Abstract
Cerebral palsy (CP) children present complex and heterogeneous motor disorders that cause gait deviations.
Clinical gait analysis (CGA) is needed to identify, understand and support the management of gait deviations in CP. CGA assesses a large amount of quantitative data concerning patients’ gait characteristics, such as video, kinematics, kinetics, electromyography and plantar pressure data.
Common gait deviations in CP can be grouped into the gait patterns of spastic hemiplegia (drop foot, equinus with different knee positions) and spastic diplegia (true equinus, jump, apparent equinus and crouch) to facilitate communication. However, gait deviations in CP tend to be a continuum of deviations rather than well delineated groups. To interpret CGA, it is necessary to link gait deviations to clinical impairments and to distinguish primary gait deviations from compensatory strategies.
CGA does not tell us how to treat a CP patient, but can provide objective identification of gait deviations and further the understanding of gait deviations. Numerous treatment options are available to manage gait deviations in CP. Generally, treatments strive to limit secondary deformations, re-establish the lever arm function and preserve muscle strength.
Additional roles of CGA are to better understand the effects of treatments on gait deviations.
Cite this article: Armand S, Decoulon G, Bonnefoy-Mazure A. Gait analysis in children with cerebral palsy. EFORT Open Rev 2016;1:448-460. DOI: 10.1302/2058-5241.1.000052.
Keywords: cerebral palsy, clinical gait analysis, gait deviations
Introduction
Cerebral palsy (CP) is the most frequent cause of motor disability among children in Europe representing 700 000 citizens.1 The prevalence of CP in Europe has been stable over the last 30 years, and ranges between 1.5 and 3.0 cases per 1000 live births.1 The motor disorders of individuals with CP are complex. They are related to primary deficits such as muscle spasticity, muscle weakness and loss of selective motor control, and secondary deficits such as muscle contractures and bony deformities. The main dysfunctions are related to motor disorders during posture and movement causing limitation in activities (e.g. walking). Around 75% of CP children are ambulatory.2 The severity and type of impairments are variable in the CP population. Clinical classifications distinguish the topography of impairments (hemiplegia, diplegia, quadriplegia), motor disorders (spastic, athetotic, dystonic, hypotonic, ataxic and a mixed group) and functional capacities with the Gross Motor Function Classification System (GMFCS).3
Walking is essential for activities of daily living and social participation; therefore, it is often considered one of the most important activities in daily life.4 Nowadays, due to the complexity of gait and especially pathological gait, clinical gait analysis (CGA) is generally used to identify, quantify and understand the deficits of a specific patient and is fully integrated into the clinical decision- making of patients with complex gait disorders.5 Moissenet and Armand have proposed a simple algorithm for the management of a patient with complex gait disorders.6 This algorithm has three steps: identify gait deviations; understand gait deviations by linking them with clinical impairments; and choose the best therapeutic option.
Based on these three steps, the aim of this paper is to present 1) methods to analyse gait and identify gait deviations in CP; 2) gait patterns and gait deviations in CP and their interpretation; and 3) an overview of gait deviations management in CP.
Clinical gait analysis
Clinical gait analysis (CGA) aims to determine what is causing a patient to walk in the way he/she does. To reach this aim, CGA collects and analyses a large amount of quantitative data concerning the gait characteristics of a patient. In fact, CGA provides detailed information on four main types of data recorded simultaneously: spatiotemporal, kinematics, kinetics and electromyography data. During the same session, standardised clinical videos are recorded with numerical video cameras generally synchronised with the opto-electronic system (Fig. 1).
Fig. 1.

Example of data in clinical gait analysis report for bilateral spastic cerebral palsy (video stills).
In addition, a standardised physical examination of the lower limbs is performed to measure anthropometry, passive range of motion, muscle force and muscle spasticity.7
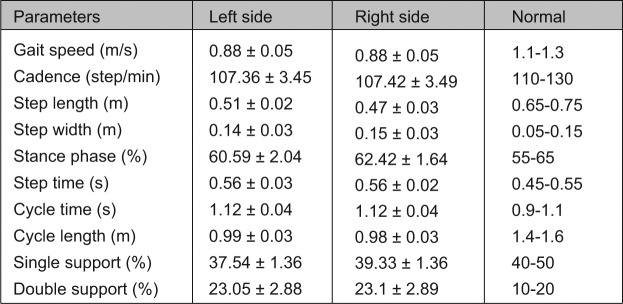
Gait can be separated into cycles. A gait cycle starts at the instant where one foot touches the floor and stops as the same foot comes into contact for the next step. It can be divided in different phases: stance phase and swing phase. Stance phase can be also sub-divided into sub-phases: first double support (both feet in contact with the floor), single support (one foot in contact with the floor) and second double support. Based on the gait cycle, temporal and spatial characteristics of gait can be calculated: walking speed, cadence, stride length, step length, step width, duration of the stance and swing phases (Fig. 2).
Fig. 2.
Example of data in clinical gait analysis report for bilateral spastic cerebral palsy: spatiotemporal parameters.
Kinematic data correspond to the movement of the body described in CGA by the angular variations of the different joints/segments: ankle, knee, hip, pelvis and trunk. The raw data are measured by opto-electronic systems from the position of passive (or active) reflective markers attached directly on the skin of the patient during CGA. These markers are fixed on the patient in an accurate standardised position relative to anatomical or technical landmarks.8 These positions are dependent on the model used to compute the kinematics. The most used models in the clinical field are the conventional gait models such as ‘Plug-InGait’.9 More advanced models and methods have been developed by different research teams in order to be more accurate. For example, calibration techniques10 or foot models11 have been developed; standardisation of joint definitions have been proposed.12
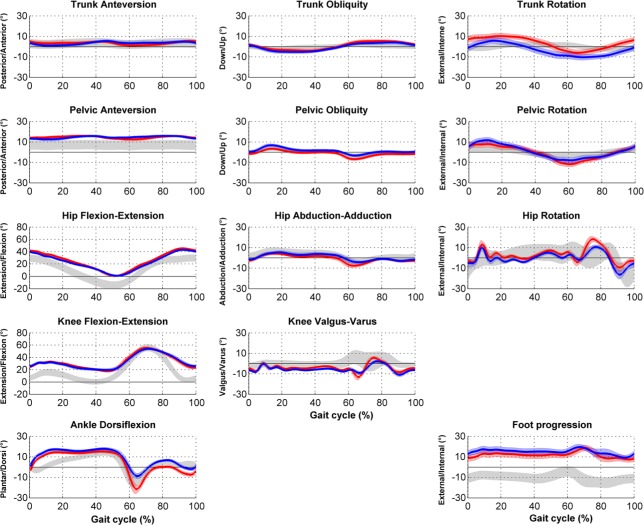
The model then permits the computation of joint angles (the angle between two adjacent segments in a specific plane). For this, the rotation around mobile axis (named Euler angles) are generally used to decompose the three-dimensional rotations in three successive elementary rotations. The three common planes used in description of the gait patterns are: the sagittal plane for flexion-extension movements; the frontal plane for adduction-abduction movements; and the transverse plane for internal-external rotations (Fig. 3).
