Abstract
Background
Endophytic fungi play an important role in balancing the ecosystem and boosting host growth. In the present study, we investigated the endophytic fungal diversity of healthy Panax notoginseng and evaluated its potential antimicrobial activity against five major phytopathogens causing root-rot of P. notoginseng.
Methods
A culture-dependent technique, combining morphological and molecular methods, was used to analyze endophytic fungal diversity. A double-layer agar technique was used to challenge the phytopathogens of P. notoginseng.
Results
A total of 89 fungi were obtained from the roots, stems, leaves, and seeds of P. notoginseng, and 41 isolates representing different morphotypes were selected for taxonomic characterization. The fungal isolates belonged to Ascomycota (96.6%) and Zygomycota (3.4%). All isolates were classified to 23 genera and an unknown taxon belonging to Sordariomycetes. The number of isolates obtained from different tissues ranged from 12 to 42 for leaves and roots, respectively. The selected endophytic fungal isolates were challenged by the root-rot pathogens Alternaria panax, Fusarium oxysporum, Fusarium solani, Phoma herbarum, and Mycocentrospora acerina. Twenty-six of the 41 isolates (63.4%) exhibited activity against at least one of the pathogens tested.
Conclusion
Our results suggested that P. notoginseng harbors diversified endophytic fungi that would provide a basis for the identification of new bioactive compounds, and for effective biocontrol of notoginseng root rot.
Keywords: biological control, endophytic fungi, fungal diversity, Panax notoginseng, root-rot disease
1. Introduction
Panax notoginseng (Burkill) F.H. Chen (Araliaceae) is a perennial herbaceous plant, cultivated mainly in Wenshan, Yunnan, China, and has been historically used as both a medicinal herb and food. Rhizome and roots of P. notoginseng are officially recorded as notoginseng in the Chinese Pharmacopoeia [1]. About 61 Chinese patent medicines contain notoginseng, including Yunnan Bai Yao, a famous hemostatic proprietary herbal remedy. The secondary metabolites of this plant include saponins [2], [3], flavones [2], amino acids [4], and polysaccharides [5], with the most emphasis being on saponins. P. notoginseng saponins are considered as the major active ingredients in notoginseng. The saponins display multiple pharmacological effects, such as hemostatic [6], [7], [8], [9], antioxidant [10], [11], neuroprotective [12], [13], antitumor [14], [15], antidiabetic [16], [17], and other activities, and have been extensively used as therapeutic agents in China. The pronounced efficacies of notoginseng saponins have led to the development of several Chinese patent medicines, such as Xuesaitong Capsules/Soft Capsules [18] and Xuesaitong Injection [19].
In recent years, the demand for notoginseng has been increasing. Unfortunately, the yield and quality of notoginseng are severely limited by replanting obstacles [20], and a number of diseases caused by a plethora of phytopathogens [21]. Among the diseases, the root-rot disease complex is the most destructive one as it results in yield reduction, no harvest, or low content of active ingredients [22]. The reported fungal pathogens causing root rot include Alternaria panax, Alternaria tenuissima, Cylindrocarpon destructans, Cylindrocarpon didynum, Rhizoctonia solani, Phytophthora cactorum, Phoma herbarum, Fusarium solani, Fusarium oxysporum [21], and Fusarium flocciferum [23]. Although diverse chemical pesticides and some biocontrol methods are used, it is difficult to control the root-rot diseases because of the pathogenic complex. More comprehensive, practical, and ecological methods to eradicate the pathogenic diseases in P. notoginseng are urgently needed. Furthermore, specific and nonspecific fungi and bacteria associated with the plant have been found with little information about their ecological functions.
Microbial communities associated with plants play an important role in balancing the ecosystem and boosting host growth. Endophytic fungi, which live in healthy plant tissues for at least a part of their life cycle, without causing any noticeable symptoms of infection or disease, may benefit their host in different ways, such as producing bioactive secondary metabolites, promoting germination and shoot growth, inducing host plants to tolerate to biotic or abiotic stresses [24], [25], [26], [27]. In a previous study, Ma et al [28] demonstrated high phylogenetic diversity and biocontrol potential of bacterial endophytes of P. notoginseng. However, little is known about the fungal community harbored in P. notoginseng.
