Abstract
Background
Compound FK506 is an immunosuppressant agent that is frequently used to prevent rejection of solid organs upon transplant. However, nephrotoxicity due to apoptosis and inflammatory response mediated by FK506 limit its usefulness. In this study, the protective effect of Korean Red Ginseng (KRG) against FK506-induced damage in LLC-PK1 pig kidney epithelial cells was investigated.
Methods
LLC-PK1 cells were exposed to FK506 with KRG and cell viability was measured. Western blotting and RT-PCR analyses evaluated protein expression of MAPKs, caspase-3, and KIM-1. TLR-4 gene expression was assessed. Caspase-3 activities were also determined. The number of apoptotic cells was measured using an image-based cytometric assay.
Results
The reduction in LLC-PK1 cell viability by 60μM FK506 was recovered by KRG cotreatment in a dose-dependent manner. The phosphorylation of p38, p44/42 MAPKs (ERK), KIM-1, cleaved caspase-3, and TLR-4 mRNA expression was increased markedly in LLC-PK1 cells treated with 60μM FK506. However, with the exception of p-ERK, elevated levels of p-p38, KIM-1, cleaved caspase-3, and TLR-4 mRNA expression were significantly decreased after cotreatment with KRG. Activity level of caspase-3 was also attenuated by KRG cotreatment. Moreover, image-based cytometric assay showed that apoptotic cell death was increased by 60μM FK506 treatment, whereas it was decreased after cotreatment with KRG.
Conclusion
Taken together, these results suggest that the molecular mechanism of KRG in the FK506-induced nephrotoxicity may lead to the development of an adjuvant for the inhibition of adverse effect FK506 in the kidney.
Keywords: FK506, Korean Red Ginseng, nephrotoxicity, MAPKs
1. Introduction
FK506 is a macrolide immunosuppressant agent isolated from the fungus Streptomyces tsukubaensis. It is a selective anti-T-lymphocyte agent and is used to prevent allograft rejection after human liver, kidney and heart transplantation, as well as autoimmune diseases [1], [2], [3], [4]. FK506 treatment induces nephrotoxicity in 17–44% of renal transplant recipients and in 18–42% of liver transplant recipients [5]. This has limited the clinical use of FK506.
The mechanisms of FK506-induced nephrotoxicity are not completely resolved. The main mechanisms causing side effects are changes in glomerular and tubular function by increases in apoptosis and inflammatory response in proximal tubular cells [3], [6]. Minimizing FK506-induced nephrotoxicity is important for improved long-term renal allograft survival. Production of nitric oxide (NO), superoxide dismutase, and catalase are significantly suppressed by green tea extract in mice and LLC-PK1 cells treated with FK506 [7], [8]. Polyphenols found in green tea significantly reduce FK506-induced cytotoxicity by suppressing the release of cytochrome c, activating caspase-3 and suppressing increased intracellular levels of reactive oxygen species (ROS) [7], [9]. Considering the gravity of the problem, few studies have examined how best to minimize FK506-induced nephrotoxicity.
Korean Red Ginseng (KRG), a steamed and dried root of Panax ginseng Meyer, has been used for millennia in Korea and other Asian countries as a traditional oriental medicine to treat illness and promote health [10], [11]. Biological and pharmacological activities can be enhanced through heat and steam processes [12]. The variety of pharmacological activities attributed to KRG include antiapoptotic [13], [14], antiinflammatory [15], [16] and antioxidant effects [12], [17], [18].
The effects and mechanisms of KRG on drug-induced nephrotoxicity have been studied. KRG can prevent nephrotoxicity induced by gentamicin by decreasing oxidative stress and apoptosis of rat renal tubular cells [19], [20]. In HK-2 proximal tubular cells, KRG-mediated reduction of nephrotoxicity induced by chronic cyclosporine, proinflammatory and profibrotic molecules including induced nitric oxide synthase and cytokines, and of apoptotic cell death [21]. KRG treatment also reportedly reduces autophagosome formation and autophagic aggregates by decreasing the expression of caspase-3 and LC3-II in mice [22]. In another study, KRG prevented nephrotoxicity induced by gentamicin through the decrease of the levels of indicators of renal dysfunction, inflammatory cytokine expression, apoptosis, and malondialdehyde content in rats [23].
The collective data prompted the present study to explore the potential of KRG as a useful agent to prevent or protect FK506-induced nephrotoxicity on the basis of KRG's antiapoptotic, antiinflammatory, and antioxidant effects.