Fig. 3.
Example of data in clinical gait analysis report for bilateral spastic cerebral palsy: kinematics.
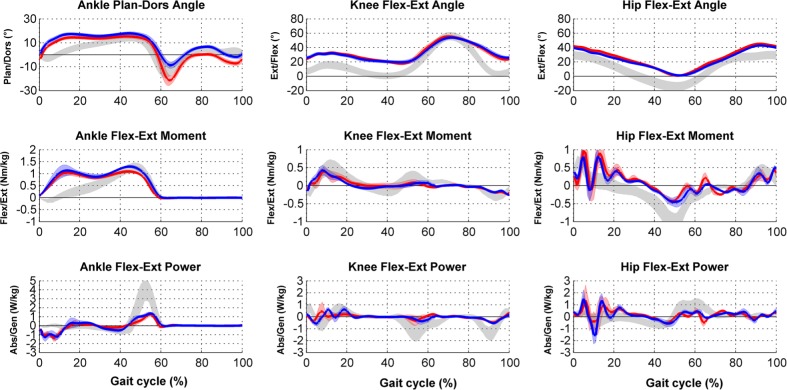
Dynamic or kinetic data describe the forces applied by the patient during his/her gait.13 This information corresponds to the ground-reaction forces, joint moments and powers of each joint. For this computation, data measured by force-plate(s) embedded in the ground of the gait laboratory are used. Using this information, it is possible to quantify ground-reaction force, which is the force exerted by the ground on the foot. With the addition of inertial parameters and kinematic data, it is possible to calculate by inverse dynamics the joint moments and joint powers applied at each joint during gait (Fig. 4).
Fig. 4.
Example of data in clinical gait analysis report for bilateral spastic cerebral palsy: kinetics.
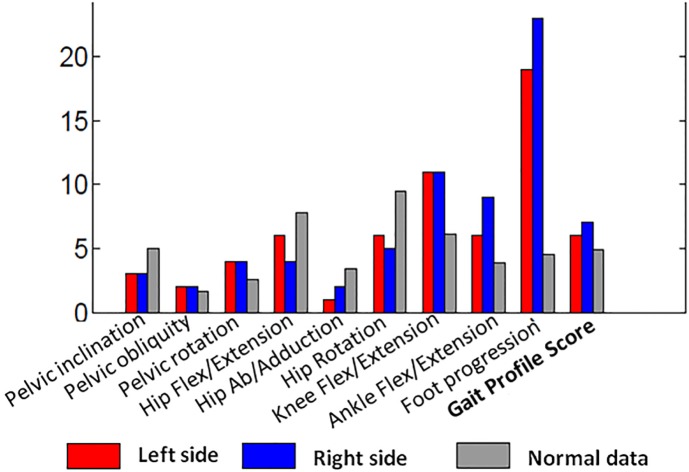
Based on kinematic data, gait scores such as Gait Profile Scores and Movement Analysis Profile14 or Gait Deviation Index15 can be computed to summarise gait deviations (Fig. 5)
Fig. 5.
Example of data in clinical gait analysis report for bilateral spastic cerebral palsy: gait scores.
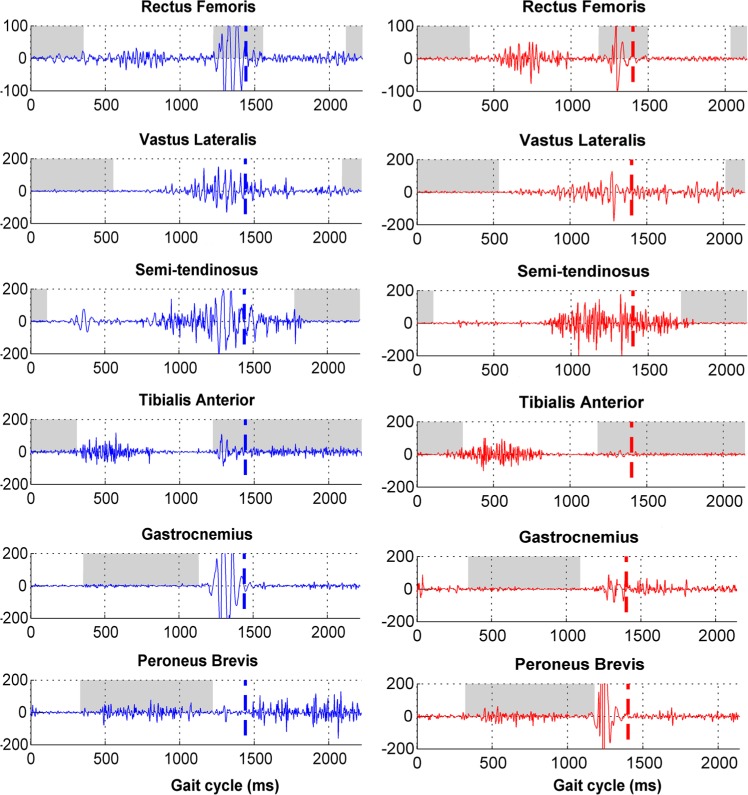
Electromyography (EMG) data show the timing and intensity of the muscle activity (Fig. 6). Thus, it is possible to describe the timing of the activity (such as lacking, delayed or permanent activity), co-contraction periods and also muscle spasticity during gait.16 In CGA, surface electrodes are generally used with specific procedures recommended by the SENIAM.17
Fig. 6.
Example of data in clinical gait analysis report for bilateral spastic cerebral palsy: electromyography.
Moreover, during a CGA, it is also possible to record pedobarography measures. These data give information about contact of the feet (without shoes) with the ground during stance phase and how plantar surface pressures are distributed (Fig. 7).
Fig. 7.
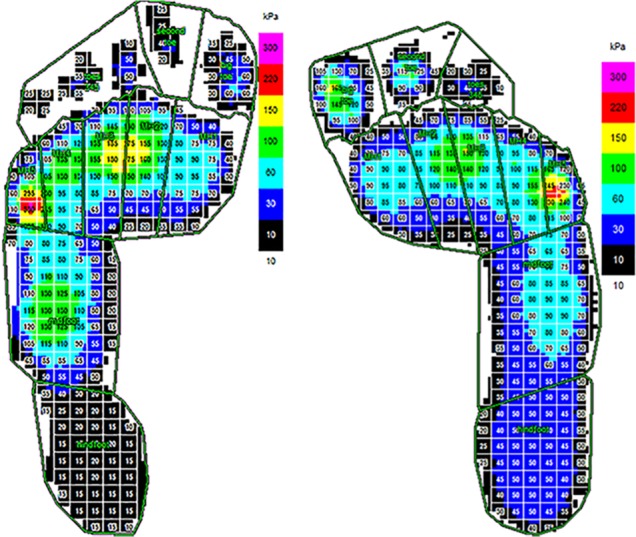
Example of data in clinical gait analysis report for bilateral spastic cerebral palsy: plantar pressures.
