Abstract
Ocotillol-type saponins are one kind of tetracyclic triterpenoids, sharing a tetrahydrofuran ring. Natural ocotillol-type saponins have been discovered in Panax quinquefolius L., Panax japonicus, Hana mina, and Vietnamese ginseng. In recent years, the semisynthesis of 20(S/R)-ocotillol-type saponins has been reported. The biological activities of ocotillol-type saponins include neuroprotective effect, antimyocardial ischemia, antiinflammatory, antibacterial, and antitumor activities. Owing to their chemical structure, pharmacological actions, and the stereoselective activity on antimyocardial ischemia, ocotillol-type saponins are subjected to extensive consideration. In this review, we sum up the discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins.
Keywords: biological activity, discovery, metabolism, ocotillol-type saponin, semisynthesis
1. Introduction
Ginseng, a perennial plant belonging to the genus Panax of the Araliaceae family, is well known for its medicinal properties that help alleviate pathological symptoms, promote health, and prevent potential diseases. Ginseng saponins are often classified into several groups: protopanaxadiol (PPD) type, protopanaxatriol (PPT) type, oleanolic acid type, and ocotillol type.
There are numerous chemical components present in Panax quinquefolius L., such as saponins, amino acids, saccharides, volatile oils, alkaloids, aliphatic acids, and mineral elements, among which ginsenosides are thought to be the main active ingredients. Ocotillol-type saponins (Fig. 1) are often used as phytochemical markers of P. quinquefolium L. to distinguish it from ginseng [1], [2].
Figure 1.
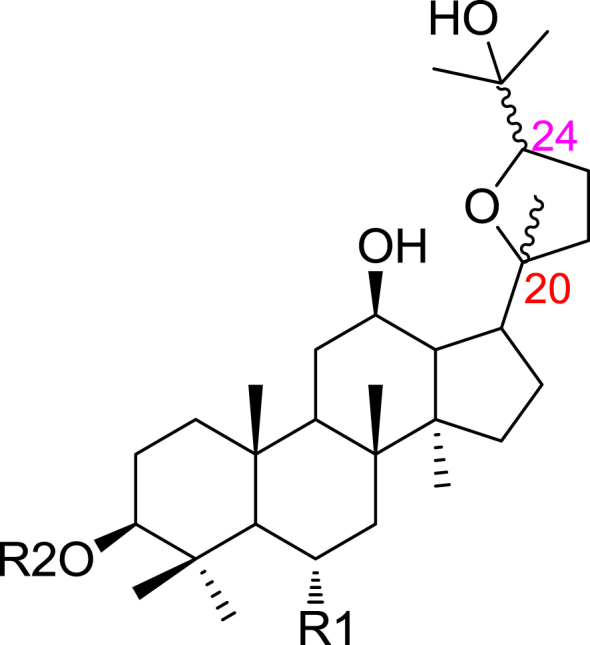
Structure of ocotillol-type saponins.
Ocotillol-type saponins, sharing a tetrahydrofuran ring and a dammarane skeleton, are one class of rare ginsenosides, which are very rarely found in natural products. We find that stereoselectivity plays a key role in pharmacological action as well as pharmacokinetics.
2. Discovery of ocotillol-type saponins
Natural ocotillol-type saponins mainly include PF11, RT2 (3), RT4 (5), RT5 (4), 24(S)-PF11 (12), vina-ginsenoside R1 (VR1; 6), VR2 (7), VR5 (8), VR6 (9), majonoside R1 (MR1; 10), MR2 (11), yesanchinoside A (13), B (14), and C (15; Table 1). The content of PF11 in American ginseng flower, pedicel, stems and leaves, pulp, and roots is 2.34%, 1.93%, 0.97% 1.54%, and 0.28%, respectively [19].
Table 1.
Natural ocotillol-type saponins
| No. | Ingredient name | R1 | R2 | C-20 | C-24 | Refs. |
|---|---|---|---|---|---|---|
| 1 | 20(S)-PF11 | -O-glc-rha | H | S | R | [3], [4], [5], [6], [7], [8] |
| 2 | 20(R)-PF11 | -O-glc-rha | H | R | R | [8], [9] |
| 3 | RT2 | -O-glc-xyl | H | S | R | [5], [10] |
| 4 | RT5 | -O-glc | H | S | R | [8], [10], [11], [12] |
| 5 | RT4 | -O-glc | H | S | S | [8], [10], [13], [14] |
| 6 | VR1 | -O-glc(-Ac)-rha | H | S | S | [13], [14] |
| 7 | VR2 | -O-glc(-Ac)-xyl | H | S | S | [13], [14] |
| 8 | VR5 | -O-glc-xyl-αrha | H | S | S | [15] |
| 9 | VR6 | -O-glc(-αglc)-xyl | H | S | S | [15] |
| 10 | MR1 | -O-glc-glc | H | S | S | [13], [16] |
| 11 | MR2 | -O-glc-xyl | H | S | S | [5], [13], [14], [16] |
| 12 | 24(S)-PF11 | -O-glc-rha | H | S | S | [5], [13], [14], [17] |
| 13 | Yesanchinoside A | -O-glc(-Ac)-glc | H | S | S | [14] |
| 14 | Yesanchinoside B | -O-glc(-αglc)-glc | H | S | S | [14] |
| 15 | Yesanchinoside C | -O-glc-glc-xyl | H | S | S | [14] |
| 16 | 3α-ocotillol | -OH | H | S | R | [18] |
Ac, acetyl; αglc, α-d-glucopyranosyl; glc, β-d-glucopyranosyl; rha, α-l-rhamnopyranosyl; xyl, β-d-xylopyranosyl
Tanaka and Yahara [3] and Chen et al [4] isolated and further identified new dammarane saponin PF11 (1) from dried leaves of Panax pseudo-ginseng subsp. himalaicus, whose sapogenin was identified as (20S,24R)-dammarane-20,24-epoxy-3β,6α,12β,25-tetraol, ocotillol (17).
Panax japonicus saponins MR1 and MR2 were afforded from Yunnan Rhizoma panacis majoris and identified by 13C nuclear magnetic resonance (NMR) and mass spectrometry (MS). Hydroxyls at C-6 of (20S,24S)-ocotillol were connected with glc2-1glc and glc2-1xyl disaccharide chain [16].