2. Materials and methods
2.1. Chemicals
FK506 was purchased from Sigma Aldrich (St. Louis, MO, USA). Ez-Cytox cell viability assay kit was purchased from Dail Lab Service Co. (Seoul, Korea). Caspase-3 colorimetric assay kit was purchased from BioVison (Milpitas, CA, USA). Tali Apoptosis Kit was purchased from Invitrogen (Carlsbad, CA, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) was purchased from Cellgro (Manassas, VA, USA) and Invitrogen Co. (Grand Island, NY, USA). KRG was provided by Korea Ginseng Corporation (Daejeon, Republic of Korea).
2.2. Preparation of KRG fractions
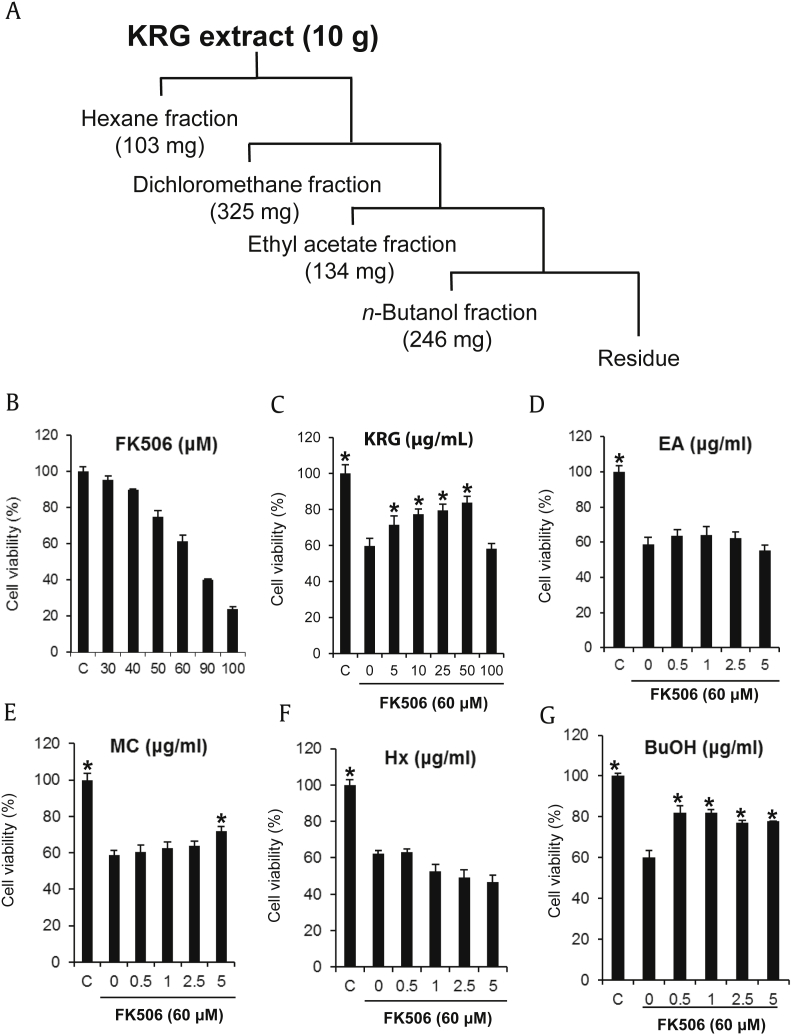
KRG extracts were manufactured from the roots of 6-year-old fresh Panax ginseng provided by Korea Ginseng Corporation. KRG extract (10 g) was solvent-partitioned in sequence with hexane, dichloromethane, ethyl acetate, and n-butanol, which yielded 103 mg, 325 mg, 134 mg, and 246 mg of dried organic fraction, respectively (Fig. 1A).
Fig. 1.
Effect of KRG and its fractions on the FK506-induced nephrotoxicity in LLC-PK1 cells. (A) Preparation of KRG fractions. (B) Cytotoxicity of FK506 in LLC-PK1 cells. (C)–(G) Comparison in protective effect of KRG and fractions against FK506-induced nephrotoxicity in LLC-PK1 cells. *p < 0.05 compared to the FK506-treated value.