Major limitations of CGA data are linked to external measurements used to estimate the movement of internal structures. These limitations include: marker placement on anatomical landmarks; skin movement (soft tissue artefacts);18 definition of joint centres;19 definition of anatomical axis respecting anatomical and clinical reality; and deformation of the segments (as for the foot20). However, CGA allows identification of gait deviations and allows further understanding of gait deviations.
To go deeper in the understanding of gait deviations, it is now technically possible to use personalised (neuro)-musculoskeletal modelling by merging data from medical imaging, CGA and clinical evaluation. Such modelling permits the simulation and testing of different hypotheses of the causes of gait deviations of a specific patient and to visualise the effect of treatments of impairments (e.g. increase length of a muscle, correction of bony deformity).21 Currently, the major clinical limitations of this approach are the validation of the models used and the time needed to build an accurate model of a specific patient.
Gait deviations in cerebral palsy
A large variety of gait deviations can be observed in CP patients. Classification systems to group common gait deviations in gait patterns are often used to facilitate communication22 but are a simplification of the reality. In CP patients, we can observe a continuum between the different classes (no strict boundaries). Some gait deviations or combinations of gait deviations are not described in the classification systems. Therefore, a more accurate definition of gait deviations and related impairments is often needed to have a precise understanding of gait deviations and to better guide treatments using CGA results. The first part of this section describes the main gait patterns observed in CP. The second part describes the main gait deviations with related impairments.
Gait patterns
Classifications in gait patterns are generally different for unilateral spastic CP and for bilateral spastic CP. Most of the classifications are based the observation of the kinematics of the sagittal plane. Impairments and typical treatments are often associated with the gait patterns.
Gait patterns in unilateral spastic CP
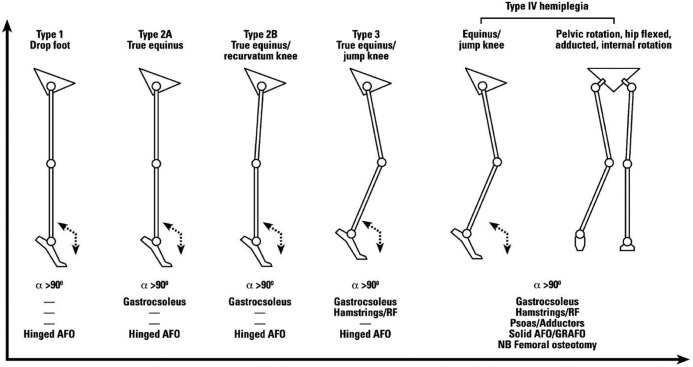
The first pattern classification for unilateral spastic CP was proposed by Winters et al.23 Four groups of gait patterns were identified based on observation of kinematic data in the sagittal plane (Fig. 8).
Fig. 8.
Gait patterns and management algorithm for unilateral spastic cerebral palsy24.
Republished from European Journal of Neurology with kind permission of John Wiley and Sons.
Original publication: Rodda J, Graham HK. Classification of gait patterns in spastic hemiplegia and spastic diplegia: a basis for a management algorithm. Eur J Neurol 2001;8(suppl 5):98-108.
There is progression of severity of impairments in the four groups and a progression of impairments from distal to proximal (from ankle to pelvis). Group I’s main characteristic is foot drop during swing phase resulting in a lack of first rocker at initial contact. The impairments associated with group I are weakness or underactivity of the anterior tibial muscle relative to overactivity of the gastrocnemius and soleus muscles. Group II’s gait pattern presents a drop foot in swing phase and a permanent plantarflexion in stance phase. Knee hyperextension is associated with this pattern. The impairments associated with group II are static or dynamic contractures of the gastrocnemius and soleus muscles. In addition to deviations of groups I and II, patients in group III have a limited flexion of the knee during the swing phase, a hyperflexion of the hip and an increased lumbar lordosis. Group IV presents all the deviations of groups I to III, plus restricted motion at the hip and knee. Patients compensate for limited motion of the hip by increasing pelvic lordosis during the terminal stance phase.
Winters’ classification was refined by Rodda and Graham in 2001 to include patients with hyperextension at the knee and transverse plane deviations at the hip.24 A schematic of gait patterns associated with possible treatments was provided by the authors (Fig. 8). Other classifications exist in the literature but have less notoriety. Some are based on subjective group placement and others on cluster analysis.22
Gait patterns in bilateral spastic CP
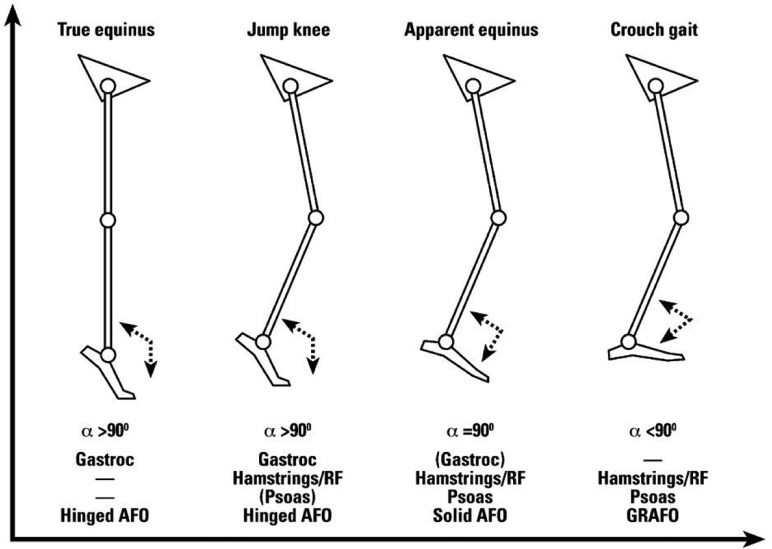
Based on Sutherland’s classification of gait abnormalities of the knee in CP,25 Rodda and Graham24 have proposed a classification for patients with bilateral spastic CP based on sagittal plane kinematics considering the ankle, knee, hip and pelvis. Four main groups were identified: true equinus; jump gait; apparent equinus; and crouch gait (Fig. 9).
