc. 2014;73:245–259. doi: 10.1017/S0029665113003881. [DOI] [PubMed] [Google Scholar]
- 23.Paredes-López O., Cervantes-Ceja M.L., Vigna-Pérez M., Hernández-Pérez T. Berries: improving human health and healthy aging, and promoting quality life–a review. Plant Foods Hum Nutr. 2010;65:299–308. doi: 10.1007/s11130-010-0177-1. [DOI] [PubMed] [Google Scholar]
- 24.Basu A., Lyons T.J. Strawberries, blueberries, and cranberries in the metabolic syndrome: clinical perspectives. J Agric Food Chem. 2012;60:5687–5692. doi: 10.1021/jf203488k. [DOI] [PubMed] [Google Scholar]
- 25.Hearing V.J. Determination of melanin synthetic pathways. J Invest Dermatol. 2011;17:E8–E11. doi: 10.1038/skinbio.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joo K.M., Lee J.H., Jeon H.Y., Park C.W., Hong D.K., Jeong H.J. Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography/mass spectrometric method. J Pharm Biomed Anal. 2010;51:278–283. doi: 10.1016/j.jpba.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Cho S.Y., Cho M., Seo D.B., Lee S.J., Suh Y. Identification of a small molecule activator of SIRT1 gene expression. Aging. 2013;5:174–182. doi: 10.18632/aging.100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho S.Y., Cho M., Kim J., Kaeberlein M., Lee S.J., Suh Y. Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1a in a FOXO3-dependent mechanism. Oncotarget. 2014;16:43–55. doi: 10.18632/oncotarget.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeds A.I., Eklund P.C., Willför S.M. Content, composition, and stereochemical characterization of lignans in berries and seeds. Food Chem. 2012;134:1991–1998. doi: 10.1016/j.foodchem.2012.03.133. [DOI] [PubMed] [Google Scholar]
- 30.Ayres D.C., Loike J.D. Cambridge University Press; Cambridge, England: 1990. Lignans: chemical, biological, and clinical proterties. [Google Scholar]
- 31.Saarinen N.M., Wärri A., Airio M., Smeds A., Mäkelä S. Role of dietary lignans in the reduction of breast cancer risk. Mol Nutr Food Res. 2007;51:857–866. doi: 10.1002/mnfr.200600240. [DOI] [PubMed] [Google Scholar]
- 32.Willför S.M., Ahotupa M.O., Hemming J.E., Reunanen M.H.T., Eklund P.C., Sjöholm R.E. Antioxidant activity of knotwood extractives and phenolic compounds of selected tree species. J Agric Food Chem. 2003;51:7600–7606. doi: 10.1021/jf030445h. [DOI] [PubMed] [Google Scholar]
- 33.Bhathena S.J., Velasquez M.T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 34.Vanharanta M., Voutilainen S., Lakka T.A., van der Lee M., Adlercreutz H., Salonen J.T. Risk of acute coronary events according to serum concentrations of enterolactone: a prospective population–based case–control study. Lancet. 1999;354:2112–2115. doi: 10.1016/S0140-6736(99)05031-X. [DOI] [PubMed] [Google Scholar]
- 35.Brunk U.T., Terman A. Lipofuscin: mechanisms of age–related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 36.Jung T., Bader N., Grune T. Lipofuscin formation, distribution, and metabolic consequences. Ann NY Acad Sci. 2007;1119:97–111. doi: 10.1196/annals.1404.008. [DOI] [PubMed] [Google Scholar]
- 37.Sitte N., Merker K., Grune T., von Zglinicki T. Lipofuscin accumulation in proliferating fibroblasts in vitro: an indicator of oxidative stress. Exp Gerontol. 2001;36:475–486. doi: 10.1016/s0531-5565(00)00253-9. [DOI] [PubMed] [Google Scholar]