2.3. Cell culture and cell viability assay
LLC-PK1 pig kidney epithelial cells (CL-101) were used to evaluate renoprotective activity against FK506-induced cytotoxicity as reported previously [9]. LLC-PK1 cells were purchased from the American Type Culture Collection (Rockville, MD, USA), and cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (Invitrogen), and 4mM L-glutamine in an atmosphere of 5% CO2 at 37°C. Viability of cells was determined by the Ez-Cytox cell viability assay, which is based on the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to insoluble formazan. Cells grown to approximately 80% confluence were seeded in wells of 96-well culture plates at 1 × 104 cells per well and incubated for 24 h for adhesion. Then cells were treated with various concentrations (30μM, 40μM, 50μM, 60μM, 90μM and 100μM) of FK506 for 24 h to confirm its appropriate concentration to induce apoptosis. After incubation, 10 μL of Ez-Cytox reagent was added to each well and incubated for 2 h. Cell viability was measured by absorbance at 450 nm using a PowerWave XS microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
To verify the protective effects of KRG and its fractions, LLC-PK1 cells seeded in wells of 96-well culture plates at 1 × 104 cells per well were exposed to FK506 with control (0.5% dimethylsulfoxide) or indicated concentrations of KRG and its fractions for 24 h. Then cell viability was measured by Ez-Cytox reagent as described above.
2.4. Western blotting analysis
LLC-PK1 cells cultured in wells of six-well plates were treated with 10 μg/mL KRG or 50 μg/mL KRG (for 24 h) and lysed with RIPA buffer [20mM Tris-HCl (pH 7.5), 150mM NaCl, 1mM Na2EDTA, 1mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5mM sodium pyrophosphate, 1mM beta-glycerophosphate, 1mM Na3VO4, 1 μg/mL leupeptin; Cell Signaling Technology, Beverly, MA, USA] supplemented with 1mM phenylmethylsulfonyl fluoride (PMSF) immediately before use. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Pittsburgh, PA, USA). Equal amounts (20 μg/lane) of protein (whole-cell extracts) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF transfer membranes. Specific proteins were analyzed with epitope-specific primary antibody to MAPK species [p38, phospho-p38, p44/42 extracellular signal-regulated kinase (ERK), and phospho-p44/42 (pERK)], cleaved caspase-3, kidney injury molecule-1 (KIM-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and horseradish peroxidase (HRP) conjugated antirabbit antibodies (Cell Signaling Technology). Bound antibodies were detected using ECL Advance Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK) and visualized using a FUSION Solo Chemiluminescence System (PEQLAB Biotechnologie GmbH, Erlangen, Germany).
2.5. RT-PCR
LLC-PK1 cells cultured in wells of six-well plates were treated with 10 μg/mL KRG or 50 μg/mL KRG for 24 h. The cells were collected and total mRNA was isolated using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the protocol of the manufacturer. The concentration and integrity of mRNA was evaluated using a spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). Total RNA (1 μg) was pretreated with RNase-free DNase I for 20 min at 37°C to remove genomic DNA prior to use in cDNA synthesis using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fischer Scientific). PCR amplification of Toll-like receptor (TLR)-4 was performed with 2 μL cDNA using Ex Taq Polymerase (TaKaRa Bio, Shiga, Japan). The PCR products were electrophoresed in a 2% polyacrylamide gel and visualized on a gel nucleic acid bioimaging device (NaBI; NEOScience, Gyeonggi-do, Korea) by ethidium bromide staining. GAPDH was used as an internal control. The primer sets were as follows: TLR-4, forward 5′-CAG ATA AGC GAG GCC GTC ATT-3′ and reverse 5′-TTG CAG CCC ACA AAA AGC A-3′: GAPDH, forward 5′-ACA TCA TCC CTG CTT CTA CTG G-3′ and reverse 5′- CTC GGA CGC CRG CTT CAC-3′.
2.6. Caspase activity assay
Caspase-3 activity was determined by using colorimetric assay kits according to the protocol of the manufacturer (BioVison, Milpitas, CA, USA). LLC-PK1 cells (1 × 106) seeded in 100-mm dishes were treated with 10 μg/mL KRG or 50 μg/mL KRG. After incubation for 2 h, 60μM FK506 was added to each well and incubated for 24 h. Cells were lysed in 50 μL of lysis buffer and incubated on ice for 10 min. Then, 50 μL of 2× reaction buffer containing 10mM dithiothreitol and 5 μL of 4mM DEVD-pNA substrate was added to each of the lysed samples and incubated for an additional 1 h at 37°C. Caspase activity was measured by absorbance at 400 nm using a PowerWave XS microplate reader (Bio-Tek Instruments).