Fig. 9.
Gait patterns and management algorithm for bilateral spastic cerebral palsy.24
Republished from European Journal of Neurology with kind permission of John Wiley and Sons.
Original publication: Rodda J, Graham HK. Classification of gait patterns in spastic hemiplegia and spastic diplegia: a basis for a management algorithm. Eur J Neurol 2001;8(suppl 5):98-108.
True equinus is defined by the ankle in plantarflexion throughout stance phase and the hips and knees extended. The authors caution that the equinus can be masked by a knee recurvatum. The jump gait pattern is characterised by equinus at the ankle, flexion at knee and hip, anterior tilt and increased lumbar lordosis. Within this pattern, the stiff knee pattern described by Sutherland and Davids25 is frequent. The apparent equinus pattern presents a normal range of dorsiflexion at the ankle, but the hip and knee are in excessive flexion throughout the stance phase leading to walking on the toes and giving an impression of equinus. Crouch gait is defined by excessive dorsiflexion at the ankle in combination with excessive flexion at the knee and hip joints (Fig. 3). The authors recognised that the two limbs can present different levels of involvement. Thus, they introduced an asymmetric gait group when the two limbs belong to two different classifications.26 They also introduced a mild gait group for kinematics within normal limits in the sagittal plane.26 These patterns established subjectively and qualitatively based on observation and experience were confirmed with statistical and clustering techniques without a priori.27-29 However, numerous classifications of CP gait children were proposed involving different numbers of joints, segments, planes and clustering techniques leading to different numbers of clusters.22
Specific gait deviations in CP
Gait deviations in CP are a continuum of deviations rather than well delineated groups. Each CP patient is specific and generally presents with a list of impairments, with varying degrees of involvement and asymmetry between the two lower limbs. To support a precise understanding of gait deviations in CP, it is mandatory to understand the links between gait deviations and impairments. Recently, we defined kinematic gait deviations observed at the individual joint/segment/plane level with probable impairments resulting from these deviations (Table 1).30
Table 1.
Frequent single joint/plane kinematic deviations and associated impairments30
| Gait deviations | Description | Impairments and coherent gait data | Confounding factor |
|---|---|---|---|
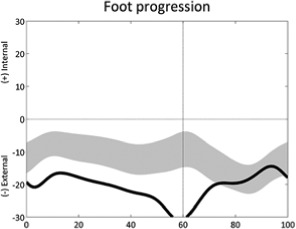 |
External foot progression (stride) | • Increased external tibial torsion • Foot deformity ◦ Increased ankle external rotation |
• Sustained pelvic retraction • Increased hip external rotation |
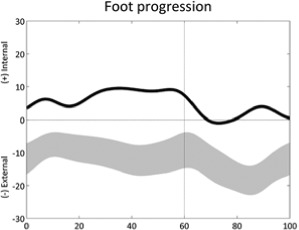 |
Internal foot progression (stride) | • Increased femoral anteversion ◦ Increased hip internal rotation • Reduced external tibial torsion • Foot deformity ◦ Increased internal ankle rotation |
• Sustained pelvic protraction |
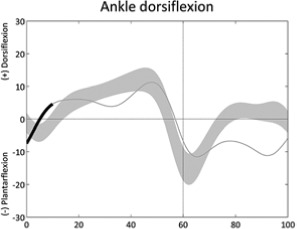 |
Absent ankle first rocker (first double support) | • Ankle dorsiflexors weakness or reduced selective motor control ◦ Excessive plantarflexion (swing) • Plantarflexors contracture or overactivity ◦ Excessive plantarflexion (stride) |
• Leg length discrepancy |
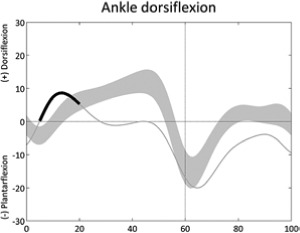 |
Early ankle plantarflexion (early stance) | • Plantarflexors overactivity ◦ Premature knee extension/hyperextension |
• Leg length discrepancy or foot clearance problem on contralateral side • Increased knee flexion |
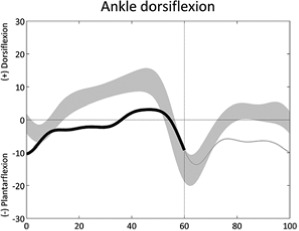 |
Lack of ankle dorsiflexion (stance) | • Plantarflexors contracture or overactivity | |
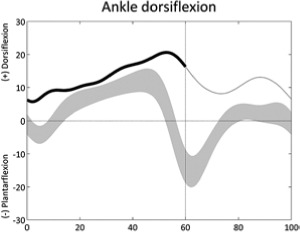 |
Increased ankle dorsiflexion (stance) | • Soleus weakness or soleus too long ◦ Increased knee flexion in mid-stance |
|
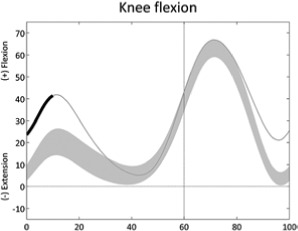 |
Increased knee flexion (first double support) | • Hamstring overactivity • Plantarflexors contracture or overactivity ◦ Excessive ankle plantarflexion |
|
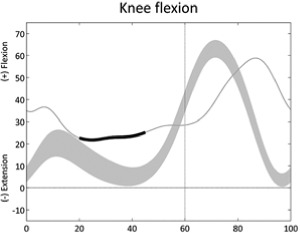 |
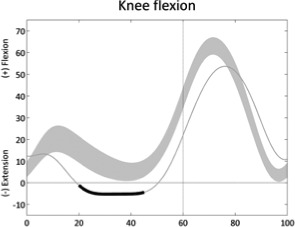
Reduced knee extension (mid-stance) | • Hamstring contracture or overactivity • Knee fixed flexion deformity • Hip extensors or knee extensors weakness ◦ Excessive hip flexion • Ankle plantarflexors weakness ◦ Excessive ankle dorsiflexion • Ankle plantarflexors overactivity or contracture ◦ Excessive ankle plantarflexion |
