Abstract
Background
Red-skin root disease has seriously decreased the quality and production of Panax ginseng (ginseng).
Methods
To explore the disease's origin, comparative analysis was performed in different parts of the plant, particularly the epidermis, cortex, and/or fibrous roots of 5-yr-old healthy and diseased red-skin ginseng. The inorganic element composition, phenolic compound concentration, reactive oxidation system, antioxidant concentrations such as ascorbate and glutathione, activities of enzymes related to phenolic metabolism and oxidation, and antioxidative system particularly the ascorbate–glutathione cycle were examined using conventional methods.
Results
Aluminum (Al), iron (Fe), magnesium, and phosphorus were increased, whereas manganese was unchanged and calcium was decreased in the epidermis and fibrous root of red-skin ginseng, which also contained higher levels of phenolic compounds, higher activities of the phenolic compound-synthesizing enzyme phenylalanine ammonia-lyase and the phenolic compound oxidation-related enzymes guaiacol peroxidase and polyphenoloxidase. As the substrate of guaiacol peroxidase, higher levels of H2O2 and correspondingly higher activities of superoxide dismutase and catalase were found in red-skin ginseng. Increased levels of ascorbate and glutathione; increased activities of l-galactose 1-dehydrogenase, ascorbate peroxidase, ascorbic acid oxidase, and glutathione reductase; and lower activities of dehydroascorbate reductase, monodehydroascorbate reductase, and glutathione peroxidase were found in red-skin ginseng. Glutathione-S-transferase activity remained constant.
Conclusion
Hence, higher element accumulation, particularly Al and Fe, activated multiple enzymes related to accumulation of phenolic compounds and their oxidation. This might contribute to red-skin symptoms in ginseng. It is proposed that antioxidant and antioxidative enzymes, especially those involved in ascorbate–glutathione cycles, are activated to protect against phenolic compound oxidation.
Keywords: ascorbate, Panax ginseng, phenolic compounds, red-skin ginseng disease
Abbreviations: AAO, ascorbic acid oxidase; Al, aluminum; APX, ascorbate peroxidase; Asc, ascorbate; CAT, catalase; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; ginseng, Panax ginseng; GPX, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; GST, glutathione-S-transferase; GuPX, guaiacol peroxidase; l-GalDH, l-galactose 1-dehydrogenase; MDA, malondialdehyde; MDHAR, monodehydroascorbate reductase; PAL, phenylalanine ammonia-lyase; PPO, polyphenoloxidase; SOD, superoxide dismutase
1. Introduction
Panax ginseng (ginseng) is a perennial aromatic herb from the Araliaceae family. It has been suggested to stimulate metabolism, and hence maintain and improve health [1]. Its use has lasted for more than 4,000 yrs and spread as an alternative to chemical agents in Western medicine [2]. Ginseng cultivation requires shade conditions and long period (5–6 yrs). The major production regions of China are in the northeast between the latitudes of 39° and 47° [3], where 50% or more of China's total ginseng crop is produced each year. The quality and price of ginseng depend on its size, shape, and overall appearance.
Red-skin root disease is frequently characterized by less fibrous roots and red to brown speckles on the superficial skin of the ginseng root. It seriously decreases both the quality and production of ginseng in the Changbai mountain area. Chinese scholars tend to identify the red-skin root disease as a noninfectious physiological disorder and differentiate it from root rusty disease, which is caused by pathogens [4], [5], [6], [7], [8]. Soil conditions such as higher moisture, nitrate concentrations, and active Al species were suggested to be closely related to the incidence of red-skin root in ginseng [9]. However, the corresponding changes that occur in the physio-biochemical level in ginseng remain unclear.
In American ginseng (Panax quinquefolius L.), rusty root symptoms infected by cylindrocarpon include raised, reddish-brown lesions [10], and physiologically caused rust has orange-brown sunken lesions [11]. The physiological basis of either pathogen-induced rusty roots or physiologically caused rust spots in American ginseng appears to relate to the accumulation and oxidation of phenolic compounds in roots [10], [12], [13]. Phenolic compounds are widely distributed in plants to protect them from adverse environments, diseases, reactive oxygen species (ROS), wounds, and UV light [14]. For example, the accumulation of phenolic compounds in roots and root exudates has been suggested to be related to Al resistance in several plant species [15], [16], [17].
The role of the ascorbate (ASC)–glutathione (GSH) cycle in stress resistance, growth, and cell signaling has been well documented by several reviews [18], [19]. For example, pumpkin roots were suggested to express higher levels of ASC–GSH system in response to the Al toxicity enhanced production of ROS [20].
Building on our previous results showing that Al toxic species in albic ginseng bed soil contributed to the incidence of red-skin root disease [9], in the present study we explored the origin of red-skin root in ginseng by comparing the phenolic metabolism, reactive oxidation system, and antioxidative system in relation to inorganic mineral element accumulation between healthy and red-skin ginseng tissues.
2. Materials and methods
2.1. Root sampling
Five-year-old ginseng roots were harvested from the first ginseng farm (41°N, 128′E) in the Malugou town of Jilin province, China. The root tissues were stored at −4°C for transport. In the laboratory, ginseng roots were rinsed five times with distilled water to remove the attached soil. After the fibrous roots were cut, the red-skin areas in main roots were scraped with a plastic ruler to collect the first three to four cell layers as epidermis, then the adjacent seven to eight cell layers as the cortex. The corresponding epidermis, cortex, and fibrous roots were sampled from healthy root tissues. Then, 1.0-g sample aliquots were stored at −80°C.
2.2. Elemental analysis
The red skin disease grade was classified according to Li et al [8]. Fibrous roots, epidermis, cortex, and central cylinder portions in main roots of different disease grades ginseng were separated using a plastic ruler. All parts were dried, weighed, and ground to a fine powder and digested with an acid mixture (HNO3:HClO4, 4:1). The element concentrations were analyzed by inductively coupled plasma optical emission spectroscopy.