2.7. Image-based cytometric assay
LLC-PK1 cells seeded in wells of six-well plates were treated with 10 μg/mL KRG or 50 μg/mL KRG. After incubation for 2 h, 60μM FK506 was added to each well and incubated for 3 h. Cells were stained with Annexin V Alexa Fluor 488 (Thermo Fischer Scientific, Waltham, MA, USA.) in darkness for 20 min after suspension in Annexin binding buffer and stained with propidium iodide in darkness for 5 min after suspension in Annexin binding buffer. The number of dead and apoptotic cells was measured using a Tali image-based cytometer (Invitrogen). This assay identified apoptotic cells stained green by Annexin V Alexa Fluor 488 from dead cells stained red from propidium iodide and green, and non-staining live cells.
2.8. Statistical analyses
Statistical significance was determined through analysis of variance (ANOVA) followed by a multiple comparison test with a Bonferroni adjustment. Values of p < 0.05 were considered statistically significant. Analyses were performed using SPSS ver. 19.0 software (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
FK506 is beneficial in avoiding solid organ transplant rejection. However, this use is hindered by the associated nephrotoxicity. The mechanisms of FK506-induced nephrotoxicity are poorly understood. Previous studies suggest that FK506 can produce ROS and can perturb antioxidant defense in the proximal tubules, which depend on oxidative phosphorylation for energy production, and which thus may be susceptible to oxidative stress [24], [25]. The result can be apoptosis and inflammation in proximal tubular cells.
In the present study, LLC-PK1 renal tubular epithelial cells were used to determine the protective effect of KRG against FK506-induced nephrotoxicity. Cytotoxicity of FK506 was explored using various concentrations of FK506. LLC-PK1 cell viability was reduced by 61.2% as compared with the control group (0.5% DMSO) after 60μM FK506 treatment (Fig. 1B), and 60μM FK506 was selected to evaluate the protective effect against FK506-induced nephrotoxicity in the following experiments. The reduction in cell viability