• Cross-plane interactions (transverse - sagittal) ◦ External tibial torsion ◦ Increased femoral neck anteversion ◦ Foot deformity |
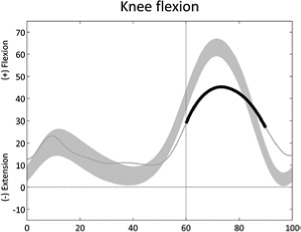 |
Reduced or delayed knee flexion (swing) | • Rectus femoris overactivity ◦ Rectus femoris EMG activity in late stance or early swing • Stiff-knee gait, hamstring/rectus co-contraction |
• Cross-plane interaction (transverse - sagittal) if retracted pelvis and hip externally rotated • Reduced push-off during ankle third rocker • Reduced speed |
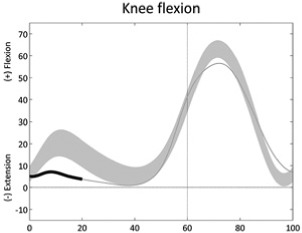 |
Reduced knee flexion (loading response) | • Quadriceps weakness or patella pain ◦ Reduced knee extensor moment (stance) |
|
 |
Knee hyper extension (mid-stance) | • Quadriceps weakness • Plantarflexors overactivity or contracture ◦ Excessive ankle plantarflexion |
|
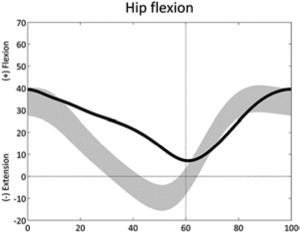 |
Increased hip flexion (stride) | • Hip flexion contracture or overactivity ◦ Anterior pelvic tilt, double bump • Hip extensor weakness |
• Sustained anterior pelvic tilt (stride) • Excessive knee flexion • Leg length discrepancy |
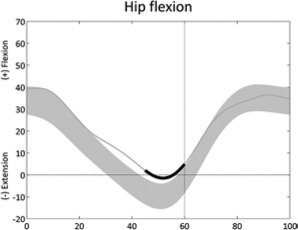 |
Lack of hip extension (second double support) | • Hip flexor contracture or overactivity ◦ Anterior pelvic tilt, double bump • Hip reduced range of movement ◦ Anterior pelvic tilt, single bump |
• Leg length discrepancy |
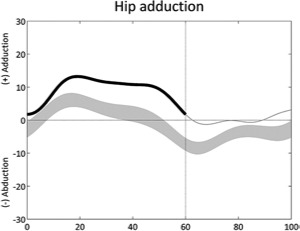 |
Increased hip adduction (stance) | • Hip abductor weakness ◦ Contralateral pelvic drop • Hip adductor contracture or overactivity |
• Increased hip internal rotation • Pelvic retraction or obliquity • Leg length discrepancy |
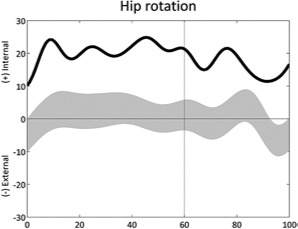 |
Increased internal hip rotation (stride) | • Increased femoral neck anteversion • Excessive external tibial torsion |
• Pelvic retraction on ipsilateral side |
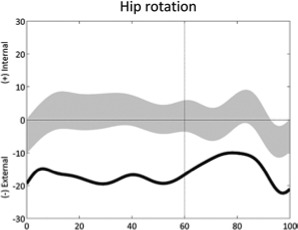 |
Increased external hip rotation (stride) | • Reduced femoral anteversion • Reduced external tibial torsion |
• Pelvic protraction on ipsilateral side • Obesity/large thighs • Foot deformity |
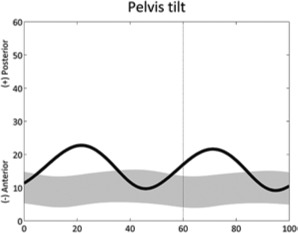 |
Pelvic tilt double bump (stride) | • Hip flexors contracture or overactivity ◦ Reduced hip extension |
|
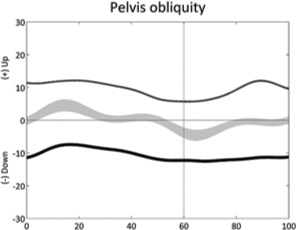 |
Pelvic obliquity down or up (stride) | • Leg length discrepancy ◦ Excessive hip abduction • Adduction contracture |
• Scoliosis • Hemiplegia |
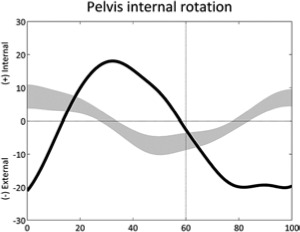 |
Reversed pelvic rotation profile (stride) | • Overall weakness ◦ Reversed hip adduction profile |
|
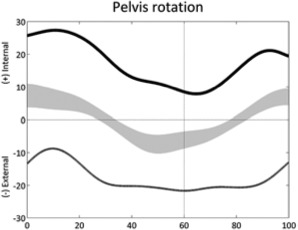 |
Sustained pelvic pro- or re-traction (stride) | • Asymmetry in overall weakness | • Femur torsional deformity • Tibia torsional deformity • Hemiplegia |
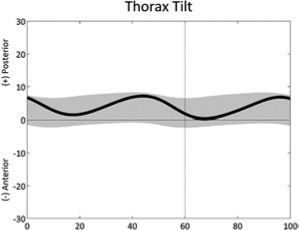 |
Trunk tilt, double bump (stride) | • Overall weakness | |
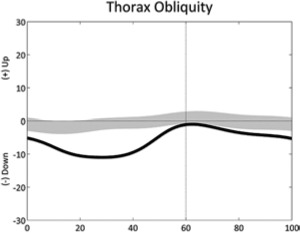 |
Sustained trunk obliquity (stride) | • Hip pain (unilateral) • Abductors weakness (unilateral) |
|
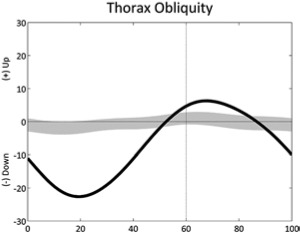 |
Excessive range of trunk obliquity, Trendelenburg (stride) | • Abductors weakness |
Table 1 is reprinted with permission from Nova Science Publishers. From: Sangeux M, Armand S. Kinematic deviations in children with cerebral palsy. In: Canavese F, Deslandes J, ed. Orthopedic management of children with cerebral palsy: A comprehensive approach. New York: Nova Science, 2015; 241-253.The table has four columns;the first presents a graph of the deviation, the second describes the gait deviation observed, the third presents the impairments (•) and lists associated deviations (◦).These confounding factors (•) are listed in the last column. One confounding factor appears several times: leg length discrepancy. Leg length discrepancy may be anatomical, when physical examination or medical imaging measures a true length difference between the legs, or functional, when the combination of joint angles during single leg support in stance phase results in altered leg length. The deviations are ordered from distal to proximal joints/segments and in the sagittal, coronal and transverse planes. In each graph, the light grey band presents the pattern of 35 typically developed children (the width equates to one standard deviation). The solid curve presents an example of altered kinematics, the part of interest is emphasised by a bolder line for the time instants of interest. Two pelvic deviations show two lines (one solid, one dashed) for the two sides of the same patient.