PF11 and ocotillol-type saponins RT2, RT4, and RT5 were collected from the rhizomes of P. pseudo-ginseng subsp. himalaicus [10]. RT5 was also obtained from the stems and leaves of American ginseng by Ma et al [11]. 24(S)-PF11, RT2, MR2, and 24(R)-PF11 were separated from wild Panax notoginseng subspecies in central of Nepal [5], [17].
VR1 and VR2 were first isolated from Vietnam ginseng rhizome, and were formulated as monoacetylated 24(S)-PF11 and monoacetylated MR2. Rare ocotillol saponin vina-ginsenoside R5 and R6 with α-glucan chains were also split by Nguyen et al [13], [15]. Yesanchinosides A, B, C and 24(S)-PF11 (12), RT4, VR1 and VR2, and MR2 were isolated from the underground part of P. japonicus collected in the south of Yunnan Province, China [14].
Ocotillol was isolated from the alkaline degradation products of American ginseng total saponins by Ma et al [6]. The C-20 configuration of ginsenosides was not changed during alkaline degradation. The 1H NMR and 13C NMR chemical shifts of ocotillol were acquired using two-dimensional NMR.
Liu et al [9] extracted 20(R)-PF11 (2) from American red ginseng, and applied patent for its extracting method and pharmaceutical activity. Compared with Asian white ginseng, steamed ginseng has stronger anticancer activities. In addition, a new minor C-3 epimer of ocotillol, 3α-ocotillol (16), was isolated from P. quinquefolium L. along with ocotillol. Its structure was elucidated as (20S,24R)-dammarane-20,24-epoxy-3α,6α,12β,25-tetraol [18].
3. Semisynthesis of 20(S)-ocotillol-type saponins
24,25-Epoxy intermediates were gained by oxidation with m-chloroperoxybenzoic acid from 20(S)-PPD and 20(R)-PPD. Tetrahydrofuran ring was formed by Baldwin's rules of molecular open-loop and close-loop response by 5-exo-tet cyclization [20], [21], [22], [23].
The ocotillol-type saponins were first semisynthesized by Liu [24] with combinatorial chemistry. PGQ (18), PHQ (19), and PDQ (20; Table 2) were obtained with oxidation cyclization of the side chain on 20(S)-Rg3, 20(S)-Rh2, and 20(S)-PPD).
Table 2.
Semisynthetic 20(S)-ocotillol-type saponins
| No. | Ingredient name | R1 | R2 | C-20 | C-24 | Refs. |
|---|---|---|---|---|---|---|
| 17 | ocotillol | -OH | -H | S | R | [6], [8], [12], [25], [26], [27] |
| 18 | 20(S)-PGQ | -H | -glc-glc | S | S | [24] |
| 19 | 20(S)-PHQ | -H | -glc | S | S | [8], [24] |
| 20 | 20(S)-PDQ | -H | -H | S | S | [24], [28], [29] |
| 21 | 24(S)-Ocotillol | -OH | -H | S | S | [8], [26], [27] |
| 22 | 24(R)-PHQ | -H | -glc | S | R | [8] |
| 23 | 24(R)-PDQ | -H | -H | S | R | [25], [28], [29] |
| 24 | 24(R)-PHQ | -H | -glc-glc | S | R | [30] |
glc, β-d-glucopyranosyl
Gao et al [25] isolated four major compounds—20(S)-PPD, 17, 20(S)-PPT, and (20S,24R)-PDQ (23)—from the oxidative residue of American ginseng’s total saponins. In 2008, (12R,20S,24R)-20,24;12,24-beisopropyl-dammarane-3β-ol (25) and 23 were obtained from the oxidative alkaline degradation products of Canadian P. quinquefolium saponins.
PF11 was afforded from 20(S)-Rg2 in yield 80% and could be used to prepare medicine for the therapy of attention deficit hyperactivity disorder, fatigue, allomnesia, etc. [7].
17 and its C-24 epimer (21) were semisynthesized from 20(S)-PPT by acetylation, oxidization, and saponification. Structures of the two epimers were identified by electrospray ionization-MS, 1H NMR, 13C NMR, and single X-ray crystal diffraction [26], [27], [31]. Meanwhile, the crystal results indicated that C24 configurations were R-form and S-form, respectively.
Ren [28] had carried out the synthesis of PDQ by (20S)-PPD. 20 and 23 were achieved in a molar ratio of 3.6ː1, whereas Meng et al [11] obtained 20 and 23 in nearly 1:1 molar ratio with acetylation, oxidization, and saponification of (20S)-PPD.
Wang [12] has studied the residue of PF11 degradation under acidic condition using chromatography and recrystallization. Ocotillol, (12R,20S,24S)-20,24;12,24-diepoxy-dammarane-3β,6α-diol (26), (20R,24R)-ocotillol (27), and 4 were obtained.
Tian [8] acquired 1, 4, 5, 17, 19, (20R,24R)-PF11, 21, and 22 by alkaline degradation and oxidation from total saponins of P. quinquefolium stems and leaves.
Compared with other ginseng plants, Panax vietnamensis has been found to have a high content of MR2, which is more than 5% of the dried rhizome, and exhibited antitumor and hepatocyte-protective activities. Zhang et al [32] conducted transcriptome sequencing of this species using Illumina next-generation sequencing, which prepared a certain amount of target compounds. The large number of transcripts provided in this study not only facilitates the study of ocotillol-type saponins biosynthesis but could also provide opportunities to engineer microorganisms for the de novo production of active ingredients. Furthermore, numerous simple sequence repeats (SSRs) were identified and will be very useful for marker-assisted selection breeding of this herb [32].
4. Semisynthesis of 20(R)-ocotillol-type saponins
20(R)-PPD (28) was degraded from P. quinquefolium L. with 50% citric acid and sodium hydrate in glycerol, respectively. Two C24 epimeric 20(R)-ocotillol type saponins, 20R,24S-epoxy-dammarane-3β,12β,25-triol (M1, 29) and 20R,24R-epoxy-dammarane-3β,12β,25-triol (M2, 30; Table 3), were synthesized from 20(R)-PPD. Suitable crystals of 29 and 30 were obtained by open-air evaporation of an acetone solution. The configurations of 29 and 30 were established as (20R,24S) and (20R,24R), respectively, using X-ray single crystal diffraction [33].
