Abstract
Background
Korean Red Ginseng (KRG) is a traditional herbal medicine made by steaming and drying fresh ginseng. It strengthens the endocrine and immune systems to ameliorate various inflammatory responses. The cyclooxygenase-2 (COX-2)/prostaglandin E2 pathway has important implications for inflammation responses and tumorigenesis. Peroxisome proliferator-activated receptor gamma (PPARγ) is a transcription factor that regulates not only adipogenesis and lipid homeostasis, but also angiogenesis and inflammatory responses.
Methods
The effects of the KRG on inhibition of hypoxia-induced COX-2 via PPARγ in A549 cells were determined by luciferase assay, Western blot, and/or quantitative reverse transcription-polymerase chain reaction (qRT-PCR). The antimigration and invasive effects of KRG were evaluated on A549 cells using migration and matrigel invasion assays.
Results and conclusion
We previously reported that hypoxia-induced COX-2 protein and mRNA levels were suppressed by KRG. This study examines the possibility of PPARγ as a cellular target of KRG for the suppression of hypoxia-induced COX-2. PPARγ protein levels and PPARγ-responsive element (PPRE)-driven reporter activities were increased by KRG. Reduction of hypoxia-induced COX-2 by KRG was abolished by the PPARγ inhibitor GW9662. In addition, the inhibition of PPARγ abolished the effect of KRG on hypoxia-induced cell migration and invasion.
Discussion
Our results show that KRG inhibition of hypoxia-induced COX-2 expression and cell invasion is dependent on PPARγ activation, supporting the therapeutic potential for suppression of inflammation under hypoxia. Further studies are required to demonstrate whether KRG activates directly PPARγ and to identify the constituents responsible for this activity.
Keywords: cyclooxygenase-2, hypoxia, Korean Red Ginseng, PPARγ
1. Introduction
Ginseng is a popular herbal medicine that has been used for over 2,000 y in Oriental countries. Its use is not confined to Asia but has expanded to Western countries as one of the top 10 best-selling herbs [1]. This popularity and its worldwide consumption indirectly demonstrate its efficacy, and accumulating scientific evidence shows that ginseng has a wide range of pharmacological activities in the cardiovascular, endocrine, immune, and central nervous systems [2]. It is especially well established that ginseng ameliorates inflammatory responses [3], [4], [5]. Data have shown that ginsenosides are pharmacological compounds with antiinflammatory and anticarcinogenic effects both in vivo and in vitro [6], [7].
Red ginseng is made by steaming and drying fresh ginseng. The pharmacological efficacy of Korean ginseng is known to be enhanced by these special processes, mostly due to changes in the characteristics of the constituent ginsenosides [8], [9]. During the steaming process, seven ginsenosides (Rg1, Re, Rb1, Rc, Rb2, Rb3, and Rd) decreased, while five ginsenosides (Rh1, Rg2, 20R-Rg2, Rg3, and Rh2) increased [10].
Hypoxia is a state of reduced overall tissue oxygen availability and a hallmark of solid tumors that leads to cell invasion and metastasis [11]. Cyclooxygenase-2 (COX-2) is induced by various stimuli such as lipopolysaccharide (LPS), cytokines, hypertonicity, and hypoxia [12], [13], [14], [15]. COX-2 increases the metastatic potential of cancer cells, and silencing COX-2 inhibits metastasis and delays tumor onset in poorly differentiated metastatic cancers [16], [17]. Mammary epithelial cells express peroxisome proliferator-activated receptor gamma (PPARγ), and its signaling is critical during breast tumorigenesis and correlated with COX-2 expression [18]. These observations indicate the importance of COX-2 inhibition in preventing hypoxia-induced cell invasion.
PPARγ, a member of a nuclear receptor superfamily, heterodimerizes with the retinoid X receptor and activates transcription by binding to the PPAR response elements of its target genes [19]. Endogenous ligands for PPARγ include fatty acids and prostanoids. PPARγ regulates adipogenesis by differentiating adipocytes, lipid metabolism, inflammation, and angiogenesis [20]. PPARγ regulates COX-2 gene expression through PPAR response elements within the promoter of COX-2 [21], [22]. However, depending on the cell type, PPARγ can both activate and inhibit COX-2 through PPARγ-dependent and -independent mechanisms [23], [24], [25]. Continuous research is required to understand these complex phenomena. PPARγ-activating natural products and plant extracts have been extensively sought after and studied because of their great potential for use in the treatment of a variety of metabolic syndromes [26], [27].