2.3. Determination of the total phenolic compounds
According to the description of Rahman and Punja [13], phenolic compounds were extracted in 80% methanol containing 8% methanoic acid, and reacted with Folin–Denis solution. The spectroscopic absorbance was monitored at 740 nm with gallic acid as analogue for the total phenolic compounds.
2.4. Determination of phenylalanine ammonia-lyase and polyphenoloxidase activities
According to Koukol and Conn [21], phenylalanine ammonia-lyase (PAL, EC 4.3.1.5) was extracted in 0.1M boric acid buffer (pH 8.8) containing 5 mM mercaptoethanol, 1mM EDTA, and 5% glycol (v/v). The enzyme extract was mixed with the boric acid buffer solution (pH 8.8) containing 15 mM l-phenylalanine. After 30 min of incubation at 37°C, HCl was added to stop the reaction. The absorbance was monitored at 290 nm, and a change of 0.01 value within 1 min was defined as 1 unit of enzyme activity.
The polyphenoloxidase (PPO, EC 1.14.16.1) activity was assayed according to Anderson and Morris [22]. PPO was extracted in 50 mM phosphate buffer solution (pH 5.6). The enzyme extract was mixed with 50 mM phosphate buffer (pH 5.6) containing 10 mM o-dihydroxybenzene. The absorbance was monitored at 420 nm, and an increase of 0.01 in the absorbance value within 1 min was defined as 1 unit of enzyme activity.
2.5. Determination of lipid peroxidation and O2•− and H2O2 concentrations
Lipid peroxidation was evaluated in terms of the malondialdehyde (MDA) concentration. Thiobarbituric acid reactive substance was measured as the final product of lipid peroxidation at 600 nm and 535 nm [23].
According to Patterson et al [24], H2O2 was extracted with ice-cold acetone and reacted with 10% TiCl4 dissolved in concentrated HCl and ammonia. The absorbance was monitored at 412 nm.
The O2•− produced was extracted in 25 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] buffer (pH 6.8) and reacted with p-aminobenzene sulfonic acid and a-naphthylamine. The absorbance was monitored at 530 nm [25].
2.6. Determination of antioxidative enzyme activities
Frozen samples (1.0 g) were homogenized with 10 mL ice-cold extraction buffer [25mM HEPES containing 2% (w/w) polyvinylpyrrolidone and 0.2mM EDTA, pH 7.8]. After centrifugation, the supernatant was collected for enzyme activity assays.
Guaiacol peroxidase (GuPX, EC 1.11.1.7) activity was assayed based on the oxidation of guaiacol by hydrogen peroxide [26]. The reaction mixture contained 2.9 mL of 50 mM potassium phosphate buffer (pH 7.0, containing 20 mM guaiacol and 10 mM H2O2), and 0.1 mL enzyme extract. An increase of 0.01 in the absorbance value within 1 min at 420 nm was defined as 1 unit of enzyme activity. Ascorbate peroxidase (APX, EC 1.11.1.11) activity was assayed at 290 nm [27]. The reaction system consisted of a mixture of 2.9 mL of 50 mM phosphate buffer (pH 7.6) containing 0.25 mM ascorbate and 0.1 mM H2O2, and 0.1 mL enzyme extract. One unit of enzyme activity was equivalent to the oxidation of 1 μM ascorbate within 1 min.
The activities of catalase (CAT, EC 1.11.3.6) and superoxide dismutase (SOD, EC 1.15.1.1) were determined according to Dhindsa et al [28]. H2O2 was monitored at 240 nm, and a decrease of 0.01 in the absorbance value within 1 min was defined as 1 unit of catalase enzyme activity. NBT was monitored at 560 nm. One unit of SOD activity was defined as 50% inhibition of nitrotetrazolium blue chloride photoreduction.
Oxidation of nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt (NADPH) was monitored at 340 nm to evaluate monodehydroascorbate reductase (MDHAR) activity with the coefficient of 6.2 mM/L/cm [29]. The formation of ASC was monitored at 265 nm to assay the activity of dehydroascorbate reductase (DHAR) with a coefficient of 14mM/L/cm [29]. According to Foyer and Halliwell [30], the activity of glutathione reductase (GR, EC.1.6.4.2) was determined by monitoring the oxidation of NADPH at 340 nm. The glutathione-S-transferase (GST) activity was monitored at 340 nm with a coefficient of 9.6 mM/L/cm. The ascorbic acid oxidase (AAO, EC 1.10.3.3) activity was assayed by monitoring at 265 nm with a coefficient of 14.3 mM/L/cm. Glutathione peroxidase (GPX, EC 1.11.1.9) activity was measured according to Griffith [31] with monitoring at 412 nm.
l-Galactose 1-dehydrogenase (l-GalDH, EC 1.1.1.316) was extracted and measured according to Gatzek et al [32]. The absorbance was monitored at 340 nM, and the coefficient was 6.2 mM/L/cm.
2.7. Determination of nonenzymatic antioxidant concentrations
ASC and dehydroascorbate (DHA) were measured spectrophotometrically at 265 nm according to the method of Hodges et al [33]. The reduced form of glutathione (GSH), glutathione disulfide (GSSH), was measured according to Griffith [31], and the absorbance was monitored at 412 nm.