Table 3.
Semisynthetic 20(R)-ocotillol-type saponins
Two C24 epimeric 3-acetyled ocotillol-type sponins, (20R,24S)-epoxy-dammarane-3β,12β,25-triol acetate (M3, 31) and (20R,24R)-epoxy-dammarane-3β,12β,25-triol acetate (M4, 32; Table 3), were obtained from 20(R)-PPD, which made it possible to isolate 29 and 30. The results indicated that the configurations of 31 and 32 were (20R,24S) and (20R,24R), respectively [34].
5. Biological activities
5.1. Effect on nervous system
Li et al [35] found that PF11 could antagonize the memory dysfunction induced by scopolamine in tests. The additional study demonstrated that morphine-induced allomnesia was markedly inhibited by PF11, and the conditioned place preference to morphine was significantly blocked [36]. Moreover, morphine-stimulated opioid receptor signaling could be antagonized directly at the cellular level by orally administered PF11 [37]; behavior sensitization was antagonized and glutamate decrease in the medial prefrontal cortex induced by morphine was blocked [38]. Meanwhile, PF11 showed no similarity to opioids, sedative-hypnosis, and stimulants because of its physical and psychological independence [39].
Wu et al [40] and Fu et al [41] reported that PF11 showed effective protection on methamphetamine-induced neurotoxicity through many experiments.
PF11 was proposed to play an important role in anti-Parkinson affection, in that it inhibited free radical formation and stimulated endogenous antioxidant release [42].
The results demonstrated that PF11 could antagonize Alzheimer disease, as measured in the Morris water maze and step-through tests, and it might serve as a promising agent for Alzheimer disease [43].
PF11 exerted antineuroinflammatory effects on lipopolysaccharide (LPS)-activated microglial cells by inhibiting TLR4-mediated transforming growth factor β activated kinase 1 (TAK1)/IkB kinases (IKK)/nuclear factor kB (NF-κB), mitogen-activated protein kinases (MPKs), and Akt signaling pathways, indicating its therapeutic implication for neurodegenerative diseases associated with neuroinflammation [44].
Peroxisome proliferator-activated receptor gamma (PPARγ) was activated with modest adipogenic activity by PF11, adiponectin oligomerization and secretion in 3T3-L1 adipocytes were promoted, and obesity-linked phosphorylation of PPARγ at Ser-273 by Cdk5 were inhibited [45].
Wang et al [46] discovered that the increased release of glutamate could mediate the ocotillol-evoked neuronal excitability, which resulted in the increased release of spontaneous locomotor activities in vivo.
MR2 exerted reversing effect on the social isolation stress-induced decrease in pentobarbital sleep, which was mediated by the neurosteroid site on the gamma aminobutyric acid (GABAA) receptor complex in mice [47].
Accumulating evidence strongly suggested that communication box paradigm-induced psychological stress- and conditioned fear stress-induced antinociception was attenuated by MR2. Numerous lines of evidence markedly indicated the involvement of central opioid, GABAA receptor, and corticotropin-releasing factor mechanisms in the effect of MR2 [48].
5.2. Protective effects on cardiovascular system
PGQ showed ameliorative effects on isoproterenol-induced and doxorubicin (DOX)-induced acute myocardial ischemia and hemorrhagic shock in rats, and transparently increased mean arterial pressure and blood oxygen content and decreased serum lipoperoxidase. In addition, it could enhance the superoxide dismutase (SOD) activities [49], [50], [51].
Previous experiments in our laboratory results indicated that ocotillol had a protective effect on myocardial ischemic injury; it significantly reduced the area of myocardial ischemia, necrosis, and level of lactate dehydrogenase (LDH) in serum, and enhanced the antifree-radical actions of heart tissues [52], [53]. Further study indicated that 17 and 23 showed better effect on myocardial injury induced by isoproterenol than 21 and 20, respectively [54], [55]. In addition, Bi et al [30], [56] found that 23 exhibited a potent protective effect on cultured myocardiocytes with anoxia/reoxygen injury, which was superior to 20(S)-panaxadiol, whereas 20 had none of this activity.
Zhao et al [57] found that ocotillol may be related to antioxidation action. It could reduce the cerebral infarction area, improve ischemic injuries induced by brain tissue pathological changes, decrease the content of malondialdehyde (MDA) in ischemic brain tissue, and increase the activity of SOD.
Cotreatment of ocotillol with DOX significantly alleviated related toxic injury, especially cardiotoxicity, whereas ocotillol alone exhibited no protective activity [58].
PF11 could significantly weaken the heart rate slowed down by posterior pituitrin-induced acute myocardial ischemia in rats, reduce elevation of the electrocardiogram T wave of myocardial ischemia, and improve myocardial ischemia in rats [59].
5.3. Antiinflammatory effects
Lee et al [60] and Jeong et al [61] measured the antiinflammatory effects of VR2, MR2, and their metabolites in LPS-stimulated mouse peritoneal macrophages, and found that only VR2 exhibited cytotoxicity against peritoneal macrophages. MR2, PRT4, and ocotillol could inhibit LPS-stimulated transcription factor (NF)-κB activation and expression of the proinflammatory cytokines tumor necrosis factor-α and interleukin-1, but they did not inhibit peptidoglycan-induced NF-κB activation in the macrophages. Among the tested ginsenosides, ocotillol exhibited a strong inhibitory effect on inflammation. VR2 and MR2 were orally administered and may be metabolized to ocotillol via PRT4. Metabolites, particularly ocotillol, showed antiinflammatory effects by prohibiting the binding of LPS to TLR4 on macrophages.
5.4. Antibacterial effects
Bi et al [62], [63], [64], [65] and Zhou et al [66] found that 20 and 23 exhibited excellent antibacterial activity in vitro against Staphylococcus aureus and Bacillus subtilis. When combined with two commercial antibiotics, kanamycin and chloramphenicol, they showed strong synergistic activity against S. aureus USA300 and B. subtilis 168 at sub–minimum inhibitory concentration levels. In addition, nitric oxide (NO) and its auto-oxidation products were known to disrupt normal bacterial function and NO releasing molecules, and they may be developed as potential antibacterial leads in drug discovery. Analysis of the in vitro data showed that the derivatives bearing the same furoxan group possessed various NO releasing capacity. Stereostructure may affect the NO release of these ocotillol-type furoxans. Compounds with nitrated aliphatic esters at C-3 displayed higher NO release than other analogues, and might be related to good antibacterial activity against both Gram-positive and Gram-negative bacteria.