We previously showed that Korean Red Ginseng (KRG) efficiently blocks hypoxia-induced COX-2 mediated by sirtuin-1 (SIRT-1), the pathway of which differs from that of dexamethasone [28]. This provides scientific evidence of KRG being effective for the suppression of the inflammatory response and tumorigenesis under hypoxia through mechanisms other than those of steroids. We present herein further evidence that KRG suppresses hypoxia-induced COX-2 and is dependent on the PPARγ signaling pathway and that PPARγ activation by KRG may reduce the tumorigenesis of pulmonary epithelial cells.
2. Materials and methods
2.1. Materials
KRG was kindly supplied by the Korea Ginseng Cooperation (Daejeon, Korea). KRG is prepared from roots of 6-yr-old KRG. Voucher specimen (KGC No. 201-3-1081) of KRG was deposited at the herbarium located at KGC Central Research Institute (Daejeon, Korea). Yield of KRG extract was 75%. The water content of the pooled extract was 36% of total weight. Phytochemical characteristics of KRG with standard ginsenosides were identified by HPLC analysis as reported previously [29], [30]. HPLC analysis result of standard ginsenosides is provided by Korea Ginseng Cooperation [28]. The ginsenoside content in KRG is 7%, and it is composed of major ginsenosides (G-Rg1, 1.79 mg/g; G-Re, 1.86 mg/g; G-Rf, 1.24 mg/g; G-Rh1, 1.01 mg/g; G-Rg2s, 1.24 mg/g; G-Rb1, 7.44 mg/g; G-Rc, 3.04 mg/g; G-Rb2, 2.59 mg/g; and G-Rd, 0.91 mg/g), and other minor ginsenoside components [29], [30]. GW9662 and celecoxib were purchased from Sigma (St. Louis, MO, USA). T0070907 was purchased from Selleckchem (Houston, TX, USA). Fetal bovine serum (FBS), Trizol Reagent, and penicillin/streptomycin were purchased from GIBCO Invitrogen (Grand Island, NY, USA). Anti-COX-2 was obtained from Cayman Chemical (Ann Arbor, MI, USA). Anti-β-actin was purchased from Sigma. Anti-sirtuin-1 (SIRT-1), anti-PPARγ and anti-peroxisome proliferator-activated receptor gamma coactivatior-1 alpha (PGC-1α) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Cell culture and hypoxic conditions
Human pulmonary epithelial A549 cells were maintained in Roswell Park Memorial Institute medium (RPMI) containing 10% FBS and penicillin/streptomycin. Cells were grown at 37°C in a humidified atmosphere of 95% air/5% CO2 and fed every 2–3 d. Before treatment, the cells were washed with phosphate-buffered saline and cultured in RPMI/5% charcoal–dextran stripped FBS (CD-FBS) for 2 d. For the hypoxic condition, cells were incubated at a CO2 level of 5% with 1% O2 balanced with N2 using a hypoxic chamber (Thermo Fisher Scientific, Waltham, MA, USA). KRG stock was prepared at 10 mg/mL in phosphate buffer saline and diluted with media to 1 mg/mL just prior to use and sterilized by filtration with a 0.22 μm bottle top filter (Thermo Fisher Scientific).
2.3. Transfection and luciferase assays
A549 cells were transiently transfected with plasmids by using the polyethylenimine (Polysciences, Warrington, PA, USA). Luciferase activity was determined 48 h after treatment with an AutoLumat LB9507 luminometer (EG & G Berthold, Bad Widbad, Germany) using the luciferase assay system (Promega Corp., Madison, WI, USA) and expressed as relative light units. PPARγ-responsive element-Luciferase (PPRE-Luc), a firefly luciferase reporter construct containing PPRE-elements, was kindly provided Dr. Ron Evans (The Salk Institute, San Diego CA, USA).
2.4. Reverse transcription-polymerase chain reaction
Total RNA was extracted using Trizol Reagent according to the manufacturer's instruction. To synthesize first strand cDNA, 3 μg total RNA was incubated at 70°C for 5 min with 0.5 μg of random hexamer and deionized water (up to 11 μL). The reverse transcription reaction was performed using 40 units of Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Promega Corp.) in 5× reaction buffer (250 mmol/L Tris-HCl; pH 8.3, 375mM KCl, 15mM MgCl2, 50mM dithiothreitol (DTT)), RNase inhibitor at 1 unit/μL, and 1mM dNTP mixtures at 37°C for 60 min. Real-time polymerase chain reaction (PCR) was performed with STEP ONE (Applied Biosystems, Foster City, CA, USA) using a SYBR green premix according to the manufacturer's instructions, as reported previously [31], [32], [33]. The primers used were: β-actin sense primer, 5′-CAAATGCTTCTAGGCGGACTATG-3′; β-actin anti-sense primer, 5′-TGCGCAAGTTAGGTTTTGTCA-3′; COX-2 sense primer, 5′-TGAAGAACTTACAGGAGAAAA-3′; COX-2 anti-sense primer, 5′-TACCAGAAGGGCAGGATACA-3′. Using the comparative threshold cycle (Ct), relative expression was calculated and normalized by the expressions of β-actin from the same samples.