3. Results
3.1. Inorganic element accumulation
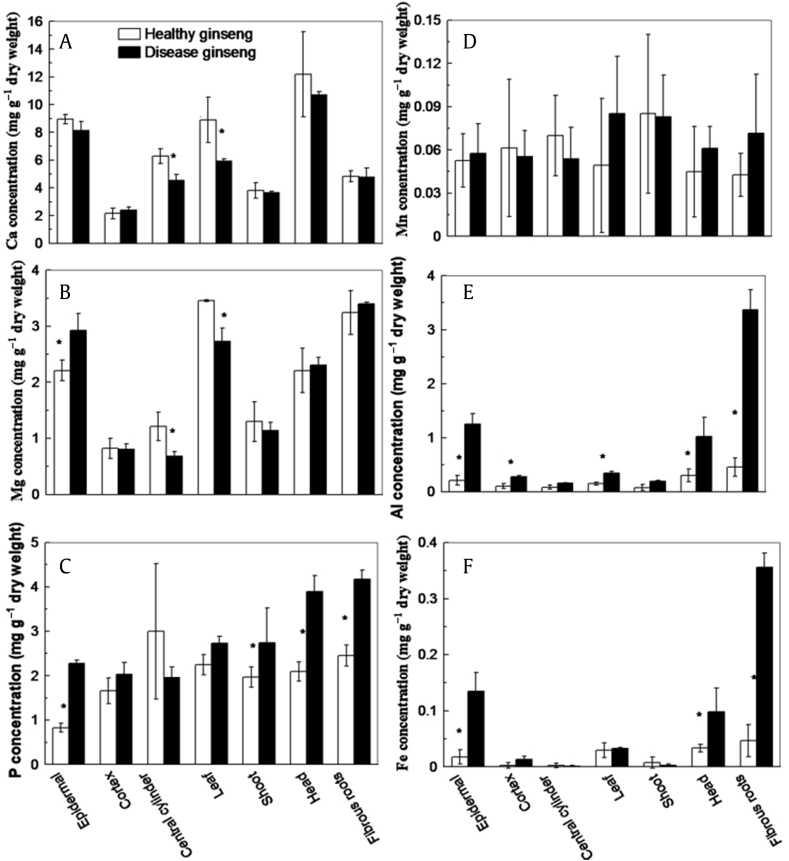
A large variation of element composition existed between healthy and red-skin ginsengs, particularly in the fibrous roots and epidermis of main root (Figs. 1A–1F). In comparison with healthy controls, lower concentration of calcium (Fig. 1A), higher concentrations of magnesium (Fig. 1B), phosphorus (Fig. 1C), Al (Fig. 1E), iron (Fig. 1F), and unchanged manganese (Mn; Fig. 1D) were found in the epidermis of red-skin ginseng. Increased Al was also observed in the head, cortex, shoot, and leaves of red-skin ginseng (Fig. 1E). In particular, the amount of Al accumulated in the epidermis of the main root and the fibrous roots of red-skin ginseng were six- and seven-fold higher, respectively, than those in the corresponding parts of healthy ginseng (Fig. 1E).
Fig. 1.
Comparison of concentrations of elements in different parts of red-skin and healthy ginseng. (A) Calcium (Ca). (B) Magnesium (Mg). (C) Phosphorus (P). (D) Manganese (Mn). (E) Aluminum (Al). (F) Iron (Fe). The epidermis, cortex, and central cylinder were separated from the main root of ginseng. Values are denoted as the mean ± standard deviation; n = 3, p < 0.05. * between the black and white columns indicates a significant difference between healthy and disease ginseng.
3.2. Phenolic compound accumulation and their metabolism
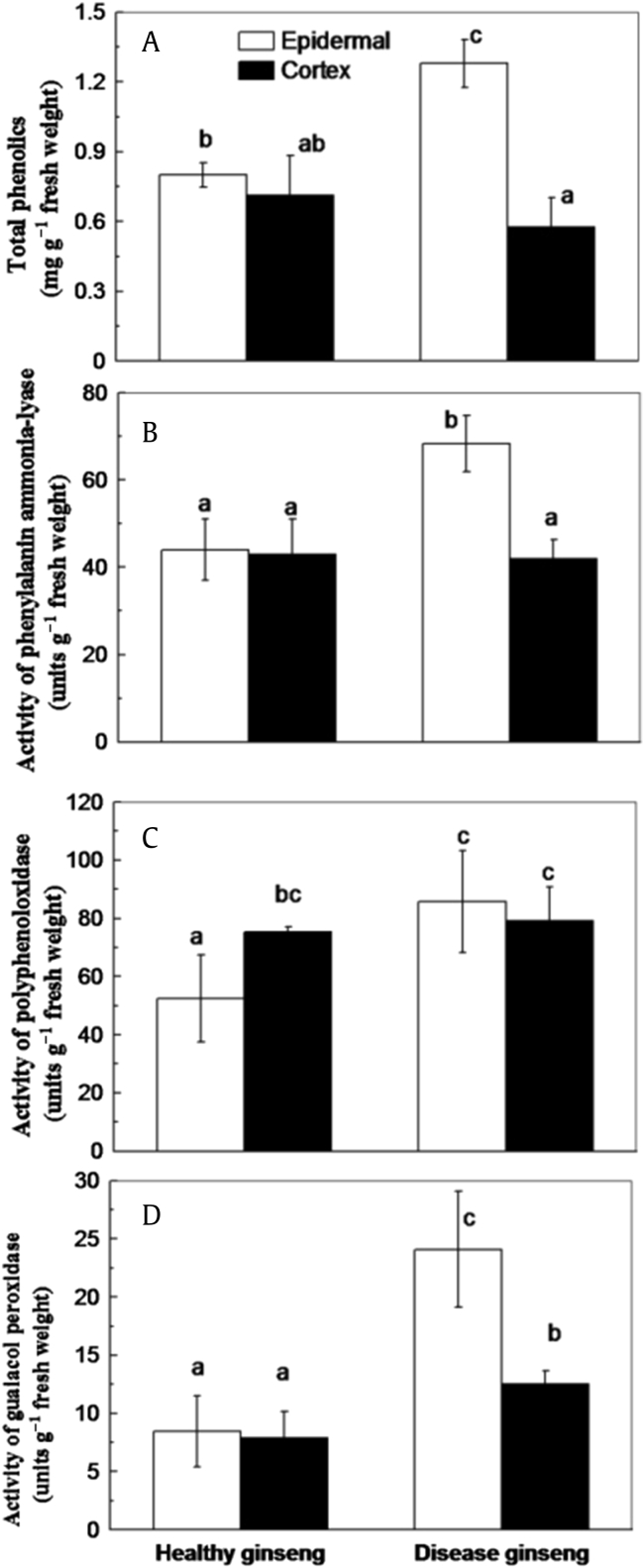
PAL is the key enzyme in the phenylpropanoid biosynthesis pathway in plants, which controls the synthesis of phenolic compounds [34]. The total phenolic compounds in the epidermis of red-skin roots were almost double those in healthy roots (Fig. 2A). Accordingly, a significant increase in PAL activity was observed in the epidermis of red-skin roots compared with the healthy roots (Fig. 2B).