5.5. Antitumor effects
The experiments suggested that MR2 showed effective influence on antitumor-promoting activity on mouse hepatic tumor and mouse skin [67], [68]. MR2 also exhibited significant inhibitory effect on Epstein–Barr virus early antigen induced by the tumor promoter phorbol acetate [69].
Ocotillol could enhance DOX-induced cell death in p53 wild-type cancer cells. Coadministration of ocotillol with DOX could induce much more cell apoptosis and activate p53 to some degree, and enhanced cytotoxic activity was partially blocked [70].
Van Le et al [71], [72] found that ocotillol-type saponins with no glycosyl moiety at C-20 were relatively stable in steaming, and the radical scavenging activity was increased continuously up to 20 h of steaming; the antiproliferative activity against A549 lung cancer cells was also improved.
Ma and Yang [73] stated that 17, 21, and 27 showed moderate cytotoxic activity against HL-60, NCI-N87, and Hep-G2 at a concentration of 1–200 μM, and indicated that cytotoxicity was related to the substitution of the hydroxyl group of C-25 and C-3, configuration of C-20.
Administration PF11 with cisplatin did not affect its anticancer effect. It could reduce cisplatin-induced renal injury in rats and prevent the DNA breakage of renal proximal tubule cell, and decrease the effects of cisplatin on mitochondrial morphology, function, and the intracellular expression of caspase family, ultimately preventing apoptosis of renal cells [74].
(20S,24R/S)-epoxy-12β,25-dihydroxy-dammarane-3β-amine (ORA and OSA) could inhibit the ABCB1 transporter. The study suggested that ORA had a stronger stimulatory effect on ATPase activity than OSA. ORA also exhibited a higher docking score as compared with OSA inside the transmembrane domain of ABCB1 [75].
Cotreatment of RT5 with CDDP might attenuate the following nephrotoxicity without inhibiting its antitumor efficiency, thereby reducing the renal tubular damage and decreasing the apoptosis by enhancing the antioxidant levels, which could provide one novel strategy for cancer treatment in clinics [76].
6. Metabolism
Wang and Li [77] found that PF11 was relatively stable in artificial gastric juice, feces, bile, and urine, and played a variety of physiological activity in prototype by intraperitoneal injection.
PGQ administered via the sublingual vein was mainly excreted in bile, accounting for 41.60% of the total dose; fecal excretion accounts for 9.97%, and only a small amount of PGQ was detected in urine. PGQ was also given as a prototype drug in the bile, urine, and feces [78].
20, 23, (20S,24S)-epoxydammarane-12,25-diol-3-one and (20S,24R)-epoxydamma rane-12,25-diol-3-one were metabolized from 20(S)-PPD in mixed human liver microsomes (HLMs) and human hepatocytes. The predominant metabolic pathway of 20(S)-PPD observed was the oxidation of 24,25-double bond to yield 24,25-epoxide, followed by hydrolysis and rearrangement to form the corresponding 24,25-vicinaldiol derivatives and the 20,24-oxide forms. Further sequential metabolites are also detected through hydroxylation and dehydrogenation. Two glucuronide conjugates, 20 and 24, were detected in human hepatocyte incubations, and their conjugation sites were tentatively assigned to the 25-hydroxyl group. The formation of 25-hydroxyl group is very important for the elimination of 20(S)-PPD [79].
After 20 or 23 was orally administered, within 48 h, fecal excretion cumulant was 17.09% and 17.69% of the dosage, respectively, whereas it was scarcely excreted through urine. Meanwhile, the bile excretion cumulant of 20 and 23 was 1.47% and 8.01% of the dosage, respectively; the former was 5.4-fold of the latter, demonstrating obvious stereoselectivity [80].
The in vitro and in vivo selectivity effects of ocotillol-type side chain and C-24 stereoconfiguration on P-glycoprotein (P-gp) were analyzed; the absolute bioavailability of 23 was about 14-fold higher than that of 20. 24(S)-Ocotillol type epimer processed poor transmembrane permeability and can be distinguished by P-gp [81].
Wang et al [82] observed the in vitro and in vivo formation and metabolism of 20 and 23. Stereoselective metabolism isoforms of CYP450 enzymes contributed to the HLMs were elucidated. 20 was the more predominant ingredient in rat plasma after the oral administration of 24(S)-PPD. The analysis data indicated that 20 had higher formation rate and lower oxygenation metabolism rate than 23, and the stereoselective differences were more obvious in HLMs than in rat liver microsomes (RLMs). The chemical inhibitor assay showed that CYP3A was the predominant isoform responsible for the further metabolism of 23 in HLMs. The biliary excretion ratio of 20 glucuronide was more than 28-fold higher than that of 23 glucuronide after intravenous administration to rats, which also indicated that 20 was preferentially metabolized to produce the glucuronide conjugates than 23.
Wang et al [83] found that the oxidative metabolites of 20(S)-PPT were identified in HLMs and rats. Enzyme kinetics experiments showed that the apparent formation Vmax of 17 was 10.4-fold and 2.4-fold higher than that of 21 in HLMs and RLMs, respectively. The depletion rate of 17 was 11-fold faster than that of 21 in HLMs, and was similar in RLMs. Hence, the remarkable species differences of 20(S)-PPT metabolism mainly resulted from the stereoselective formation and further metabolic elimination of 17 and 21.
7. Conclusion and recommendation
In conclusion, the available data suggest that antiinflammatory activity, inhibiting the increase of MDA, reduction of myocardial ischemia and necrosis area, level of LDH, SOD, glutathione peroxidase, and total antioxidant capacity of ocotillol, play vital role in myocardial ischemia. Among the reported ocotillol-type saponins, ocotillol exhibits excellent and stereoselective activity in configuration against myocardial ischemia reperfusion injury and possesses further research value. However, owing to the lack of clinical studies on ocotillol, it is difficult to make a clear decision. We recommend that further study on ocotillol should concentrate on the following areas: (1) molecular mechanisms underlying the beneficial role of ocotillol on myocardial ischemia; (2) pharmacokinetic research on utilizing different delivery systems to increase its bioavailability; (3) pharmacodynamic study on clinical studies about myocardial ischemia. In addition, it is also necessary to study the scale-up preparation method of ocotillol.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 81473104).