Some of these gait deviations are also presented in the book by Perry.31 We recognise that some kinematic deviations may come secondary to another problem or as compensation for another deviation. We also recognise that this table is not exhaustive and is based more on experience than evidence. Further studies are needed to confirm these links. Moreover, impairments are rarely isolated; therefore, gait deviations are often the results of combinations of impairments. This table considers only kinematic gait deviations and not kinetic, EMG and plantar pressure gait deviations.
Management of gait deviations in CP
Management of gait deviations in CP is complex and requires identification and understanding the gait deviations. Even if CGA supports these requirements, one can question the reliability of treatment decisions based on CGA.32,33 As for many clinical diagnostic tools (e.g. imagery), CGA does not tell us how to treat a patient, but does help to identify and understand an impairment.34 The choice of treatments depends on each physician.
Moreover, CGA helps to better understand the effects of treatments on gait deviations. This objective assessment of the patient’s gait allows comparison of pre- and post-operative results. This information helps to determine if there is an improvement in patients’ walking capacity. From this, the physician can critically analyse the therapeutic strategies as demonstrated by Vuillermin et al.35 Before the introduction of CGA in their hospital in 1995, the prevalence of crouch gait was 25%. Thanks to CGA, they better understood the complexity of the walking patterns in CP patients. Thus, they realised how important the ankle-knee extension couple is. They stopped lengthening the Achilles tendon and they decreased considerably the prevalence of crouch gait in their CP patients.
Currently, there are no biological therapies to correct the brain lesion. Therefore, the management of CP children concerning motor disabilities is focussed on the primary consequences of the brain lesions (muscle spasticity and loss of selective motor control) and secondary consequences (muscle weaknesses, muscle contractures, bony deformities).
It is difficult to provide general considerations for the treatments of gait deviations in CP due to multiple gait deviations coming from different combinations of impairments. Moreover, even if the brain lesion is static, the chronic neurological impairments affect the development of muscles and bones. Therefore, CP is a progressive musculoskeletal pathology. To present the treatment options, we have divided the treatment considerations according types of treatments and gait patterns.
Main treatments in CP to address gait deviations
CP patients present numerous possible combinations of impairments that can be managed by numerous types of treatments. Physiotherapy is certainly the most common treatment in CP. The main objectives are to maintain range of motion with adequate muscle length, to preserve or restore strength, and to improve balance and co-ordination. Orthoses are also used frequently to manage gait deviations in CP, in particular ankle-foot orthoses.36
Spasticity management is addressed with a large spectrum of therapies that can have a focal or global effect and a permanent or reversible effect. Botulinim Neurotoxin-A (BoNT-A) is largely used to treat local spasticity.37 BoNT-A injection should be reversible, but currently the long-term implications of denervation atrophy are not fully understood.38 Gough et al recommends taking a cautious approach, particularly with ambulant children with spastic diplegic CP.39 BoNT-A can also be used as a pre-operative test injection before muscle-tendon lengthening.40 Hence, application of the drug is a possible step to test whether weakness causes deterioration in gait function prior to an operation. Selective dorsal rhizotomy has a permanent effect with a global impact on the lower limb. It is reserved for selected patients with diplegic CP.41 Intrathecal baclofen has a general effect that can be reversible. This treatment is used to treat severe spasticity in CP.42
Bony deformities and muscle contractures can be addressed by surgery. One general principle is to preserve or restore lever arms. Abnormal lever arms result from bony deformities that lead to additional deformities and difficulties in efficiently-using forces exerted by the muscles.43
The growth of the skeleton associated with spasticity, reduced level of activity and weakness of muscles leads to muscle contractures. Muscle contractures should be evaluated and treated as conservatively as possible to prevent over lengthening and muscle weakness.
To avoid ‘birthday syndrome’, which is the correction of one deviation after another occurring with growth, the current treatment is to perform a single event multi-level surgery (SEMLS) to manage musculoskeletal deformities in CP.44 SEMLS addresses all the impairments of both lower limbs in the same surgical procedure.45-47
Main surgical treatments in CP according gait patterns
We chose to present the main surgical treatments according three common gait patterns in CP: true equinus,48-50 crouch gait51,52 and stiff knee gait;51,52 and two impairments: torsional deformities55-58 and varus feet59,60 (Table 2).
Table 2.
Main surgical treatments in cerebral palsy (CP) according gait patterns
| Gait patterns Impairments | Treatment options | Comments |
|---|---|---|
| True equinus | - Gastrocnemius recession rather than tendo-Achilles lengthening | - Important to distinguish true equinus from apparent equinus - Excessive dorsiflexion can occur over time without surgical intervention, secondary to progressive weakness and increasing weight |
| Crouch gait | - Re-establish the lever arm function: ◦ Stabilise flat foot deformity to restore foot lever arm ◦ Correct torsional tibial deformity ◦ Patella advancement to reinforce the quadriceps (Reinforce the Achilles tendon with a floor reaction orthosis) |
- Caution with hamstring lengthening because CP patients can develop knee recurvatum and an anterior pelvic tilt |
| Stiff knee gait | - Rectus femoris transfer is indicated in patients functioning at GMFCS level I or II. (Patellar advancement should be considered when there is an important knee extension moment in stance) |
- Rectus femoris transfer is not helpful in patients functioning at GMFCS level IV and some patients at level III, due to increased knee flexion in stance post-operatively |
| Torsional deformities | - Derotational osteotomy of tibia and/or femur | - Good correlation with tibial de-rotational osteotomy correction and the foot progression angle correction - Weak correlation between femoral derotational osteotomy correction and the foot progression angle correction - Soft tissue surgeries alone will not fully correct torsional deformities |
| Varus feet | - Tendon transfers to correct muscular forces that cause deformity | - Frequent deformities in cerebral palsy caused by tibialis anterior or tibialis posterior or a combination of both |
GMFCS, gross motor function classification system
Conclusion
Cerebral palsy children present complex gait deviations that evolve with growth. Having an in-depth understanding of these gait deviations is necessary to choose the most appropriate therapeutic approach. Therefore, CGA has become a fundamental part of the clinical management of gait deviations in CP patients. By using a precise quantification of a person’s gait characteristics, CGA allows the identification of gait deviations and gives a better understanding of these gait deviations. Moreover, CGA has allowed better evaluation and understanding of the effects of treatments on gait deviations. Indeed, thanks to CGA, management of gait deviations in CP children has changed and continues to improve.
Acknowledgments
The authors would like to thank Charlotte Elsworth-Edelsten for her English reviewing.
Footnotes
Conflict of Interest: None declared.