2.5. Western blot analysis
Protein was isolated in lysis buffer (150mM NaCl, 50mM Tris-HCl, 5mM EDTA, 1% Nonidet P-40, 0.5% deoxycholate, 1% SDS) with protease inhibitor cocktail (Sigma) on ice for 1 h and then centrifuged for 20 min at 13,000g. Supernatant was collected and protein concentrations were measured using the Bradford method (Bio-Rad, Hercules, CA, USA). Proteins were dissolved in sample buffer and boiled for 5 min prior to loading onto an acrylamide gel. After SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane, blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 60 min at room temperature. The membranes were incubated for 2 h at room temperature with antibody. Equal lane loading was assessed using β-actin monoclonal antibody (Sigma). After washing with TBST, blots were incubated with 1:5,000 dilution of the horseradish peroxidase conjugated-secondary antibody (Invitrogen, Grand Island, NY, USA), and washed again three times with TBST. The transferred proteins were visualized with an enhanced chemiluminescence detection kit (GE Healthcare Life Sciences, Pittsburgh, PA, USA).
2.6. Cell migration and invasion assays
The migration assay was performed with transwell inserts that have 6.5 mm polycarbonate membranes with 8.0 μm pores (Corning Inc., NY, USA). Matrigel invasion assay was performed using membranes coated with matrigel matrix (BD Science, Sparks, MD, USA). A549 cells were seeded into the upper chamber in serum-free media. The lower chambers consisted of RPMI media containing 10% FBS. After incubation under normoxia or hypoxia for 24–48 h, noninvasive cells present on the upper surface of the membrane were scraped with cotton swabs and the invasive cells present on the lower side of the membrane were fixed with ice cold methanol, stained with 0.1% crystal violet. The cells that migrated and invaded to the lower side of the filter were observed using a light microscope and counted.
2.7. Statistical analysis
All data were analyzed and expressed as means and standard deviations. The two-tailed, unpaired Student t test was applied using SPSS software (version 23.0; IBM, Armonk, NY, USA). The t test was used to compare data between the hypoxia and KRG-treated groups. The criterion for statistical significance was p < 0.05.
3. Results
3.1. KRG induces PPARγ and inhibits hypoxic induction of COX-2 expression in A549 cells
COX-2 is transcriptionally induced by hypoxia and has been implicated in tumor progression and angiogenesis in tumor cells. We have previously shown that KRG inhibits COX-2 expression under hypoxia in A549 lung cancer cells, where COX-2 is also strongly implicated in tumorigenesis [28]. In the course of studying the mechanism of KRG inhibition of COX-2 under hypoxia, the protein levels of PPARγ were examined. A549 cells were preincubated with KRG for 1 h and cotreated with hypoxia. KRG at doses of 500–2,000 μg/mL increased PPARγ protein levels. At the same time, as observed previously, KRG efficiently blocked the expression of hypoxia-induced COX-2 protein (Fig. 1A). KRG at doses of 100–2,000 μg/mL activated PPARγ luciferase reporter activity. To confirm that the activities of KRG are PPARγ-mediated, we cotreated the cells with the PPARγ antagonist T0070907 at a concentration of 5μM, which is enough to block almost all the PPARγ on the cells. A known PPARγ agonist, rosiglitazone, was used as a positive control. The transcriptional activation of the reporter plasmid by KRG was blocked by T0070907, indicating that luciferase gene activation is PPARγ-specific (Fig. 1B). The concentration of 100–500 μg/mL KRG was chosen as the treatment condition in further experiments because KRG exerted its efficacy with no effect on cell viability at these concentrations (Fig. 1C).
Fig. 1.