References
- 1.Yao H., Li X., Liu Y., Wu Q., Jin Y. An optimized microwave-assisted extraction method for increasing yields of rare ginsenosides from Panax quinquefolius L. J Ginseng Res. 2016;40:415–422. doi: 10.1016/j.jgr.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y.X., Liu S.F., Wang X.N. Psedoginsenoside F11—an outstanding symbol to distinguish ginseng (Panax ginseng) from American ginseng (P. quinquefolius) Chin Tradit Herbal Drugs. 1995;26:540–541. [Google Scholar]
- 3.Tanaka O., Yahara S. Dammarane saponins of leaves of Panax psuedo-ginseng subsp. himalaicus. Phytochemistry. 1978;17:1353–1358. [Google Scholar]
- 4.Chen S.E., Staba E.J., Taniyasu S., Kasai S.R., Tanaka O. Further study on dammarane-saponins of leaves and stems of American ginseng, Panax quinquefolium. Planta Med. 1981;42:406–411. doi: 10.1055/s-2007-971664. [DOI] [PubMed] [Google Scholar]
- 5.Toshinobu M., Kong Y.C., Paul P.B., Ng K.H., Yip T.T., Ryoji K., Osamu T. Saponins of plants of Panax species collected in central Nepal and their chemotaxonomical significance: II. Chem Pharm Bull. 1986;34:4368–4372. doi: 10.1248/cpb.48.889. [DOI] [PubMed] [Google Scholar]
- 6.Ma S.G., Jiang Y.T., Song S.J., Wang Z.H., Bai J., Xu S.X., Liu K. Alkaline-degradation products of ginsenosides from leaves and stems of Panax quinquefolium. Acta Pharm Sin. 2005;40:924–930. [PubMed] [Google Scholar]
- 7.Li PY. Semi synthetic approach intends to ginsenoside PF11. CN1015194192008, 2.
- 8.Tian X. Jilin University; Jilin: 2012. Studies on ocotillol-type ginsenoside and its related compounds. MS thesis. [in Chinese] [Google Scholar]
- 9.Liu J.P., Wang F., Li P.Y., Lu D. A new ocotillol-type triterpenoid saponin from red American ginseng. Nat Prod Res. 2012;26:731–735. doi: 10.1080/14786419.2010.551644. [DOI] [PubMed] [Google Scholar]
- 10.Osamu T., Toshinobu M., Ryoji K., Junko K., Shuichi S., Yoshiteru I., Junzo S. Study on saponins of rhizomes of panax psedo-ginseng subsp. Himalaicus collected at Tzatogang and Parila, Bhutan-Himalaya. Chem Pharm Bull. 1985;33:2323–2330. [Google Scholar]
- 11.Ma X.Y., Shao C.J., Xu J.D. The chemical study on Panax quinquefolium saponins—isolation and structural identification of Pseudoginsenoside-RT5. Gin Res. 1991;4:9–15. [in Chinese] [Google Scholar]
- 12.Wang C.C. Jilin University; Jilin: 2011. Studies on the structural modification of psedo-ginsenoside-F11. MS thesis. [in Chinese] [Google Scholar]
- 13.Nguyen M.D., Nguyen T.N., Ryoji K., Aiko I., Kazuo Y., Osamu T. Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. Collected in central Vietnam: I. Chem Pharm Bull. 1993;41:2010–2014. doi: 10.1248/cpb.41.2010. [DOI] [PubMed] [Google Scholar]
- 14.Zou K., Zhu S., Chihiro T., Cai S.Q., Katsuko K. Dammarane-type riterpene saponins from panax japonicus. J Nat Prod. 2002;65:346–351. doi: 10.1021/np010354j. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen M.D., Ryoji K., Kazuhiro O., Aiko I., Nguyen T.N., Kazuo Y., Osamu T. Saponins from vietnamese ginseng, panax vietnamensis Ha et Grushv. Collected in central Vietnam: II. Chem Pharm Bull. 1994;42:115–122. doi: 10.1248/cpb.42.115. [DOI] [PubMed] [Google Scholar]
- 16.Toshinobu M., Ryoji K., Osamu T., Zhou J., Yang T.R., Junzo S. Saponins of zu-tziseng, rhizomes of panax japonicus C.A. Meyer var. major (Burk.) C.Y. Wu et K.M. Feng, collected in Yunnan, China. Chem Pharm Bull. 1982;30:4341–4346. [Google Scholar]
- 17.Tsuneo N., Katsumichi M., Toshinobu M., Osamu T. Saponins of plants of panax species collected in central Nepal and their chemotaxonomical significance: I. Chem Pharm Bull. 1986;34:730–738. doi: 10.1248/cpb.48.889. [DOI] [PubMed] [Google Scholar]
- 18.Han L., Lin M.Y., Zheng Q., Liu H.Y., Liu H.Y., Dong G., Liu J.P., Li P.Y. A new epimer of ocotillol from stems and leaves of American ginseng. Nat Prod Res. 2014;28:935–939. doi: 10.1080/14786419.2014.896008. [DOI] [PubMed] [Google Scholar]
- 19.Li X.G., Zhang L.X., Meng X.Y., Hou J.R., Zhang J. Isolation, identification and content determination of pseudoginsenoside F11 in American ginseng. J Jilin Agric Univ. 2006;27:645–648. [Google Scholar]
- 20.Ivan V., Timothy F.J. Epoxide-opening cascades in the synthesis of polycyclic polyether natural products. Angew Chem Int Ed Engl. 2009;48:5250–5281. doi: 10.1002/anie.200900600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulrich K., Holger W., Matthias S. An enantiomerically pure epoxy organolithium reagent for the synthesis of Oligo (tetrahydrofurans) by an epoxide-cascade reaction. Tetrahedron Lett. 1994;35:7629–7632. [Google Scholar]
- 22.Kassoum N., Michel B., Chantal Z., Liliane G., Joel J. Lactonisation and lactone ether formation of nerol geraniol compounds. Use of 13C to identify the cyclisation process. Tetrahedron. 1999;55:5129–5138. [Google Scholar]