Fig. 2.
Comparison between red-skin ginseng and healthy ginseng. (A) Total phenolic compound concentrations. (B) Activities of phenylalanine ammonia-lyase. (C) Polyphenoloxidase activities. (D) Guaiacol peroxidase (GuPX) activities. Values are denoted as the mean ± standard deviation; n = 3. The different letters above column represented statistically different at p = 0.05.
GuPX and PPO contribute to the catalysis of phenolic compound oxidation in plants through the reduction of H2O2 and molecular oxygen, respectively [35], [36]. Higher activities of both PPO (Fig. 2C) and GuPX (Fig. 2D) were observed in the epidermis of red-skin ginseng roots. However, no significant difference was found in the concentrations of phenolic compound and the activities of PPO, PAL, and POD in the cortex between healthy and diseased ginseng (Figs. 2A–2D). The increase in PAL activity (Fig. 2B), the total phenolic compounds concentrations (Fig. 2A), and the activities of PPO (Fig. 2C) and GuPX (Fig. 2D) are consistent with the assumption that phenolics and their oxidation are related to the reddish-brown speckle symptoms in red-skin ginseng roots.
Higher concentrations of H2O2 were detected in both epidermis and cortex of main root of red skin ginseng compared with the corresponding part of healthy ginseng (Fig. 3A). There was no significant difference in O2•− concentrations between healthy and red-skin ginseng (Fig. 3B). The MDA concentration, which indicates lipid peroxidation, was increased in the epidermis and cortex of red-skin ginseng roots (Fig. 3C). Thus, the presence of more ROS and higher levels of lipid peroxidation were found in the epidermis of red-skin ginseng roots (Figs. 3A–3C). Increased H2O2 can provide substrates for GuPX to involve in phenolic compound oxidation.
Fig. 3.
Comparison between red-skin ginseng and healthy ginseng. (A) Concentration of H2O2. (B) Concentration of superoxide anion. (C) Concentration of malondialdehyde. (D) Activities of catalase. (E) Activities of superoxide dismutase. The values are denoted as the mean ± standard deviation; n = 3. The different letters above columns represent statistically different at p = 0.05.
The activities of H2O2-related antioxidative enzymes were analyzed. Higher activities of CAT (Fig. 3D) and SOD (Fig. 3E) were found in the epidermis of red-skin ginseng roots. The activities of CAT in the red-skin root epidermis were two-fold higher than those in healthy ginseng (Fig. 3D).
3.3. ASC metabolism and activities of enzymes in the ASC–GSH cycle
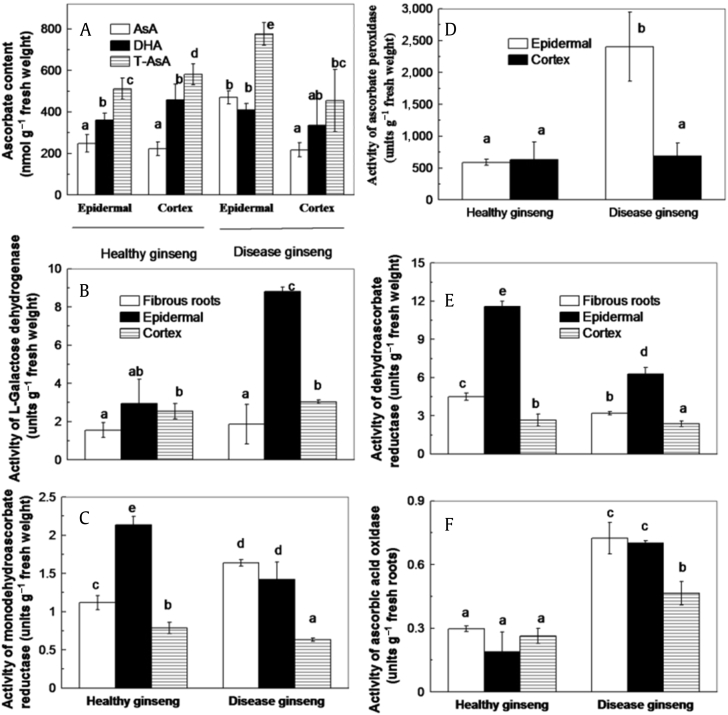
The ASC–GSH cycle plays an important role in the detoxification of ROS by successive oxidation and reduction reactions involving ASC and GSH. Their main cellular redox buffers including ASC and GSH with their respective oxidized forms MDHA, DHA, and GSSH. A higher accumulation of ASC and DHA was observed in the epidermis of red-skin ginseng (Fig. 4A).
Fig. 4.
Comparison between red-skin ginseng and healthy ginseng. (A) Ascorbate concentration. (B) Activity of l-galactose dehydrogenase. (C) Activity of monodehydroascorbate reductase. (D) Activity of ascorbate peroxidase. (E) Dehydroascorbate reductase. (F) Activity of ascorbic acid oxidase. The values are denoted as the mean ± standard deviation, n = 3. The different letters above columns represent statistically different at p = 0.05.
The activities of related enzymes in the ASC–GSH cycle were measured (Figs. 4B–4F). l-GalDH and AAO are the key enzymes responsible for ASC synthesis and degradation, respectively. Comparing the healthy ginseng control, activities of both l-GalDH and AAO were significantly higher in the epidermis of red-skin roots (Figs. 4B and 4F). In particular, the activity of l-GalDH is almost three-fold higher in the epidermis of red-skin ginseng than that in healthy ginseng (Fig. 4B). APX can remove H2O2 with ASC as the electron donor. MDHAR and DHAR involved in the regeneration of ASC [37] substantially increased APX activity (Fig. 4D), whereas decreased activities of DHAR and MDHAR were found in the epidermis of red-skin ginseng (Figs. 4C and 4E). These findings were consistent with the higher ASC + DHA and DHA concentrations in the epidermis of red-skin ginseng (Fig. 4A). No significant differences between healthy and red-skin ginseng were noted regarding the activities of the antioxidative enzymes and the antioxidant concentrations in the cortex (Figs. 4A–4F).