Korean Red Ginseng (KRG) induces peroxisome proliferator-activated receptor gamma (PPARγ) activity and its expression. (A) A549 cells were treated with KRG at 100–2,000 μg/mL for 24 h under hypoxia and analyzed by Western using indicated antibodies. (B) A549 cells were transiently transfected with the PPARγ-responsive element (PPRE)-luciferase reporter gene. The following day, A549 cells were cultured in medium containing vehicle or KRG (100–2,000 μg/mL) or PPARγ agonist rosiglitazone (1μM) or PPARγ antagonist T0070907 (5μM) for 48 h and luciferase activities were determined. (C) A549 cells were incubated with KRG at 10–2,000 μg/mL for 24 h under hypoxia and 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay was performed. All experiments were repeated at least three times.
To further confirm the involvement of PPARγ in inhibiting the hypoxic induction of COX-2, COX-2 was examined after treatment with another PPARγ antagonist, GW9662. In accordance with our previous report, SIRT-1 levels were increased with KRG treatments. The enhanced protein levels of SIRT-1, PPARγ, and COX2 were blocked by GW9662, suggesting that the response involves PPARγ (Fig. 2A). Induction of COX-2 occurs mostly at the transcription level. Inhibition of COX-2 at the mRNA level by 500 μg/mL KRG was blocked by GW9662 (Fig. 2B). These results indicate that the inhibition of hypoxia-induced COX-2 regulation by KRG at the transcription and translation levels in A549 cells is dependent on PPARγ.
Fig. 2.
Korean Red Ginseng (KRG) downregulates cyclooxygenase-2 (COX-2) expression through peroxisome proliferator-activated receptor gamma (PPARγ) activation. (A) A549 cells were pretreated with KRG (100 μg/mL or 500 μg/mL) and/or GW9662 (5μM) for 1 h before treatment with hypoxia for 24 h. Total proteins were prepared and protein levels of sirtuin-1 (SIRT-1), PPARγ, and COX-2 were determined by western blot. (B) A549 cells were pretreated with KRG (500 μg/mL) and/or GW9662 (5μM) for 1 h before treatment with hypoxia for 24 h. Total RNA from A549 cells were analyzed for COX-2 mRNA expression by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). All experiments were repeated at least three times.
3.2. KRG inhibits cellular migration and invasion of A549 cells under hypoxia
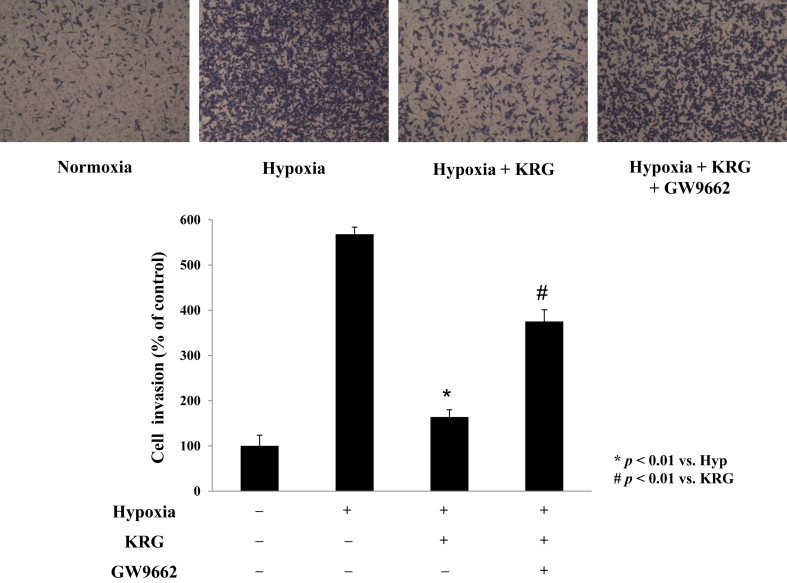
To determine whether hypoxia induces cell migration via COX-2–dependent PGE2 production in A549 cells, cell migration was examined with the COX-2 inhibitor, celecoxib. Hypoxia-induced cell migration was inhibited by celecoxib, suggesting that COX-2 plays an important role in hypoxia-induced cell migration (Fig. 3A). KRG decreased the migration of A549 cells under hypoxic conditions by approximately 50%. KRG inhibition of cell migration was significantly blocked by GW9662 and T0070907, indicating that the effects of KRG on hypoxia-induced migration are mediated by PPARγ (Fig. 3B). Similarly, hypoxia enhanced A549 cell invasion and KRG treatments significantly decreased this effect (Fig. 4). Inhibition of invasiveness was absent in the presence of GW9662, indicating that the anti-invasive effects of KRG under hypoxic conditions are dependent on PPARγ. The invasiveness was absent in the presence of PPARγ inhibitors, indicating that the anti-invasive properties of KRG under hypoxic microenvironments require PPARγ activation and occur via a COX-2/PGE2-dependent pathway. However, this migration and invasion were also inhibited by SIRT-1 inhibitors [28], suggesting that this cellular movement is a complicated phenomenon relying on multiple factors.