- 23.Ivan V., Timothy F.J. Synthesis of marine polycyclic polyethers via endo-selective epoxide-opening cascades. Mar Drugs. 2010;8:763–809. doi: 10.3390/md8030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J.P. Shenyang Pharm Univ; Shenyang: 2005. Studies on isolation, structure modification and pharmacological activities of saponins from the leaves and stems of Panax quinquefolium L. cultivated in China. PhD thesis. [in Chinese] [Google Scholar]
- 25.Gao L.S., Li N., Li X. Alkaline-degradation products of total ginsenosides from leaves and stems of Panax quinquefolium L. J Shenyang Pharm Univ. 2007;24:552–555. [Google Scholar]
- 26.Meng Q.G., Bi Y., Wang L., Jiang N.C., Jiang Y.T., Zhang J.F., Yi S.T., Sun H.J. Synthesis, structural determination of a new ocotillol derivative and its epimer. Lett Org Chem. 2011;8:682–685. [Google Scholar]
- 27.Zhang L., Guo H.M., Li W.J., Gao Y.J., Meng Q.G. (3R,6R,12R,20S,24R)-20, 24-epoxy-dammarane-3,6,12,25-tetraol. Acta Crystallogr Sect E Struct Rep Online. 2011;67:o846. doi: 10.1107/S1600536811008609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren Y.Y. Jilin University; Jilin: 2012. Studies on the preparation process and the related substances of pseudo-sapogenin DQ. MS thesis. [in Chinese] [Google Scholar]
- 29.Meng Q.G., Tan W.J., Hou G.G., Zhang X.Y., Hu X.Y., Yang F., Bai G.J., Zhu W.W., Cai Y., Bi Y. Synthesis and structural characterization of two epimers driven from 20(S)-protopanaxadiol. J Mol Struct. 2013;2013:1054–1055. [Google Scholar]
- 30.Bi Y., Tian J.W., Wang L., Zhao F.L., Zhang J.F., Wang N., Sun H.J., Meng Q.G. Synthesis, structural determination and protective effects on cultured anoxia/reoxygen injury myocardiocytes of ocotillol-type derivatives. J Med Plants Res. 2011;5:2424–2429. [Google Scholar]
- 31.Meng Q.G., Liu L.D., Guo H.M., Bi Y., Wang L. (3R,6R,12R,20S,24S)-20,24-epoxy- dammarane-3,6,12, 25-tetral dihydrate. Acta Crystallogr Sect E Struct Rep Online. 2010;66:o3210. doi: 10.1107/S1600536810046362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang G.H., Ma C., Zhang J.L., Chen J.W., Tang Q.Y., He M.H., Xu X.Z., Jiang N.H., Yang S.C. Transcriptome analysis of Panax vietnamensis var. Fuscidicus discovers putative ocotillol-type ginsenosides biosynthesis genes and genetic markers. BMC Genomics. 2015;16:159–178. doi: 10.1186/s12864-015-1332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y.R., Yang J.J., Liu J., Hou G.G., Meng Q.G. Synthesis and crystal structure of ocotillol-type metabolites driven from 20(R)-protopanaxadiol. Acta Crystallogr. 2016;C72:498–503. [Google Scholar]
- 34.Yang J.J., Xu Y.R., Li X.L., Zhang K.X., Zhang R.M., Wang W.Z., He X.Y., Meng Q.G., Hou G.G. Synthesis and crystal structures of two C24 epimeric 3-acetyled 20(R)-ocotillol type sapogenins obtained from 20(R)-protopanaxadiol. J Chem Res. 2016;40:235–238. [Google Scholar]
- 35.Li Z., Guo Y.Y., Wu C.F., Li X., Wang J.H. Protective effects of pseudoginsenoside-F11 on scopolamine-induced memory impairment in mice and rats. J Pharm Pharmacol. 1999;51:435–440. doi: 10.1211/0022357991772484. [DOI] [PubMed] [Google Scholar]
- 36.Li Z., Wu C.F., Pei G., Guo Y.Y., Li X. Antagonistic effect of pseudoginsenoside-F11 on the Behavioral actions of morphine in mice. Pharmacol Biochem Behav. 2000;66:595–601. doi: 10.1016/s0091-3057(00)00260-4. [DOI] [PubMed] [Google Scholar]
- 37.Li Z., Xu N.J., Wu C.F., Ying X., Fan H.P., Zhang W.B., Sun Y., Pei G. Pseudoginsenoside-F11 attenuates morphine-induced signalling in Chinese hamster ovary-μ cells. Neuroreport. 2001;12:1453–1456. doi: 10.1097/00001756-200105250-00031. [DOI] [PubMed] [Google Scholar]
- 38.Hao Y., Yang J.Y., Wu C.F., Wu M.F. Pseudoginsenoside-F11 decreases morphine-induced behavioral sensitization and extracellular glutamate levels in the medial. Pharmacol Biochem Behav. 2007;86:660–666. doi: 10.1016/j.pbb.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Zhu D. Shenyang Pharm University; Shenyang: 2004. Studies of PF11 on the dependence potential. MS thesis. [in Chinese] [Google Scholar]
- 40.Wu C.F., Liu Y.L., Song M., Liu W., Wang J.H., Li X., Yang J.Y. Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice. Pharmacol Biochem Behav. 2003;76:103–109. doi: 10.1016/s0091-3057(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 41.Fu K.Q., Lin H.Y., Yoshiaki M., Wu C.F., Yang J.Y., Kyosuke U., Atsumi N. Pseudoginsenoside-F11 inhibits methamphetamine-induced behaviors by regulating dopaminergic and GABAergic neurons in the nucleus accumbens. Psychopharmacology. 2016;233:831–840. doi: 10.1007/s00213-015-4159-8. [DOI] [PubMed] [Google Scholar]