In this study, the diversity of endophytic fungi isolated from different tissues of healthy P. notoginseng was evaluated, and the isolates were screened for their potential antimicrobial activity against five major phytopathogens causing root rot of P. notoginseng. To the best of our knowledge, this is the first report of the biodiversity, phylogeny, and assessment of biocontrol potential of endophytic fungi harbored in P. notoginseng.
2. Materials and methods
2.1. Isolation of endophytic fungi
Three-year-old healthy P. notoginseng plant samples were collected in October 2013, from a plantation in Wenshan, Yunnan, Southwest China. The collected plants were excised into roots, stems, leaves, and seeds, put into plastic bags, transferred to the laboratory within 24 h, and stored at 4°C until the isolation procedure of endophytic fungi was carried out.
The surface sterilization and isolation of fungal endophytes were carried out by following the procedures described by Park et al [29]. The plant samples were washed thoroughly with running tap water to remove soil particles and rinsed six times with distilled water. The separated parts (roots, stems, leaves, and seeds; Fig. 1A) were immersed in 75% ethanol solution for 2–3 min, and subsequently transferred to 5.5% sodium hypochlorite solution for 1–2 min, depending on the different tissues. The surface-sterilized samples were rinsed three times with sterile distilled water and dried with sterile filter paper. Sterile samples were aseptically crumbled into small fragments, evenly placed on Petri dishes containing potato dextrose agar (PDA) with 100 mg/L ampicillin to inhibit bacterial growth. The Petri dishes were incubated at 28 ± 1°C until fungal growth started. To confirm that the disinfection process was successful, a 0.1-mL aliquot of the water used for the last washing step was spotted on PDA plates supplemented with 100 mg/L ampicillin, and incubated under the same conditions in parallel. Plates that were not detected as contaminated by cultivable microorganisms were considered as successfully surface disinfected and used for isolation of endophytes [30]. The cultures were monitored every day to monitor the growth of endophytic fungi. Each colony that emerged from the fragments was transferred to antibiotic-free PDA medium (Fig. 1B), for subculture, and brought into pure culture.
Fig. 1.
Panax notoginseng, endophytic fungi and bioactivities. (A) Different parts of 3-year-old healthy plant of P. notoginseng; (B) isolation of endophytic fungi; (C) fermentation; (D) screening of antagonistic endophytic fungi against host phytopathogens of root-rot disease.
The pure cultures were recorded and maintained on PDA in active form for further investigation. For long-term storage, the fungal colonies were stored in 15% (v/v) glycerol at −80°C. All the fungal isolates were deposited in the Yunnan Institute of Microbiology, Yunnan University, Kunming, China.
2.2. Fungal DNA extraction, PCR amplification and sequencing
The pure cultures were used for DNA extraction. DNA was extracted from 0.5–1.0 g mycelia chilled in liquid nitrogen by using the sodium dodecyl sulfate–cetyltrimethyl ammonium bromide method [31]. The internal transcribed spacer (ITS) region was amplified using primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [32]. The polymerase chain reaction (PCR) was performed in a 50-μL reaction mixture containing 1 μL template DNA, 1 μL forward primer (10μM), 1 μL reverse primer (10μM), 5 μL reaction buffer (10×), 4 μL dNTP (each 2.5μM), 0.5 μL Taq DNA Polymerase (5 U/μL), and 37.5 μL sterile double-distilled water. The PCR cycling protocol consisted of initial denaturation at 94°C for 4 min, followed by 30 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 2 min, and a final elongation step of 72°C for 10 min. As a negative control, the template DNA was replaced by sterile double-distilled water. The PCR amplified products were separated by agarose gel electrophoresis, and sequenced on an ABI Prism 3730 sequencer at Sangon Biotech (Shanghai, China).