Fig. 3.
Korean Red Ginseng (KRG) inhibits migration ability of A549 cells under hypoxia. (A) A549 cells were pretreated with KRG (100 μg/mL) and/or celecoxib (5μM) treatment with hypoxia for 24 h. (B) A549 cells were pretreated with KRG (100 μg/mL) and/or GW9662 (5μM) or T0070907 (5μM) treatment with hypoxia for 24 h. Cells that migrated through the membranes were fixed and counted using light microscopy. Bar graph shows relative migrated cells. The cells in the lower side were counted and are graphed below. Values represent the mean ± standard deviation (n = 3). * p < 0.05 vs. hypoxia, ** p < 0.01 vs. hypoxia, # p < 0.01 vs. KRG. All experiments were repeated at least three times.
Fig. 4.
Korean Red Ginseng (KRG) inhibits cellular invasion of A549 cells under hypoxia. A549 cells were pretreated with KRG (100 μg/mL) and/or GW9662 (5μM) for 1 h before treatment with hypoxia for 48 h. Matrigel invasion assay was done under normoxia or hypoxia. The cells in the lower side were counted and are graphed below. Values represent the mean ± standard deviation (n = 3). ** p < 0.01 vs. hypoxia, # p < 0.01 vs. KRG. All experiments were repeated at least three times.
4. Discussion
We have recently reported that SIRT-1 is an important player in the suppression of hypoxia-induced COX-2 by KRG in A549 cells [28]. We suggested that SIRT-1 activation by KRG has potential therapeutic value in the suppression of inflammation and in cancer therapies under hypoxic conditions. COX-2 is rapidly induced by various stimuli such as LPS, high osmolarity, and hypoxia, and plays a pivotal role in the production of proinflammatory eicosanoids. High levels of PGE2 synthesized by COX-2 are an important mediator in airway inflammatory responsiveness and angiogenesis involved in tumor development [34]. Many reports have shown that KRG possesses antiinflammatory and antioxidant properties both in vitro and in vivo [35], [36]. However, better understanding of the molecular mechanisms underlying the inhibitory effect of KRG on hypoxia-induced COX-2 is still needed. In this study, we examined the PPARγ-mediated KRG inhibition of COX-2 under hypoxia and showed that PPARγ activation is responsible for COX-2 suppression under hypoxia.
Peroxisome Proliferator-Activated Receptors (PPARs) is a ligand-activated transcription factor and regulates diverse biological functions including adipocyte differentiation, lipogenesis, inflammation, and insulin sensitivity. PPARγ is one pathway that modulates the thriving of cancer cells by multiple complex pathways, thereby sustaining uncontrolled tumor growth. Endogenous ligands including prostaglandins (15d-PGJ2) and synthetic ligands including the antidiabetic thiazolidinediones are known to bind PPAR. Upon ligand binding, PPAR is modified by phosphorylation, sumoylation, ubiquitination, and acetylation. PPARγ is acetylated by CBP/p300 and deacetylated by SIRT-1 [37].
Some PPARγ-activating ligands that modulate inflammation have been discovered from natural products such as curcumin, alpha-linolenic acid, magnolol, and orange peel extract [38], [39], [40], [41]. A recent report showed that fisetin, a flavonol present in vegetables and fruits, upregulates adiponectin with antiobesity, antidiabetic, and antiatherosclerotic functions through the activation of SIRT-1 and PPAR [42]. Ginsenosides from ginseng showed differential response to PPAR activation. Ginsenosides Re and Rg1 showed inhibitory effects on the differentiation of 3T3-L1 adipocytes [43]. However, ginsenosides Rb2, Rb3, and Rc displayed promotional activities [44]. Ginsenoside-Rg3 induces inhibition of adipogenesis through the activation of AMP-activated protein kinase (AMPK) and the inhibition of PPARγ transcriptional activity in 3T3-L1 adipocytes [45]. Protopanaxatriol is a novel PPARγ antagonist with moderate binding activity [44]. By contrast, Ginsenoside-Rb1 binds to PPARγ, attenuates central inflammation and leptin resistance, reduces the release of free fatty acid, and alleviates the ectopic deposit of triglyceride by upregulating the expression of perilipin in adipose tissue [26]. Ginsenoside-Re reduces insulin resistance through activation of PPARγ pathway and inhibited the production of inflammatory cytokine [43]. Ginsenoside-Rg1 can increase the insulin-degrading enzyme expression in the hippocampus by upregulating PPARγ, attenuated hippocampal histopathological abnormalities, and improved learning and memory in a rat model of Alzheimer's disease [46].