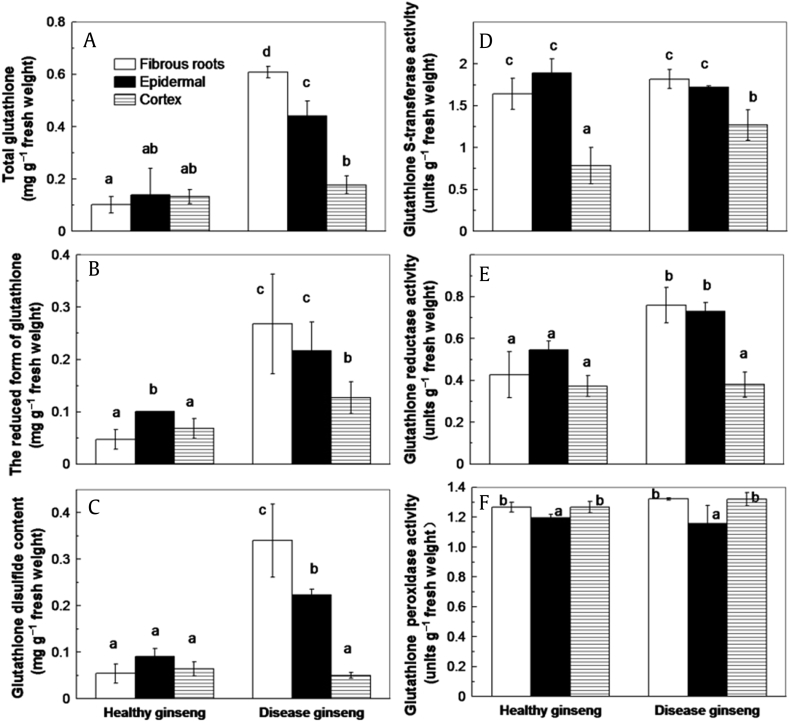
GSH is critical for the regeneration of ASC in the ASC–GSH cycle. GPX reduces H2O2 to water with GSH as reductant [38]. The conversion of GSH into oxidized GSSH by GPX can be reversed by GR. GST was suggested to detoxify lipid peroxidation and release O2•− in conjunction with cytosolic peroxidases [39], [40]. Increased concentrations of GSH + GSSH (Fig. 5A), GSH (Fig. 5B), and GSSH (Fig. 5C) were found in the fibrous roots and epidermis of red-skin ginseng. Unchanged activities of GST (Fig. 5D) and GPX (Fig. 5E), and higher GR (Fig. 5F) activity were found in red-skin ginseng.
Fig. 5.
Comparison between red-skin ginseng and healthy ginseng. (A) Concentration of total glutathione. (B) Concentration of reduced form of glutathione. (C) Concentration of glutathione disulfide. (D) Activity of glutathione S-transferase. (E) Activity of glutathione reductase. (F) Activity of glutathione peroxidase (GPX). The values are denoted as the mean ± standard deviation; n = 3. The different letters above columns represented statistically different at p = 0.05.
4. Discussion
In this work, the origin of the ginseng red-skin root disease was studied in relation to inorganic element composition and the accumulation and oxidation of phenolic compounds. Particular attention was paid to antioxidative enzymes, especially those involved in the ASC–GSH cycles, to explore the process of protecting against phenolic oxidation.
Phenolics in plants were significantly increased under a variety of stresses, such as Al toxicity [16], [17]. The phenylpropanoid pathway is responsible for the synthesis of various secondary metabolites, including phenolic esters, coumarins, flavonoids, and lignin [34]. In the present study, higher phenolic compound concentrations and PAL activities were observed in the epidermis of red-skin ginseng (Figs. 2A and 2B). The increased accumulation of phenolic compounds might result from increased PAL activity and could be related to Al toxicity.
Metals, including Al, Fe, and Mn, form complexes with phenolic compounds, which usually contribute to their colorful appearance [41]. For example, Ayala-Silva and Al-Hamadani [42] reported that Azolla caroliniana incubated with Al2(SO4)3 accumulated nine times more anthocyanins than control plants. Earlier reports demonstrated the presence of elevated phenolics and cations, especially Fe, in rusty tissues compared to healthy tissues of P. ginseng Meyer and P. quinquefolius L. [12], [43], [44]. Yang et al [43] reported two- to three-fold higher Fe, Al, and Si levels among the inorganic elements in the epidermis of red-colored ginseng compared to healthy roots.
Our previous report revealed that a large amount of exchangeable Al existed in the albic ginseng bed soils [9]. Furthermore, the occurrence of red-skin root disease was closely related to multiple factors including Al toxicity [45]. Consistently, more Al and Fe were observed in the epidermis, cortex, head, and fibrous roots of red-skin ginseng (Figs. 1E and 1F). The amount of Fe was rather low in both healthy and red-skin ginseng compared with Al (Figs. 1E and 1F). Al and Fe are suggested to play important roles in contributing to the occurrence of the red-skin symptom in ginseng. The large variation in Ca, Mg, and P concentrations in the epidermis, central cylinder, or leaf between healthy and red-skin ginseng might be a secondary effect of Al or Fe toxicity (Figs. 1A–1C). Higher phosphorus concentrations might immobilize the extra metal cations. No significant difference was observed in the Mn concentration between the healthy and diseased ginseng (Fig. 1D).