Funding
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Himmelmann K, Hagberg G, Uvebrant P. The changing panorama of cerebral palsy in Sweden. X. Prevalence and origin in the birth-year period 1999-2002. Acta Paediatr 2010;99(9):1337-43. [DOI] [PubMed] [Google Scholar]
- 2. Hutton JL, Colver AF, Mackie PC. Effect of severity of disability on survival in north east England cerebral palsy cohort. Arch Dis Child 2000;83(6):468-474.PMID:11087278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214-23. [DOI] [PubMed] [Google Scholar]
- 4. Chiou II, Burnett CN. Values of activities of daily living. A survey of stroke patients and their home therapists. Phys Ther 1985;65:901-06. [DOI] [PubMed] [Google Scholar]
- 5. Wren TA, Gorton GE, III, Ounpuu S, Tucker CA. Efficacy of clinical gait analysis: A systematic review. Gait Posture 2011;34:149-53. [DOI] [PubMed] [Google Scholar]
- 6. Moissenet M, Armand S. Qualitative and quantitative methods of assessing gait disorders. In: Canavese F, Deslandes J, ed. Orthopedic management of children with cerebral palsy: A comprehensive approach. New York: Nova Science, 2015; 215-239. [Google Scholar]
- 7. Fosang AL, Galea MP, McCoy AT, Reddihough DS, Story I. Measures of muscle and joint performance in the lower limb of children with cerebral palsy. Dev Med Child Neurol 2003;45(10):664-70. [DOI] [PubMed] [Google Scholar]
- 8. Van Sint Jan S. Color atlas of skeletal landmarks definitions: Guidelines for reproductible manual and virtual palpitations. London: Elsevier, 2007. [Google Scholar]
- 9. Baker R. Measuring walking: a handbook of clinical gait analysis. London: Mac Keith Press, 2013. [Google Scholar]
- 10. Cappozzo A, Catani F, Leardini A, Benedetti MG, Croce UD. Position and orientation in space of bones during movement: experimental artefacts. Clin Biomech (Bristol, Avon) 1996;11:90-100. [DOI] [PubMed] [Google Scholar]
- 11. Carson MC, Harrington ME, Thompson N, O’Connor JJ, Theologis TN. Kinematic analysis of a multi-segment foot model for research and clinical applications: a repeatability analysis. J Biomech 2001;34:1299-1307. [DOI] [PubMed] [Google Scholar]
- 12. Wu G, Siegler S, Allard P, et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion–part I: ankle, hip, and spine. International Society of Biomechanics. J Biomech 2002;35:543-8. [DOI] [PubMed] [Google Scholar]
- 13. Winter DA. Biomechanics and motor control of human movement. Hoboken: Wiley, 2009. [Google Scholar]
- 14. Baker R, McGinley JL, Schwartz MH, et al. The gait profile score and movement analysis profile. Gait Posture 2009;30:265-9. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz MH1, Rozumalski A. The Gait Deviation Index: a new comprehensive index of gait pathology. Gait Posture 2008;28:351-7. [DOI] [PubMed] [Google Scholar]
- 16. Davis RB. Reflections on clinical gait analysis. J Electromyogr Kinesiol 1997;7:251-257. [DOI] [PubMed] [Google Scholar]
- 17. Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 2000;10:361-74. [DOI] [PubMed] [Google Scholar]
- 18. Leardini A, Chiari L, Della Croce U, Cappozzo A. Human movement analysis using stereophotogrammetry. Part 3. Soft tissue artifact assessment and compensation. Gait Posture 2005;21:212-25. [DOI] [PubMed] [Google Scholar]
- 19. Sangeux M, Peters A, Baker R. Hip joint centre localization: evaluation on normal subjects in the context of gait analysis. Gait Posture 2011;34:324-8. [DOI] [PubMed] [Google Scholar]
- 20. Pothrat C, Authier G, Viehweger E, Berton E, Rao G. One- and multi-segment foot models lead to opposite results on ankle joint kinematics during gait: implications for clinical assessment. Clin Biomech (Bristol, Avon) 2015;30:493-9. [DOI] [PubMed] [Google Scholar]
- 21. Fregly BJ, Boninger ML, Reinkensmeyer DJ. Personalized neuromusculoskeletal modeling to improve treatment of mobility impairments: a perspective from European research sites. J Neuroeng Rehabil 2012;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dobson F, Morris ME, Baker R, Graham HK. Gait classification in children with cerebral palsy: a systematic review. Gait Posture 2007;25:140-52. [DOI] [PubMed] [Google Scholar]
- 23. Winters TF, Jr, Gage JR, Hicks R. Gait patterns in spastic hemiplegia in children and young adults. J Bone Joint Surg [Am] 1987;69:437-41. [PubMed] [Google Scholar]
- 24. Rodda J, Graham HK. Classification of gait patterns in spastic hemiplegia and spastic diplegia: a basis for a management algorithm. Eur J Neurol 2001;8(suppl 5):98-108. [DOI] [PubMed] [Google Scholar]
- 25. Sutherland DH, Davids JR. Common gait abnormalities of the knee in cerebral palsy. Clin Orthop Relat Res 1993;(288):139-147. [PubMed] [Google Scholar]
- 26. Rodda JM, Graham HK, Carson L, Galea MP, Wolfe R. Sagittal gait patterns in spastic diplegia. J Bone Joint Surg [Br] 2004;86:251-8. [DOI] [PubMed] [Google Scholar]
- 27. Bonnefoy-Mazure A, Sagawa Y, Jr, Lascombes P, De Coulon G, Armand S. Identification of gait patterns in individuals with cerebral palsy using multiple correspondence analysis. Res Dev Disabil 2013;34:2684-93. [DOI] [PubMed] [Google Scholar]
- 28. Sangeux M, Rodda J, Graham HK. Sagittal gait patterns in cerebral palsy: the plantarflexor-knee extension couple index. Gait Posture 2015;41:586-91. [DOI] [PubMed] [Google Scholar]
- 29. Toro B, Nester CJ, Farren PC. Cluster analysis for the extraction of sagittal gait patterns in children with cerebral palsy. Gait Posture 2007;25:157-65. [DOI] [PubMed] [Google Scholar]
- 30. Sangeux M, Armand S. Kinematic deviations in children with cerebral palsy. In: Canavese F, Deslandes J, ed. Orthopedic management of children with cerebral palsy: A comprehensive approach. New York: Nova Science, 2015; 241-253. [Google Scholar]
- 31. Perry J, Burnfield JM. Gait analysis - normal and pathological function. New Jersey: Slack Incorporated, 2010. [Google Scholar]