by 60μM FK506 was ameliorated by KRG cotreatment in a dose-dependent manner. Particularly, the reduced LLC-PK1 cell viability was ameliorated by up to 81.8% after cotreatment with 50 μg/mL KRG (Fig. 1C). In a comparative experiment with KRG fractions, the butanol fraction mainly consisting of ginsenosides showed the strongest activity. LLC-PK1 cell viability was improved by up to 82.0% by pretreatment with 0.5 μg/mL of butanol fraction (Fig. 1C–G). Therefore, ginsenosides may be the main active ingredients of KRG to protect FK506-induced nephrotoxicity.
FK506 promotes death, proinflammatory response, and profibrotic responses as well as nephrotoxic responses in tubular cells by activation of protein kinases, such as the TAK1/JNK/AP-1 and TLR4/Myd88/IRAK pathways [26]. KIM-1 is a type I transmembranous protein that can be exploited as a sensitive biomarker of FK506-induced nephrotoxicity in rat proximal tubule epithelial cells [4]. FK506 also causes a significant increase in apoptosis of LLC-PK1 cells through a release of cytochrome c and activation of caspase-3 [7], [9].
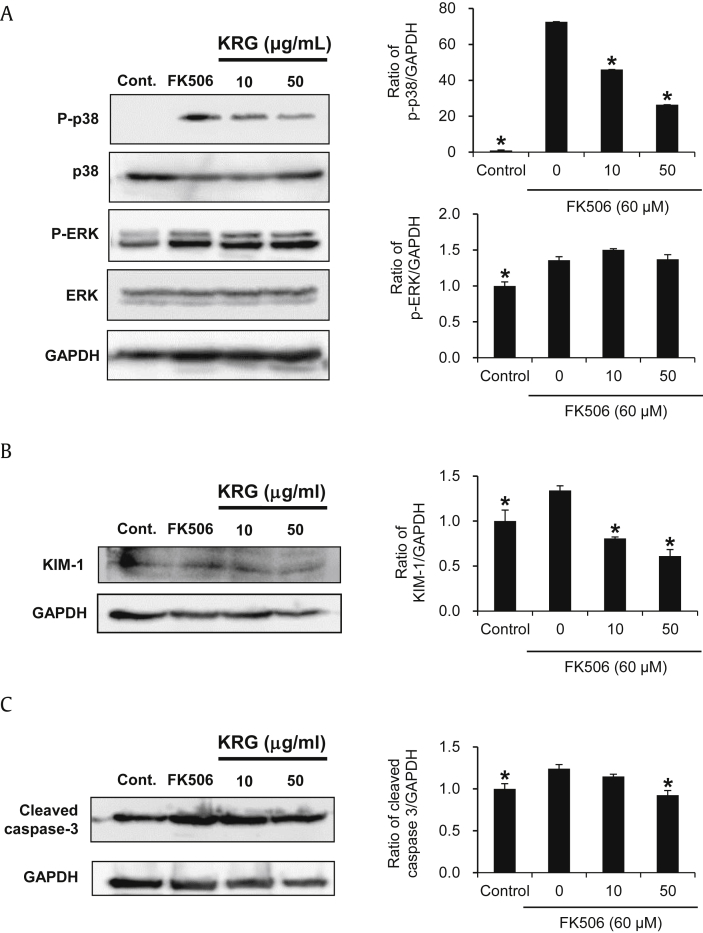
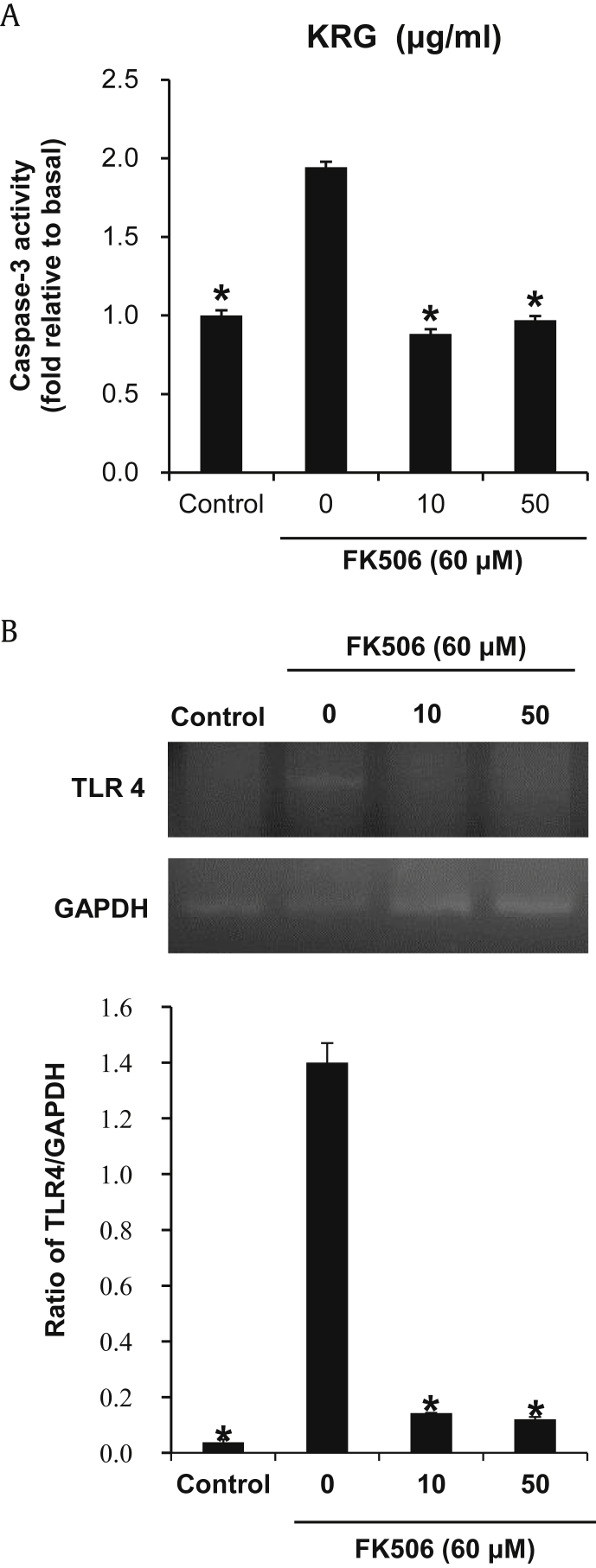
Considering the aforementioned protective effects of KRG on cell viability, we further evaluated the effect of KRG on MAPKs, caspase-3 activation, and TLR-4 mRNA expression on the FK506-induced nephrotoxicity in LLC-PK1 cells. The phosphorylation of p38 MAPK and p44/42 MAPK (ERK), and the production of KIM-1 and cleaved caspase-3 were increased markedly in LLC-PK1 cells treated with 60μM FK506. However, with the exception of p-ERK, elevated levels of p-p38, KIM-1, cleaved caspase-3, and TLR-4 mRNA expression were significantly decreased after cotreatment with KRG (Fig. 2A–C). Caspase-3 activity supported the Western blot result; activity was increased significantly after treatment with 60μM FK506, whereas either concentration of KRG completely abrogated FK506-induced caspase-3 activity to near basal levels (Fig. 3A). Similarly, increased TLR-4 mRNA expression also decreased by treatment with either concentration of KRG (Fig. 3B).