- 42.Wang J.Y., Yang J.Y., Wang F., Fu S.Y., Hou Y., Jiang B., Ma J., Song C., Wu C.F. Neuroprotective effect of Pseudoginsenoside-F11 on a rat model of Parkinson’s disease induced by 6-Hydroxydopamine. Evid Based Complement Alternat Med. 2013;2013:1–9. doi: 10.1155/2013/152798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C.M., Liu M.Y., Wang F., Wei M.J., Wang S., Wu C.F., Yang J.Y. Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer's disease. Pharmacol Biochem Behav. 2013;106:57–67. doi: 10.1016/j.pbb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Wang X.X., Wang C.M., Wang J.M., Zhao S.Q., Zhang K., Wang J.M., Zhang W., Wu C.F., Yang J.Y. Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4-mediated TAK1/IKK/NF-κB, MAPKs and Akt signaling pathways. Neuropharmacology. 2014;79:642–656. doi: 10.1016/j.neuropharm.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 45.Wu G.Y., Yi J.Y., Wang P.C., Zhang Z.J., Li Z. Pseudoginsenoside F11, a novel partial PPAR agonist, promotes adiponectin oligomerization and secretion in 3T3-L1 Adipocytes. PPAR Res. 2013;2013:1–8. doi: 10.1155/2013/701017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z.J., Sun L., Peng W., Ma S., Zhu C., Fu F.H., Heinbockel T. Ginseg derivatives ocotillol enhances neuronal activity through increased glutamate release: a possible mechanism underlying increased spontaneous locomotor activity of mice. Neuroscience. 2011;195:1–8. doi: 10.1016/j.neuroscience.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen T.T., Matsumoto K., Yamasaki K., Watanabe H. Majonoside-R2 reverses social isolation stress-induced decrease in pentobarbital sleep in mice: possible involvement of neuroactive steroids. Life Sci. 1997;61:395–402. doi: 10.1016/s0024-3205(97)00396-2. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen T.T., Matsumoto K., Watanable H. The antistress effect of majonoside-R2, a major saponin component of Vietnamese ginseng: neuronal mechanisms of action. Method Find Exp Clin. 1998;20:65. doi: 10.1358/mf.1998.20.1.485634. [DOI] [PubMed] [Google Scholar]
- 49.Liu J.P. Ameliorative effects of pseudoginsenoside GQ on isoproterenol-induced acute myocardial ischemia in rats. J Jilin Univ (Med. Ed.) 2006;32:64–67. [in Chinese] [Google Scholar]
- 50.Liu J.P., Lu D., Zhao Y., Li P.Y., Li X. A new semisynthetic ocotillol-type saponin and resuscitation of haemorrhagic shock. J Asian Nat Prod Res. 2007;9:103–113. doi: 10.1080/10286020500251741. [DOI] [PubMed] [Google Scholar]
- 51.Jin X., Shen W.Z., Jin L.F., Jia J.Y., Li X.F., Wang X.L., Di X., Zhang H.J., Li P.Y. Protective effect of pseudo-ginsenoside GQ on doxorubicin-induced acute myocardial injury in rats. J Jilin Univ (Med. Ed.) 2013;39:1164–1168. [in Chinese] [Google Scholar]
- 52.Yu C., Fu F.H., Jiang Y.T., Yu X., Zhu M., Han B. Protective effect of ocotillol on acute myocardial injury. Chin Tradit Herbal Drugs. 2007;38:576–578. [in Chinese] [Google Scholar]
- 53.Yu C., Fu F.H., Yu X., Zhu M. Protective effect of ocotillol on acute myocardial injury induced by LAD in rat. J Mol Cell Cardiol. 2007;42:S215. [Google Scholar]
- 54.Han B., Meng Q.G., Li Q., Zhang J.F., Bi Y., Jiang N.C. Effect of 20(S)-protopanaxatriol and its epimeric derivatives on myocardial injury induced by isoproterenol. Arzneimittel-Forsch. 2011;61:148–152. doi: 10.1055/s-0031-1296181. [DOI] [PubMed] [Google Scholar]
- 55.Wang T., Meng Q., Zhang J.F., Bi Y., Jiang N.C. Study on the structure–function relationship of 20(S)-panaxadiol and its epimeric derivatives in myocardial injury induced by isoproterenol. Fitoterapia. 2010;81:783–787. doi: 10.1016/j.fitote.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Bi Y., Wang T., Meng Q.G., Zhang J.F., Wang L., Li Q., Zhao F.L., Sun H.J. Synthesis and myocardial ischemia protective effect of ocotillol-type derivatives. Rec Nat Prod. 2012;6:242–254. [Google Scholar]
- 57.Zhao B., Fu F.H., Wei X.B., Chen L., Zhang X.M. Protective effects of ocotillol on focal cerebral ischemic injury in rats. Chin Pharmacol Bull. 2008;24:87–90. [in Chinese] [Google Scholar]
- 58.Fu X.Y., Kong L., Tang M.T., Zhang J.Q., Zhou X.Y., Li G., Wang H.B., Fu F.H. Protective effect of ocotillol against doxorubicin-induced acute and chronic cardiac injury. Mol Med Rep. 2014;9:360–364. doi: 10.3892/mmr.2013.1791. [DOI] [PubMed] [Google Scholar]
- 59.Dai L. Jilin University; Jilin: 2010. Studies on isolation, modification and bioactivities of protopanaxatriol saponins in leaves and stems of panax quinquefolium L. MS thesis. [in Chinese] [Google Scholar]
- 60.Lee S.Y., Jeong J.J., Le T.H.V., Eun S.H., Nguyen M.D., Park J.H., Kim D.H. Ocotillol, a Majonoside R2 metabolite, ameliorates 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by restoring the balance of Th17/Treg cells. J Agric Food Chem. 2015;63:7024–7031. doi: 10.1021/acs.jafc.5b02183. [DOI] [PubMed] [Google Scholar]
- 61.Jeong J.J., Le T.H.V., Lee S.Y., Eun S.H., Nguyen M.D., Park J.H., Kim D.H. Anti-inflammatory effects of vina-ginsenoside R2 and majonoside R2 isolated from Panax vietnamensis and their metabolites in lipopolysaccharide-stimulated macrophages. Int Immunopharmacol. 2015;28:700–706. doi: 10.1016/j.intimp.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 62.Bi Y., Ma C., Zhang H.Y., Zhou Z.W., Yang J., Zhang Z.L., Meng Q.G., Lewis P.J., Xu J.Y. Novel 3-substituted ocotillol-type triterpenoid derivatives as antibacterial candidates. Chem Biol Drug Des. 2014;84:489–496. doi: 10.1111/cbdd.12337. [DOI] [PubMed] [Google Scholar]