Oxidation of phenolic compounds contributes to browning and leads to formation of brown pigments in plant tissues [35]. Wissemeier and Horst [46] have suggested that accumulations of oxidized Mn and oxidized phenolic compounds in the cell wall contributed to the local brown spots in cowpea leaves. Generally, the oxidation of phenolic compounds involved in two enzymes. On the one hand, with molecular oxygen as the electron acceptor, PPO catalyzes phenolic compounds oxidizing into quinones with toxic oxygen species formation [47]. On the other hand, the GuPX-catalyzed phenol oxidation with H2O2 as the electron acceptor results in the formation of phenoxy radicals, which can form polyphenols to express browning, or combine with metal ions to cause lipid peroxidation [48], [49]. For example, Al, Ca, Mg, and Cd have been found to stimulate phenoxy radical-induced lipid peroxidation [50]. Al was shown to have a spin-stabilizing effect on the phenoxy radical [51]. In the present study, higher activities of GuPX and PPO were found in the red-skin root of ginseng (Figs. 2C and 2D), indicating their coinvolvement in phenolic compound oxidation with the expression of red to brown speckles. Consistently, the important oxidative species H2O2 was also observed to be enhanced in the red-skin root (Fig. 3A) and could act as a substrate for GuPX. Increased MDA concentration in red-skin epidermis reflected an increase in membrane lipid peroxidation (Fig. 3C), which might be caused by Al or Fe toxic ions or by the combination of phenoxy radical and Al ions.
Considering the importance of phenoxy radicals in polyphenol formation, the redox environment was studied here. Antioxidative enzymes that are involved in scavenging for phenoxy radicals and clearing the H2O2 production pathway were selected to investigate how the process of phenolic compound oxidation is controlled.
The combined actions of SOD and CAT are crucial for mitigating the effects of oxidative stress, as the former involves the dismutation of O2− into H2O2 and the latter decomposes H2O2 to water and O2. Both CAT and SOD showed increased activity in the red-skin ginseng epidermis layers (Figs. 3D and 3E), indicating their important role in controlling H2O2 accumulation.
The ASC–GSH cycle plays an important role in ROS detoxification by successive oxidation and reduction reactions involving ASC and GSH. With ASC as the electron donor, APX effectively removes H2O2 and scavenges phenoxy radicals to inhibit phenol oxidation [48], [52]. When ASC is also oxidized to DHA, quinoic compounds can be reduced back to their phenol form [53]. MDHAR and DHAR catalyze the reduction of monodehydroascorbate (MHDA) radical or DHA to regenerate the parent compound ASC [37]. Red-skin ginseng exhibited higher activities of the ASC synthetic enzyme l-GalDH (Fig. 4B) and the degradation enzymes, APX (Fig. 4E) and AAO (Fig. 4F), indicating the important role of ASC in controlling phenol radicals and H2O2. Lower activities of the regenerating enzymes DHAR (Fig. 4D) and MDHAR (Fig. 4C) suggested that less DHA was reduced to ASC, which is consistent with the higher total ASC concentrations and lower ASC/DHA ratio in red-skin ginseng (Fig. 4A).
GSH is also critical for the regeneration of ASC in the ASC–GSH cycle. The high GSH/GSSH ratio in plants could help maintain an appropriate redox environment. In the present study, the higher concentrations of total GSH and oxidized GSSH observed in the epidermis and fibrous roots of red-skin ginseng (Figs. 5A–5C) suggested that GSH might play an important role in protecting against phenolics oxidation.
GPX reduces H2O2 to water by converting GSH to GSSH [38]. The GSSH can be reduced to GSH by GR. GST combines with cytosolic peroxidases to detoxify lipid peroxidation products and release O2− [39], [40]. The activities of GPX and GST remained unchanged in healthy and red-skin ginseng (Figs. 5D and 5F), reflecting their lower contribution of scavenging H2O2 and O2− production. Higher GR activity was found in the red-skin ginseng, which is consistent with its higher reduced GSH concentration (Fig. 5E).
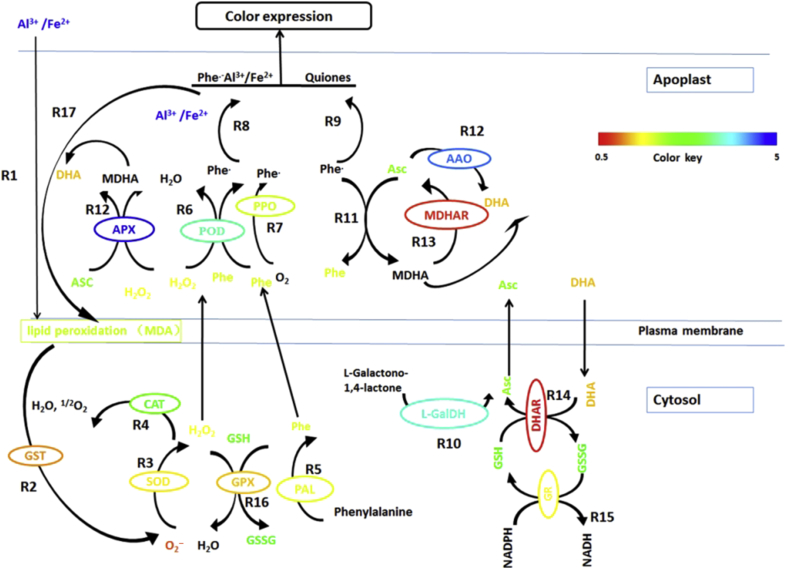
In conclusion, a model was proposed to explain the occurrence of red-skin symptoms in ginseng (Fig. 6). The Al and/or Fe stress that caused phenolic compound accumulation and oxidation was suggested to contribute to the formation of red-skin disease. The ASC–GSH system was suggested to play an important role in controlling the phenolic oxidation process and thus the occurrence of red-skin symptoms. The regulation of redox environment in ginseng might be useful to alleviate or control the development of red skin ginseng disease.
Fig. 6.