Fig. 2.
Effect of KRG on MAPKs, KIM-1 and caspase-3 activation in the FK506-induced nephrotoxicity in LLC-PK1 cells. (A)–(C) Protein expression levels of p-p38, p38, p-ERK, ERK, KIM-1, cleaved caspase-3 and GAPDH. *p < 0.05 compared to the FK506-treated value.
Fig. 3.
Effect of KRG on caspase-3 activity and TLR-4 mRNA expression on the FK506-induced nephrotoxicity in LLC-PK1 cells. (A) Activity level of caspase-3; (B) mRNA level of TLR-4. *p < 0.05 compared to the FK506-treated value.
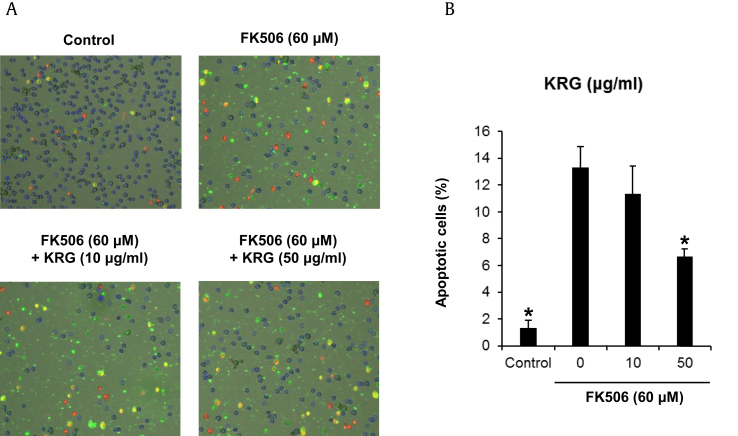
In the study about the protective effects of green tea extract and tea polyphenols against FK506-induced cytotoxicity, treatment of the cells with 50μM FK506 induced a significant increase in apoptotic cell death as demonstrated by Annexin V binding from 2.6% to 14.5% in LLC-PK1 cells [7]. To explore whether KRG could decrease FK506-induced apoptosis in LLC-PK1 cells through Annexin V Alexa Fluor 488 and propidium iodide staining, LLC-PK1 cells were treated with we treated with 10 μg/mL KRG or 50 μg/mL KRG. Apoptotic cell death evident by Annexin V staining was increased from 1.3% to 13.3% by treatment with 60μM FK506 treatment, whereas apoptosis decreased 6.6% after cotreatment with either concentration of KRG (Fig. 4A). The percentage of apoptotic cells is depicted in the bar graph of Fig. 4B.
Fig. 4.
Effects of KRG on apoptosis on the FK506-induced nephrotoxicity in LLC-PK1 cells. (A) Visualization of apoptosis detection. (B) Percentage of apoptotic cells stained with Annexin V and shown in green cells. *p < 0.05 compared to the FK506-treated value.
In an earlier study of the beneficial effects of KRG on drug-induced renal toxicity, KRG treatment significantly reduced the expression of both active caspase-3 and LC3-II in cyclosporine-induced autophagic cell death in mice [22]. Also, KRG treatment significantly reduced apoptotic cell death, as demonstrated by Annexin V binding by reducing oxidative stress in mice and proximal tubular cells (HK-2) [21]. In gentamicin-induced nephrotoxicity, KRG treatment significantly attenuated oxidative stress-mediated apoptosis by reducing the expression of mitochondrial Bax, cytosolic cytochrome c, and cleaved caspase-9 and caspase-3, along with a decrease in bcl-2 expression in rat NRK-52E renal tubular epithelial cells [20]. In cisplatin-induced nephrotoxicity, KRG treatment significantly decreased the levels of indicators of renal dysfunction, inflammatory cytokine expression, apoptosis, and malondialdehyde content in rats [23].
In summary, the protective effect and mechanism of KRG against FK506-induced nephrotoxicity was demonstrated in LLC-PK1 cells. KRG has an antiapoptotic effect by inhibition of p38 phosphorylation and activation of caspase, and an antiinflammatory effect by inhibition of TLR-4 expression. Also, the elevated KIM-1 expression by FK506 is decreased by cotreatment with KRG. Thus, KGR can be used as an adjuvant for the inhibition of adverse effect FK506 in the kidney.
Acknowledgments
This work was supported by the 2014 grant from the Korean Society of Ginseng.