Our study showed that total ginseng extract exhibited PPARγ and SIRT-1 activation with simultaneous suppression of COX-2. Our observation was derived from the sum of activity from each component and interactions between the components. These results will aid our understanding of how ginseng is beneficial in the treatment of metabolic disorders. Our data imply that KRG inhibits COX-2 expression by increasing SIRT-1 deacetylase activity, leading to increased interactions with the PPAR complex and ultimately transcriptional activation. The activation of SIRT-1 by KRG may deacetylate PGC-1α, which in turn may increase its interaction with the PPAR complex to suppress COX-2 transcription. Our ongoing study is designed to characterize the COX-2 suppression and PPARγ activation functions of each ginsenoside in A549 cells, as used in this study, and 3T3L1 preadipocytes. Our findings are important for the clinical usage of ginseng and provide a mechanistic explanation of its effects on metabolic disorders and cancer.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by a 2014 grant from the Korean Society of Ginseng and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2015M3C8A6A06014500; 2015R1A2A2A04003620) to Y.J.L.
References
- 1.Qi L.W., Wang C.Z., Yuan C.S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72:689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya S.K., Mitra S.K. Anxiolytic activity of Panax ginseng roots: an experimental study. J Ethnopharmacol. 1991;34:84–92. doi: 10.1016/0378-8741(91)90193-h. [DOI] [PubMed] [Google Scholar]
- 3.Jin Y., Kotakadi V.S., Ying L., Hofseth A.B., Cui X., Wood P.A., Windust A., Matesic L.E., Pena E.A., Chiuzan C. American ginseng suppresses inflammation and DNA damage associated with mouse colitis. Carcinogenesis. 2008;29:2351–2359. doi: 10.1093/carcin/bgn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J.S., Shin J.A., Jung J.S., Hyun J.W., Van Le T.K., Kim D.H., Park E.M., Kim H.S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J Pharmacol Exp Ther. 2012;341:59–67. doi: 10.1124/jpet.111.189035. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y., Yang W.S., Yu T., Sung G.H., Park K.W., Yoon K., Son Y.J., Hwang H., Kwak Y.S., Lee C.M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J Ethnopharmacol. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Kim H.J., Kim P., Shin C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37:8–29. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee B., Sur B., Park J., Kim S.H., Kwon S., Yeom M., Shim I., Lee H., Hahm D.H. Ginsenoside Rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol Ther (Seoul) 2013;21:381–390. doi: 10.4062/biomolther.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenthal M. Asian ginseng: potential therapeutic uses. Adv Nurse Pract. 2001;9:26–28. [PubMed] [Google Scholar]
- 9.Baek K.S., Hong Y.D., Kim Y., Sung N.Y., Yang S., Lee K.M., Park J.Y., Park J.S., Rho H.S., Shin S.S. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J Ginseng Res. 2015;39:155–161. doi: 10.1016/j.jgr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C.Z., Zhang B., Song W.X., Wang A., Ni M., Luo X., Aung H.H., Xie J.T., Tong R., He T.C. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 11.Arsenault D., Brochu-Gaudreau K., Charbonneau M., Dubois C.M. HDAC6 deacetylase activity is required for hypoxia-induced invadopodia formation and cell invasion. PLoS One. 2013;8:e55529. doi: 10.1371/journal.pone.0055529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredenburgh L.E., Ma J., Perrella M.A. Cyclooxygenase-2 inhibition and hypoxia-induced pulmonary hypertension: effects on pulmonary vascular remodeling and contractility. Trends Cardiovasc Med. 2009;19:31–37. doi: 10.1016/j.tcm.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaidi A., Qualtrough D., Williams A.C., Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.J., Natsuizaka M., Ohashi S., Wong G.S., Takaoka M., Michaylira C.Z., Budo D., Tobias J.W., Kanai M., Shirakawa Y. Hypoxia activates the cyclooxygenase-2-prostaglandin E synthase axis. Carcinogenesis. 2010;31:427–434. doi: 10.1093/carcin/bgp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L., Wu Y., Xu Z., Wang H., Zhao Z., Li Y., Yang P., Wei X. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J Cell Mol Med. 2012;16:1840–1855. doi: 10.1111/j.1582-4934.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stasinopoulos I., Shah T., Penet M.F., Krishnamachary B., Bhujwalla Z.M. COX-2 in cancer: Gordian knot or Achilles heel? Front Pharmacol. 2013;4:34. doi: 10.3389/fphar.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsujii M., Kawano S., DuBois R.N. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apostoli A.J., Roche J.M., Schneider M.M., SenGupta S.K., Di Lena M.A., Rubino R.E., Peterson N.T., Nicol C.J. Opposing roles for mammary epithelial-specific PPARγ signaling and activation during breast tumour progression. Mol Cancer. 2015;14:85. doi: 10.1186/s12943-015-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyagi S., Gupta P., Saini A.S., Kaushal C., Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu G., Yi J., Liu L., Wang P., Zhang Z., Li Z. Pseudoginsenoside F11, a novel partial PPARγ agonist, promotes adiponect in oligomerization and secretion in 3T3-L1 adipocytes. PPAR Res. 2013;2013:701017. doi: 10.1155/2013/701017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazra S., Dubinett S.M. Ciglitazone mediates COX-2 dependent suppression of PGE2 in human non-small cell lung cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2007;77:51–58. doi: 10.1016/j.plefa.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel L., Pass I., Coxon P., Downes C.P., Smith S.A., Macphee C.H. Tumor suppressor and anti-inflammatory actions of PPARgamma agonists are mediated via upregulation of PTEN. Curr Biol. 2001;11:764–768. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 23.Bren-Mattison Y., Meyer A.M., Van Putten V., Li H., Kuhn K., Stearman R., Weiser-Evans M., Winn R.A., Heasley L.E., Nemenoff R.A. Antitumorigenic effects of peroxisome proliferator-activated receptor-gamma in non-small-cell lung cancer cells are mediated by suppression of cyclooxygenase-2 via inhibition of nuclear factor-kappaB. Mol Pharmacol. 2008;73:709–717. doi: 10.1124/mol.107.042002. [DOI] [PubMed] [Google Scholar]
- 24.Patel K.M., Wright K.L., Whittaker P., Chakravarty P., Watson M.L., Ward S.G. Differential modulation of COX-2 expression in A549 airway epithelial cells by structurally distinct PPAR(gamma) agonists: evidence for disparate functional effects which are independent of NF-(kappa)B and PPAR(gamma) Cell Signal. 2005;17:1098–1110. doi: 10.1016/j.cellsig.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Meade E.A., McIntyre T.M., Zimmerman G.A., Prescott S.M. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J Biol Chem. 1999;274:8328–8334. doi: 10.1074/jbc.274.12.8328. [DOI] [PubMed] [Google Scholar]
- 26.Mu Q., Fang X., Li X., Zhao D., Mo F., Jiang G., Yu N., Zhang Y., Guo Y., Fu M. Ginsenoside Rb1 promotes browning through regulation of PPARγ in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2015;466:530–535. doi: 10.1016/j.bbrc.2015.09.064. [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Waltenberger B., Pferschy-Wenzig E.M., Blunder M., Liu X., Malainer C., Blazevic T., Schwaiger S., Rollinger J.M., Heiss E.H. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem Pharmacol. 2014;92:73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim W., Shim M.K., Kim S., Lee Y. Red ginseng represses hypoxia-induced cyclooxygenase-2 through sirtuin1 activation. Phytomedicine. 2015;22:597–604. doi: 10.1016/j.phymed.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Kang T.H., Park H.M., Kim Y.B., Kim H., Kim N., Do J.H., Kang C., Cho Y., Kim S.Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Kim I.W., Sun W.S., Yun B.S., Kim N.R., Min D., Kim S.K. Characterizing a full spectrum of physico-chemical properties of (20S)- and (20R)-ginsenoside Rg3 to be proposed as standard reference materials. J Ginseng Res. 2013;37:124–134. doi: 10.5142/jgr.2013.37.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noh H., Park J., Shim M., Lee Y. Trichostatin A enhances estrogen receptor-alpha repression in MCF-7 breast cancer cells under hypoxia. Biochem Biophys Res Commun. 2016;470:748–752. doi: 10.1016/j.bbrc.