The proposed phenolics oxidation process and the protection afforded by ascorbate (ASC)–glutathione (GSH) cycles in red-skin roots in Panax ginseng. Al and or Fe stress caused lipid peroxidation (R1). Cytosolic peroxidases (cPX) conjugated with glutathione-S-transferase (GST) to detoxify the lipid peroxidation and release O2•− (R2). The production of the reactive oxygen system and the higher activities of superoxide dismutase (SOD) and catalase (CAT) were induced to produce and scavenge H2O2, respectively (R3, R4). Simultaneously, higher PAL activity was induced to increase the production of phenolics (R5). The phenol was oxidized to phenol radicals in a reaction catalyzed by guaiacol peroxidase (GuPX; R6) and polyphenoloxidase (PPO; R7) with H2O2 and O2 as the substrate, respectively. The phenol radicals combined with Al or Fe ions (R8) or dismutated to quinone (R9), resulting in the expression of red or brown color. More ASC was produced via a reaction catalyzed by l-GalDH (R10). ASC can reduce phenoxy radicals to their original phenol forms (R11) and can also remove H2O2 by APX by undergoing oxidation to malondialdehyde (MDA) and dehydroascorbate (DHA). ASC can also be oxidized to DHA by ascorbic acid oxidase (AAO) (R12). ASC can be regenerated from MDA or DHA by MDAR (R13) and DHAR (R14), whereas GSH can be oxidized to glutathione disulfide (GSSH). The reduction of GSSH to GSG can be realized by GR, which is involved in the ASC regeneration system (R15). GPX reduces H2O2 to water by consuming GSH and converting it to GSSH (R16). Lipid peroxidation might also result from the complexation of phenol radicals with Al and/or Fe ions (R17). The color keys represent the ratio of activities or concentrations in epidermal between red skin and healthy ginseng. The black letters represent untested factors.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
National Natural Science Foundation of China (No. 40903029), International Foundation for Science (C4711-1) and Jilin Natural Science Foundation of China (20130101084JC) provided financial support for this research.
References
- 1.Hu S.Y. A contribution to our knowledge of ginseng. Am J Chin Med. 1977;5:1–23. doi: 10.1142/s0192415x77000026. [DOI] [PubMed] [Google Scholar]
- 2.Baranov A. Recent advances in our knowledge of the morphology, cultivation and uses of ginseng (Panax ginseng C.A. Meyer) Econ Bot. 1966;20:403–406. [Google Scholar]
- 3.Evans B.L. Ginseng: root of Chinese–Canadian relations. Can Hist Rev. 1985;66:1–26. [Google Scholar]
- 4.Wang Y.Q. A preliminary study on cause of ginseng red skin disease in the second ginseng farm of Jinyu county. Spec Wild Econ Anim Plant Res. 1963;2:9–15. [in Chinese] [Google Scholar]
- 5.Zhang Y.C., Wang Z.S., Li J.F., Gao K.J., Sun S.J., Wang Y.L. An investigation of cause and protective method of ginseng red skin disease. Spec Wild Econ Anim Plant Res. 1984;1:21–24. [in Chinese] [Google Scholar]
- 6.Wu K.Y. The relationship between ginseng red skin disease and inorganic ions. Chin Trad Med. 1989;12:13–14. [in Chinese] [Google Scholar]
- 7.Zhao Y.F. The progress of diagnosis and comprehensive control of ginseng red skin disease. Spec Wild Econ Anim Plant Res. 1998;1:41–46. [in Chinese] [Google Scholar]
- 8.Li Z.H., Tian S.Z., Sun Y.J., Guo S.Y., Liu Z.R. The relationship between ginseng red skin root disease and soil ecology conditions. Ecol J. 1999;19:864–869. [in Chinese] [Google Scholar]
- 9.Liu X., Yang Z.M., Gao L.L., Xiang W.Y., Zhang B., Xie Z.L., You J.F. Comparison of the characteristics of artificial ginseng bed soils in relation to the incidence of ginseng red skin disease. Exp Agric. 2014;50:59–71. [Google Scholar]
- 10.Parke J.L. Shot well KM. Diseases of cultivated ginseng. Univ Wisconsin Madison Coll Agric Life Sci Res Bull. 1989;3465:16. [Google Scholar]
- 11.Campeau C.A. University of Guelph; Ontario, Canada: 2002. Effects of ethephon on floral abscission and root quality of North American ginseng (Panax quinquefolius L.) MS dissertation. [Google Scholar]
- 12.Campeau C.A., Proctor J.T.A., Jackson C.J.C., Rupasinghe H.P.V. Rust-spotted north American ginseng roots: phenolic, antioxidant, ginsenoside and mineral nutrient. Hort Sci. 2003;38:179–182. [Google Scholar]
- 13.Rahman M., Punja Z.K. Biochemistry of ginseng root tissues affected by rusty root symptoms. Plant Physiol Biochem. 2005;43:1103–1114. doi: 10.1016/j.plaphy.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Dixon R.A., Paiva N.L. Stress-induced phenyl propanoid metabolism. Plant Cell. 1995;7:1805–1897. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidarabadi M.D., Ghanati F., Fujiwara T. Interaction between boron and aluminum and their effects on phenolic metabolism of Linum usitatissimum L. roots. Plant Physiol Biochem. 2011;49:1377–1383. doi: 10.1016/j.plaphy.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Ofei-Manu P., Wagatsuma T., Ishikawa S., Tawaraya K. The plasma membrane strength of the root-tip cells and root phenolic compounds are correlated with Al tolerance in several common woody plants. Soil Sci Plant Nutr. 2001;47:359–376. [Google Scholar]
- 17.Kidd P.S., Lugany M., Poschenreder C., Gunse B., Barcelo J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea may L.) J Exp Bot. 2001;52:1339–1352. [PubMed] [Google Scholar]
- 18.Horemas N., Foyer C.H., Asard H. Transport and action of ascorbate at the plasma membrane. Trends in Plant Sci. 2000;5:263–267. doi: 10.1016/s1360-1385(00)01649-6. [DOI] [PubMed] [Google Scholar]
- 19.Pignocchi C., Foyer C.H. Apoplastic ascorbate metabolism and its role in the regulation of cell signaling. Curr Opin Plant Biol. 2003;6:379–389. doi: 10.1016/s1369-5266(03)00069-4. [DOI] [PubMed] [Google Scholar]