Contributor Information
Ki Hyun Kim, Email: khkim83@skku.edu.
Noriko Yamabe, Email: norikoy@gachon.ac.kr.
Conflicts of interest
The authors have declared no conflicts of interest.
References
- 1.Wallemacq P.E., Reding R. FK506 (tacrolimus), a novel immunosuppressant in organ transplantation: clinical, biomedical, and analytical aspects. Clin Chem. 1993;39:2219–2228. [PMC free article] [PubMed] [Google Scholar]
- 2.Dumont F.J. FK506, an immunosuppressant targeting calcineurin function. Curr Med Chem. 2000;7:731–748. doi: 10.2174/0929867003374723. [DOI] [PubMed] [Google Scholar]
- 3.Tada H., Nakashima A., Awaya A., Fujisaki A., Inoue K., Kawamura K., Itoh K., Masuda H., Suzuki T. Effects of thymic hormone on reactive oxygen species-scavengers and renal function in tacrolimus-induced nephrotoxicity. Life Sci. 2002;70:1213–1223. doi: 10.1016/s0024-3205(01)01495-3. [DOI] [PubMed] [Google Scholar]
- 4.Cosner D., Zeng X., Zhang P.L. Proximal tubular injury in medullary rays is an early sign of acute tacrolimus nephrotoxicity. J Transplant. 2015;2015:142521. doi: 10.1155/2015/142521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randhawa P.S., Starzl T.E., Demetris A.J. Tacrolimus (FK506)-associated renal pathology. Adv Anat Pathol. 1997;4:265–276. doi: 10.1097/00125480-199707000-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen F.T., Leyssac P.P., Kemp E., Starklint H., Dieperink H. Nephrotoxicity of FK-506 in the rat. Studies on glomerular and tubular function, and on the relationship between efficacy and toxicity. Nephrol Dial Transplant. 1995;10:334–340. [PubMed] [Google Scholar]
- 7.Hisamura F., Kojima-Yuasa A., Kennedy D.O., Matsui-Yuasa I. Protective effect of green tea extract and tea polyphenols against FK506-induced cytotoxicity in renal cells. Basic Clin Pharmacol Toxicol. 2006;98:192–196. doi: 10.1111/j.1742-7843.2006.pto_284.x. [DOI] [PubMed] [Google Scholar]
- 8.Back J.H., Ryu H.H., Hong R., Han S.A., Yoon Y.M., Kim D.H., Hong S.J., Kim H.L., Chung J.H., Shin B.C., Kwon Y.E. Antiproteinuric effects of green tea extract on tacrolimus-induced nephrotoxicity in mice. Transplant Proc. 2015;47:2032–2034. doi: 10.1016/j.transproceed.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Hisamura F., Kojima-Yuasa A., Huang X., Kennedy D.O., Matsui-Yuasa I. Synergistic effect of green tea polyphenols on their protection against FK506-induced cytotoxicity in renal cells. Am J Chin Med. 2008;36:615–624. doi: 10.1142/S0192415X08006028. [DOI] [PubMed] [Google Scholar]
- 10.Jeon B.R., Kim S.J., Hong S.B., Park H.J., Cho J.Y., Rhee M.H. The inhibitory mechanism of crude saponin fraction from Korean Red Ginseng in collagen-induced platelet aggregation. J Ginseng Res. 2015;39:279–285. doi: 10.1016/j.jgr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang Y., Lee W.J., Hong G.S., Shim W.S. Red ginseng extract blocks histamine-dependent itch by inhibition of H1R/TRPV1 pathway in sensory neurons. J Ginseng Res. 2015;39:257–264. doi: 10.1016/j.jgr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J.Y., Lee S., Yang J.H., Kim S., Sim J., Kim M.G., Jeong T.C., Ku S.K., Cho I.J., Ki S.H. Korean Red Ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J Ginseng Res. 2015;39:105–115. doi: 10.1016/j.jgr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S.E., Park C., Kim S.H., Hossain M.A., Kim M.Y., Chung H.Y., Son W.S., Kim G.Y., Choi Y.H., Kim N.D. Korean red ginseng extract induces apoptosis and decreases telomerase activity in human leukemia cells. J Ethnopharmacol. 2009;121:304–312. doi: 10.1016/j.jep.