- 63.Bi Y., Ma C., Zhou Z.W., Zhang H.Y., Zhang X.C., Lu J., Meng Q.G., Lewis P.J., Xu J.Y. Synthesis and antibacterial evaluation of novel hydrophilic ocotillol-type triterpenoid derivatives from 20(S)-protopanaxadiol. Rec Nat Prod. 2015;9:356–368. [Google Scholar]
- 64.Bi Y., Ma C., Zhang T.T., Zhang X.C., Lu J., Meng Q.G. Design, synthesis and in vitro NO-releasing activities of ocotillol-type furoxans. Pharmazie. 2015;70:213–218. [PubMed] [Google Scholar]
- 65.Bi Y., Yang X., Zhang T.T., Liu Z.Y., Zhang X.C., Lu J., Cheng K.G., Xu J.Y., Wang H.B., Lewis P.J. Design, synthesis, nitric oxide release and antibacterial evaluation of novel nitrated ocotillol-type derivatives. Eur J Med Chem. 2015;101:71–80. doi: 10.1016/j.ejmech.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Z.W., Ma C., Zhang H.Y., Bi Y., Chen X., Tian H., Xie X.X., Meng Q.G., Lewis P.J., Xu J.Y. Synthesis and biological evaluation of novel ocotillol-type triterpenoid derivatives as antibacterial agents. Eur J Med Chem. 2013;68:444–453. doi: 10.1016/j.ejmech.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 67.Takao K., Midori T., Eiichiro I., Teruo M., Harukuni T., Hoyoku N., Nguyen M.D., Ryoji K., Kazuo Y. Cancer chemopreventive activity of majonoside-R2 from Vietnamese ginseng, Panax vietnamensis. Cancer Lett. 1999;147:11–16. doi: 10.1016/s0304-3835(99)00257-8. [DOI] [PubMed] [Google Scholar]
- 68.Tran Q.L. Triterpene saponins from Vietnamese ginseng (Panax vietnamensis) and their hepatocytoprotective activity. J Nat Prod. 2001;64:456–461. doi: 10.1021/np000393f. [DOI] [PubMed] [Google Scholar]
- 69.Kazuo Y. Bioactive saponins in Vietnamese ginseng, Panax vietnamensis. Pharm Biol. 2000;38:16–24. doi: 10.1076/phbi.38.6.16.5956. [DOI] [PubMed] [Google Scholar]
- 70.Wang H.B., Yu P.F., Bai J., Zhang J.Q., Kong L., Zhang F.X., Du G.Y., Pei S.Q., Zhang L.X., Jiang Y.T. Ocotillol enhanced the antitumor activity of doxorubicin via p53-dependent apoptosis. Evid Based Compl Alt. 2013;2013:1–8. doi: 10.1155/2013/468537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Le T.H., Lee S.Y., Kim T.R., Kim T., Kim J.Y., Kwon S.W., Nguyen N.K., Park J.H., Nguyen M.D. Processed Vietnamese ginseng: preliminary results in chemistry and biological activity. J Ginseng Res. 2014;38:154–159. doi: 10.1016/j.jgr.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Le T.H., Lee S.Y., Lee G.J., Nguyen N.K., Park J.H., Nguyen M.D. Effects of steaming on saponin compositions and anti-proliferative activity of Vietnamese ginseng. J Ginseng Res. 2015;39:274–278. doi: 10.1016/j.jgr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma L.Y., Yang X.W. Six new dammarane-type triterpenes from acidic hydrolysate of the stems–leaves of Panax ginseng and their inhibitory-activities against three human cancer cell lines. Phytochem Lett. 2015;13:406–412. [Google Scholar]
- 74.Kong L. Shandong Tradit Chin Med; Shandong: 2014. Protective effect of Pseudoginsenoside F11 on cisplatin induced nephrotoxicity and its molecular mechanism. MS thesis. [in Chinese] [Google Scholar]
- 75.Zhang Y.K. Semi-synthetic ocotillol analogues as selective ABCB1-mediated drug resistance reversal agents. Oncotarget. 2015;6:24277–24290. doi: 10.18632/oncotarget.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang Y.T., Qiu X.L., Ma J.B., Lv G.Y., Wang Z.L., Zhang J.W., Fu F.H., Wang H.B. Ameliorative effect of ginsenoside RT5 on CDDP-induced nephrotoxicity. J Wuhan Univ (Nat Sci Ed) 2015;20:343–349. [Google Scholar]
- 77.Wang J.H., Li X. Study on the metabolism of pseudo-ginsenoside F11 in rats. Acta Pharmacol Sin. 2001;36:427–431. [in Chinese] [PubMed] [Google Scholar]
- 78.Zhao C.F., Liu J.P., Zhao Y., Li P.Y. Study on excretion of pseudo-ginsenoside GQ. China J Chin Mater Med. 2008;33:432–435. [in Chinese] [PubMed] [Google Scholar]
- 79.Li L., Chen X.Y., Li D., Zhong D.F. Identification of 20(S)-Protopanaxadiol metabolites in human liver microsomes and human hepatocytes. Drug Metab Dispos. 2011;39:472–483. doi: 10.1124/dmd.110.036723. [DOI] [PubMed] [Google Scholar]
- 80.Wu X.M., Wang L., Ni Y.Y., Wang H., Wang W.Y., Meng Q.G. Study on excretion of 20(S)-protopanaxadiol ocotillol type epimers in rats. China J Chin Mater Med. 2014;39:1306–1310. [in Chinese] [PubMed] [Google Scholar]
- 81.Wang W.Y., Wu X.M., Wang L., Meng Q.G., Liu W.H. Stereoselective property of 20(S)-protopanaxadiol ocotillol type epimers affects its absorption and also the inhibition of P-glycoprotein. PLos One. 2014;9:1–10. doi: 10.1371/journal.pone.0098887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang W.Y., Wang L., Wu X.M., Xu L.X., Meng Q.G., Liu W.H. Stereoselective formation and metabolism of 20(S)-protopanaxadiol ocotillol type epimers in vivo and in vitro. Chirality. 2015;27:170–209. doi: 10.1002/chir.22407. [DOI] [PubMed] [Google Scholar]
- 83.Wang W.Y., Ni Y.Y., Che X., Liu W.H., Meng Q.G. Stereoselective oxidation metabolism of 20(S)-protopanaxatriol in human liver microsomes and in rats. Xenobiotica. 2014;45:385–395. doi: 10.3109/00498254.2014.986562. [DOI] [PubMed] [Google Scholar]