- 20.Dipierro N., Mondelli D., Paciolla C., Brunetti G., Dipierro S. Changes in the ascorbate system in the response of pumpkin (Cucurbita pepo L.) roots to aluminum stress. J Plant Physiol. 2005;162:529–536. doi: 10.1016/j.jplph.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Koukol J., Conn E.E. The metabolism of aromatic compounds in higher plants, purification and properties of the phenylalanine deaminase of Herdeum vulagare. J Biol Chem. 1961;236:2690–2698. [PubMed] [Google Scholar]
- 22.Anderson J.V., Morris C.F. An improved whole seed assay for screening wheat germplasm for polyphenol oxidase activity. Crop Sci. 2001;41:1697–1705. [Google Scholar]
- 23.Hodges D.M., Delong J.M., Forney C.F. Improving the thiobarbituric acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- 24.Patterson B.D., MacRae E.A., Ferquson I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV) Anal Biochem. 1984;139:487–492. doi: 10.1016/0003-2697(84)90039-3. [DOI] [PubMed] [Google Scholar]
- 25.Able A.J., Guest D.I., Sutherland M.W. Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var nicotinae. Plant Physiol. 1998;117:491–499. doi: 10.1104/pp.117.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castillo F.I., Penel I., Greppin H. Peroxidase release induced by ozone in Swdum album leaves. Plant Physiol. 1984;74:846–851. doi: 10.1104/pp.74.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asada K. Chloroplasts: formation of active oxygen and its scavenging. Methods Enzymol. 1984;105:422–429. [Google Scholar]
- 28.Dhindsa R.S., Plumb-Dhindsa P., Throne T.A. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32:93–101. [Google Scholar]
- 29.Hossain M.A., Nakano Y., Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395. [Google Scholar]
- 30.Foyer C.H., Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- 31.Griffith O.W. Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinypyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 32.Gatzek S., Wheeler G.L., Smirnoff N. Antisense suppression of l-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated l-galactose synthesis. Plant J. 2002;30:541–553. doi: 10.1046/j.1365-313x.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- 33.Hodges D., Andrews C., Johnson D., Hamilton R. Antioxidant compound response to chilling stress in differentially sensitive inbred maize 23 lines. Physicol Plant. 1996;98:685–692. [Google Scholar]
- 34.Zhang X.B., Liu C.J. Multifaceted regulations of gateway enzyme phenylalanine Ammonia-lyase in the biosynthesis of phenylpropanoids. Mol Plant. 2015;8:17–27. doi: 10.1016/j.molp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Pourcel L., Routaboul J.M., Cheynier V., Lepiniec L., Debeaujon I. Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci. 2006;12:29–36. doi: 10.1016/j.tplants.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Marusck C.M., Trobough N.M., Flurkey W.H., Inlow J.K. Comparative analysis of polyphenol oxidase from plant and fungal species. J Inorg Chem. 2006;100:108–123. doi: 10.1016/j.jinorgbio.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Asada K. The role of ascorbate peroxidase and monodehydroascorbate reducases in H2O2 scavenging in plants. In: Scandalions J.G., editor. Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1987. pp. 715–735. [Google Scholar]
- 38.Noctor G., Foyer C.H. Ascorbic and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 39.Richards K.D., Schott E.J., Sharma Y.K., Davis K.R., Gardner R.C. Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol. 1998;116:409–418. doi: 10.1104/pp.116.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakihama Y., Yamasaki H. Lipid peroxidation induced by phenolics in conjunction with aluminum ions. Biol Plant. 2002;45:249–254. [Google Scholar]
- 41.Vermerris W., Nicholson R. 2009. Phenolic compound biochemistry (Chapter 2). Chemical properties of phenolic compounds; p. p.43.Spinger.com e-ISBN: 978-1-4020-5164-7. [Google Scholar]
- 42.Ayala-Silva T., Al-Hamadani S. Interactive effects of polyactic acid with different aluminum concentrations on growth, pigment concentrations, and carbohydrate accumulation of Azolla. Am Fern J. 1997;87:120–126. [Google Scholar]
- 43.Yang D.C., Kim Y.H., Yun K.Y., Lee S.S., Kwon J.N., Kang H.M. Red-colored phenomena of ginseng (Panax ginseng C. A. Meyer): root and soil environment. J Ginseng Sci. 1997;21:91–97. [Google Scholar]
- 44.Wang Y.P., Li Z.H., Sun Y.J., Guo S.W., Tian S.Z., Liu Z.R. Studies on the genesis of ginseng rust spots. Korean J Ginseng Sci. 1997;21:69–77. [Google Scholar]
- 45.You J.F., Liu X., Zhang B., Xie Z.L., Hou Z.G., Yang Z.M. Seasonal changes in soil acidity and related properties in ginseng artificial bed soils under a plastic shade. J Ginseng Res. 2015;39:81–88. doi: 10.1016/j.jgr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wissemeier A.H., Horst W.J. Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata (L.) Walp.) Plant Soil. 1992;143:299–309. [Google Scholar]
- 47.Appel H.M. Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol. 1993;9:1521–1552. doi: 10.1007/BF00984895. [DOI] [PubMed] [Google Scholar]
- 48.Takahama U. Redox state of ascorbic acid in the apoplast of stems of Kalanchoe daigremontiana. Physiol Plant. 1993;89:791–798. [Google Scholar]
- 49.Takahama U., Oniki T. Regulation of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol. 1992;33:379–387. [Google Scholar]
- 50.Sakihama Y., Cohen M.F., Grace S.C. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/s0300-483x(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 51.Kalyanaraman B. Characterization of O-semiquinoe radicals in biological systems. Methods Enzymol. 1990;186:333–343. doi: 10.1016/0076-6879(90)86127-h. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez M., Queijeiro E., Revilla G., Zarra I. Changes in ascorbate levels in apoplastic fluid during growth of pine hypocotyls. Effect on peroxides activities associated with cell walls. Physiol Plant. 1997;101:815–820. [Google Scholar]
- 53.Isaacs N.S., van Eldik R. A mechanistic study of the reduction of quinines by ascorbic acid. J Chem Soc Dalton Trans. 1997;2:1465–1468. [Google Scholar]