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Jung S.W., Kim H.J., Lee B.H., Choi S.H., Kim H.S., Choi Y.K., Kim J.Y., Kim E.S., Hwang S.H., Lim K.Y., Kim H.C., Jang M., Park S.K., Cho I.H., Nah S.Y. Effects of Korean Red Ginseng extract on busulfan-induced dysfunction of the male reproductive system. J Ginseng Res. 2015;39:243–249. doi: 10.1016/j.jgr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang C.S., Hong S.H., Suk K.T., Kim J.B., Han S.H., Sung H., Kim E.J., Kim M.J., Kim M.Y., Baik S.K., Kim D.J. Effects of Korean Red Ginseng (Panax ginseng), urushiol (Rhus vernicifera Stokes), and probiotics (Lactobacillus rhamnosus R0011 and Lactobacillus acidophilus R0052) on the gut-liver axis of alcoholic liver disease. J Ginseng Res. 2014;38:167–172. doi: 10.1016/j.jgr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H., Choi J. Shik Shin S, Yoon M. Effects of Korean red ginseng (Panax ginseng) on obesity and adipose inflammation in ovariectomized mice. J Ethnopharmacol. 2015;S0378–8741:30270–30271. doi: 10.1016/j.jep.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Park H.M., Kim S.J., Mun A.R., Go H.K., Kim G.B., Kim S.Z., Jang S.I., Lee S.J., Kim J.S., Kang H.S. Korean red ginseng and its primary ginsenosides inhibit ethanol-induced oxidative injury by suppression of the MAPK pathway in TIB-73 cells. J Ethnopharmacol. 2012;141:1071–1076. doi: 10.1016/j.jep.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Jun Y.L., Bae C.H., Kim D., Koo S., Kim S. Korean Red Ginseng protects dopaminergic neurons by suppressing the cleavage of p35 to p25 in a Parkinson's disease mouse model. J Ginseng Res. 2015;39:148–154. doi: 10.1016/j.jgr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y.K., Chin Y.W., Choi Y.H. Effects of Korean red ginseng extract on acute renal failure induced by gentamicin and pharmacokinetic changes by metformin in rats. Food Chem Toxicol. 2013;59:153–159. doi: 10.1016/j.fct.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Shin H.S., Yu M., Kim M., Choi H.S., Kang D.H. Renoprotective effect of red ginseng in gentamicin-induced acute kidney injury. Lab Invest. 2014;94:1147–1160. doi: 10.1038/labinvest.2014.101. [DOI] [PubMed] [Google Scholar]
- 21.Doh K.C., Lim S.W., Piao S.G., Jin L., Heo S.B., Zheng Y.F., Bae S.K., Hwang G.H., Min K.I., Chung B.H., Yang C.W. Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am J Nephrol. 2013;37:421–433. doi: 10.1159/000349921. [DOI] [PubMed] [Google Scholar]
- 22.Lim S.W., Doh K.C., Jin L., Jin J., Piao S.G., Heo S.B., Chung B.H., Yang C.W. Ginseng treatment attenuates autophagic cell death in chronic cyclosporine nephropathy. Nephrology (Carlton) 2014;19:490–499. doi: 10.1111/nep.12273. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y.J., Lee M.Y., Son H.Y., Park B.K., Ryu S.Y., Jung J.Y. Red ginseng ameliorates acute cisplatin-induced nephropathy. Planta Med. 2014;80:645–654. doi: 10.1055/s-0034-1368571. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X., Yang G., Davis C.A., Doi S.Q., Hirszel P., Wingo C.S., Agarwal A. Hydrogen peroxide mediates FK506-induced cytotoxicity in renal cells. Kidney Int. 2004;65:139–147. doi: 10.1111/j.1523-1755.2004.00380.x. [DOI] [PubMed] [Google Scholar]
- 25.Al-Harbi N.O., Imam F., Al-Harbi M.M., Iqbal M., Nadeem A., Al-Shahrah O.A., Korashy H.M., Al-Hosaini K.A., Ahmed M., Bahashwar S. Treatment with aliskiren ameliorates tacrolimus-induced nephrotoxicity in rats. J Renin Angiotensin Aldosterone Syst. 2015;16:1329–1336. doi: 10.1177/1470320314530178. [DOI] [PubMed] [Google Scholar]
- 26.González-Guerrero C., Ocaña-Salceda C., Berzal S., Carrasco S., Fernández-Fernández B., Cannata-Ortiz P., Egido J., Ortiz A., Ramos A.M. Calcineurin inhibitors recruit protein kinases JAK2 and JNK, TLR signaling and the UPR to activate NF-κB-mediated inflammatory responses in kidney tubular cells. Toxicol Appl Pharmacol. 2013;272:825–841. doi: 10.1016/j.taap.2013.08.011. [DOI] [PubMed] [Google Scholar]