- 32. Noonan KJ, Halliday S, Browne R, O’Brien S, Kayes K, Feinberg J. Interobserver variability of gait analysis in patients with cerebral palsy. J Pediatr Orthop 2003;23:279-87. [PubMed] [Google Scholar]
- 33. Skaggs DL, Rethlefsen SA, Kay RM, Dennis SW, Reynolds RA, Tolo VT. Variability in gait analysis interpretation. J Pediatr Orthop 2000;20:759-64. [DOI] [PubMed] [Google Scholar]
- 34. Theologis T, Wright J. Is 3-D gait analysis essential? Gait Posture 2015;42:227-9. [DOI] [PubMed] [Google Scholar]
- 35. Vuillermin C, Rodda J, Rutz E, Shore BJ, Smith K, Graham HK. Severe crouch gait in spastic diplegia can be prevented: a population-based study. J Bone Joint Surg [Br] 2011;93:1670-75. [DOI] [PubMed] [Google Scholar]
- 36. Wingstrand M, Hägglund G, Rodby-Bousquet E. Ankle-foot orthoses in children with cerebral palsy: a cross sectional population based study of 2200 children. BMC Musculoskelet Disord 2014;15:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molenaers G, Fagard K, Van Campenhout A, Desloovere K. Botulinum toxin A treatment of the lower extremities in children with cerebral palsy. J Child Orthop 2013;7:383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gough M. Does botulinum toxin prevent or promote deformity in children with cerebral palsy? Dev Med Child Neurol 2009;51:89-90. [DOI] [PubMed] [Google Scholar]
- 39. Gough M, Fairhurst C, Shortland AP. Botulinum toxin and cerebral palsy: time for reflection? Dev Med Child Neurol 2005;47:709-12. [DOI] [PubMed] [Google Scholar]
- 40. Rutz E, Hofmann E, Brunner R. Preoperative botulinum toxin test injections before muscle lengthening in cerebral palsy. J Orthop Sci 2010;15:647-53. [DOI] [PubMed] [Google Scholar]
- 41. Roberts A, Stewart C, Freeman R. Gait analysis to guide a selective dorsal rhizotomy program. Gait Posture 2015;42:16-22. [DOI] [PubMed] [Google Scholar]
- 42. Hasnat MJ, Rice JE. Intrathecal baclofen for treating spasticity in children with cerebral palsy. Cochrane Database Syst Rev 2015;11:CD004552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Novacheck TF, Gage JR. Orthopedic management of spasticity in cerebral palsy.Childs Nerv Syst 2007;23:1015-31. [DOI] [PubMed] [Google Scholar]
- 44. McGinley JL, Dobson F, Ganeshalingam R, Shore BJ, Rutz E, Graham HK. Single-event multilevel surgery for children with cerebral palsy: a systematic review. Dev Med Child Neurol 2012;54:117-28. [DOI] [PubMed] [Google Scholar]
- 45. Rutz E, Tirosh O, Thomason P, Barg A, Graham HK. Stability of the Gross Motor Function Classification System after single-event multilevel surgery in children with cerebral palsy. Dev Med Child Neurol 2012;54:1109-13. [DOI] [PubMed] [Google Scholar]
- 46. Thomason P, Baker R, Dodd K, et al. Single-event multilevel surgery in children with spastic diplegia: a pilot randomized controlled trial. J Bone Joint Surg [Am] 2011;93:451-60. [DOI] [PubMed] [Google Scholar]
- 47. Thomason P, Selber P, Graham HK. Single event multilevel surgery in children with bilateral spastic cerebral palsy: a 5 year prospective cohort study. Gait Posture 2013;37:23-28. [DOI] [PubMed] [Google Scholar]
- 48. Firth GB, Passmore E, Sangeux M, et al. Multilevel surgery for equinus gait in children with spastic diplegic cerebral palsy: medium-term follow-up with gait analysis. J Bone Joint Surg [Am] 2013;95:931-8. [DOI] [PubMed] [Google Scholar]
- 49. Rutz E, Baker R, Tirosh O, Romkes J, Haase C, Brunner R. Tibialis anterior tendon shortening in combination with Achilles tendon lengthening in spastic equinus in cerebral palsy. Gait Posture 2011;33:152-7. [DOI] [PubMed] [Google Scholar]
- 50. Shore BJ, White N, Kerr Graham H. Surgical correction of equinus deformity in children with cerebral palsy: a systematic review. J Child Orthop 2010;4:277-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sossai R, Vavken P, Brunner R, Camathias C, Graham HK, Rutz E. Patellar tendon shortening for flexed knee gait in spastic diplegia. Gait Posture 2015;41:658-65. [DOI] [PubMed] [Google Scholar]
- 52. Stout JL, Gage JR, Schwartz MH, Novacheck TF. Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy. J Bone Joint Surg [Am] 2008;90:2470-84. [DOI] [PubMed] [Google Scholar]
- 53. Dreher T, Wolf SI, Maier M, et al. Long-term results after distal rectus femoris transfer as a part of multilevel surgery for the correction of stiff-knee gait in spastic diplegic cerebral palsy. J Bone Joint Surg [Am] 2012;94-A:e142(1-10). [DOI] [PubMed] [Google Scholar]
- 54. Presedo A, Megrot F, Ilharreborde B, Mazda K, Penneçot GF. Rectus femoris distal tendon resection improves knee motion in patients with spastic diplegia. Clin Orthop Relat Res 2012;470:1312-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dreher T, Wolf SI, Heitzmann D, et al. Long-term outcome of femoral derotation osteotomy in children with spastic diplegia. Gait Posture 2012;36:467-70. [DOI] [PubMed] [Google Scholar]
- 56. Er MS, Abousamra O, Rogers KJ, et al. Long-term outcome of internal tibial derotation osteotomies in children with cerebral palsy. J Pediatr Orthop 2015;10. PMID:26491913. [DOI] [PubMed] [Google Scholar]
- 57. Er MS, Bayhan IA, Rogers KJ, et al. Long-term outcome of external tibial derotation osteotomies in children with cerebral palsy. J Pediatr Orthop 2015;1: 1-5. [DOI] [PubMed] [Google Scholar]
- 58. Carty CP, Walsh HP, Gillett JG, et al. The effect of femoral derotation osteotomy on transverse plane hip and pelvic kinematics in children with cerebral palsy: a systematic review and meta-analysis. Gait Posture 2014;40:333-40. [DOI] [PubMed] [Google Scholar]
- 59. Michlitsch MG, Rethlefsen SA, Kay RM. The contributions of anterior and posterior tibialis dysfunction to varus foot deformity in patients with cerebral palsy. J Bone Joint Surg [Am] 2006;88:1764-68. [DOI] [PubMed] [Google Scholar]
- 60. Sees JP, Miller F. Overview of foot deformity management in children with cerebral palsy. J Child Orthop 2013;7:373-77. [DOI] [PMC free article] [PubMed] [Google Scholar]