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Shim M., Bae J.Y., Lee Y.J., Ahn M.J. Tectoridin from Maackia amurensis modulates both estrogen and thyroid receptors. Phytomedicine. 2014;21:602–606. doi: 10.1016/j.phymed.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 33.Kim B., Kim J.E., Choi B.K., Kim H.S. Anti-inflammatory effects of water chestnut extract on cytokine responses via nuclear factor-κB-signaling pathway. Biomol Ther. 2015;23:90–97. doi: 10.4062/biomolther.2014.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu C.K., Lin C.C., Hsiao L.D., Yang C.M. Mevastatin ameliorates sphingosine 1-phosphate-induced COX-2/PGE2-dependent cell migration via FoxO1 and CREB phosphorylation and translocation. Br J Pharmacol. 2015;172:5360–5376. doi: 10.1111/bph.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim E.H., Kim I.H., Lee M.J., Thach Nguyen C., Ha J.A., Lee S.C., Choi S., Choi K.T., Pyo S., Rhee D.K. Anti-oxidative stress effect of red ginseng in the brain is mediated by peptidyl arginine deiminase type IV (PADI4) repression via estrogen receptor (ER) β up-regulation. J Ethnopharmacol. 2013;148:474–485. doi: 10.1016/j.jep.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 36.Hong C.E., Lyu S.Y. Anti-inflammatory and anti-oxidative effects of Korean Red Ginseng extract in human keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmons G.E., Jr., Pruitt W.M., Pruitt K. Diverse roles of SIRT1 in cancer biology and lipid metabolism. Int J Mol Sci. 2015;16:950–965. doi: 10.3390/ijms16010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin M.H., Chen M.C., Chen T.H., Chang H.Y., Chou T.C. Magnolol ameliorates lipopolysaccharide-induced acute lung injury in rats through PPAR-γ-dependent inhibition of NF-κB activation. Int Immunopharmacol. 2015;28:270–278. doi: 10.1016/j.intimp.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z.J., Liu W., Liu L., Xiao C., Wang Y., Jiao J.S. Curcumin protects neuron against cerebral ischemia-induced inflammation through improving PPAR-gamma function. Evid Based Complement Alternat Med. 2013;2013:470975. doi: 10.1155/2013/470975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Yuan J., Liu L., Shi C., Wang L., Tian F., Liu F., Wang H., Shao C., Zhang Q. α-linolenic acid inhibits human renal cell carcinoma cell proliferation through PPAR-γ activation and COX-2 inhibition. Oncol Lett. 2013;6:197–202. doi: 10.3892/ol.2013.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshizaki N., Fujii T., Masaki H., Okubo T., Shimada K., Hashizume R. Orange peel extract, containing high levels of polymethoxyflavonoid, suppressed UVB-induced COX-2 expression and PGE2 production in HaCaT cells through PPAR-γ activation. Exp Dermatol. 2014;23(Suppl 1):18–22. doi: 10.1111/exd.12394. [DOI] [PubMed] [Google Scholar]
- 42.Jin T., Kim O.Y., Shin M.J., Choi E.Y., Lee S.S., Han Y.S., Chung J.H. Fisetin up-regulates the expression of adiponectin in 3T3-L1 adipocytes via the activation of silent mating type information regulation 2 homologue 1 (SIRT1)-deacetylase and peroxisome proliferator-activated receptors (PPARs) J Agric Food Chem. 2014;62:10468–10474. doi: 10.1021/jf502849j. [DOI] [PubMed] [Google Scholar]
- 43.Gao Y., Yang M.F., Su Y.P., Jiang H.M., You X.J., Yang Y.J., Zhang H.L. Ginsenoside Re reduces insulin resistance through activation of PPAR-γ pathway and inhibition of TNF-α production. J Ethnopharmacol. 2013;147:509–516. doi: 10.1016/j.jep.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Yu L., Cai W., Fan S., Feng L., Ji G., Huang C. Protopanaxatriol, a novel PPARγ antagonist from Panax ginseng, alleviates steatosis in mice. Sci Rep. 2014;4:7375. doi: 10.1038/srep07375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang J.T., Lee M.S., Kim H.J., Sung M.J., Kim H.Y., Kim M.S., Kwon D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother Res. 2009;23:262–266. doi: 10.1002/ptr.2606. [DOI] [PubMed] [Google Scholar]
- 46.Quan Q., Wang J., Li X., Wang Y. Ginsenoside Rg1 decreases Aβ (1-42) level by upregulating PPARγ and IDE expression in the hippocampus of a rat model of Alzheimer's disease. PLoS One. 2013;8:e59155. doi: 10.1371/journal.pone.0059155. [DOI] [PMC free article] [PubMed] [Google Scholar]