2.3. Phylogenetic analysis of endophytic fungi
For strain identification, the ITS sequences were compared with those in the NCBI database (http://www.ncbi.nlm.nih.gov/) using the BLAST search program for the final identification of the fungal endophytes program. A phylogenetic tree was constructed by the neighbor-joining method using MEGA version 5.0 software (http://www.megasoftware.net/), after pairwise alignments using the CLUSTAL X program, version 1.8 (http://www.clustal.org/) [33]. The stability of the internal branches was assessed with 1,000 bootstrap replications. The sequences of this study were deposited in the GenBank database under the accession numbers: KP714353–KP714393 (Table 1).
Table 1.
Analysis of 89 isolates from Panax notoginseng with the related species and their isolation information
| Phylogenetic group (genus) |
Representative isolate (accession No.) |
Closest species in GenBank (accession No.) |
Similarity (%) | Tissues of P. notoginseng |
No. of isolates | |||
|---|---|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | Seeds | |||||
| ASCOMYCETES | ||||||||
| Capnodiales | ||||||||
| Cladosporium | PH30450 (KP714353) | Cladosporium cladosporioides (KJ767066 ) | 100 | 3 | 8 | |||
| PH30409 (KP714354) | Cladosporium oxysporum (KJ475816) | 100 | 3 | |||||
| PH30412 (KP714355) | Cladosporium oxysporum (KJ475816) | 100 | 2 | |||||
| Chaetothyriales | ||||||||
| Phialophora | PH30403 (KP714356) | Phialophora mustea (KF850364) | 100 | 1 | 1 | |||
| Diaporthales | ||||||||
| Phomopsis | PH30406 (KP714357) | Phomopsis columnaris (KC145883) | 100 | 2 | 2 | |||
| Eurotiales | ||||||||
| Aspergillus | PH30427 (KP714358) | Aspergillus versicolor (KJ152174) | 100 | 1 | 3 | 4 | ||
| Penicillium | PH30444 (KP714359) | Penicillium chrysogenum (LN482541) | 100 | 2 | 10 | |||
| PH30424 (KP714360) | Penicillium crustosum (LN482509) | 100 | 3 | |||||
| PH30378 (KP714361) | Penicillium cordubense (KJ191427) | 100 | 3 | |||||
| PH30390 (KP714362) | Penicillium lapidosum (KJ676451) | 100 | 2 | |||||
| Glomerellales | ||||||||
| Colletotrichum | PH30420 (KP714363) | Colletotrichum gloeosporioides (KF864554) | 100 | 2 | 2 | |||
| Helotiales | ||||||||
| Botryotinia | PH30439 (KP714364) | Botryotinia fuckeliana (KF802809) | 100 | 2 | 2 | |||
| Hypocreales | ||||||||
| Acremonium | PH30454 (KP714365) | Acremonium implicatum (HQ914932) | 98 | 1 | 1 | |||
| Fusarium | PH30410 (KP714366) | Fusarium acuminatum (KF887097) | 100 | 1 | 8 | |||
| PH30386 (KP714367) | Fusarium oxysporum (KJ026700) | 100 | 3 | |||||
| PH30436 (KP714368) | Fusarium solani (KF939488) | 100 | 2 | 2 | ||||
| Ilyonectria | PH30396 (KP714369) | Ilyonectria macrodidyma (HQ703420) | 100 | 2 | 3 | |||
| PH30398 (KP714370) | Ilyonectria macrodidyma (HQ703420) | 100 | 1 | |||||
| Myrothecium | PH30382 (KP714371) | Myrothecium sp. (HQ631058) | 99 | 2 | 1 | 3 | ||
| Plectosphaerella | PH30464 (KP714372) | Plectosphaerella sp. (JX535104) | 100 | 1 | 1 | 2 | 5 | |
| PH30457 (KP714373) | Plectosphaerella sp. (JX535104) | 99 | 1 | |||||
| Trichoderma | PH30435 (KP714374) | Trichoderma longibrachiatum (KJ767089) | 100 | 1 | 6 | |||
| PH30441 (KP714375) | Trichoderma koningiopsis (KM079603) | 100 | 2 | 1 | 1 | |||
| PH30432 (KP714376) | Trichoderma spirale (KM079610) | 100 | 1 | |||||
| Microascales | ||||||||
| Periconia | PH30428 (KP714377) | Periconia byssoides (KC954160) | 99 | 1 | 1 | |||
| Pleosporales | ||||||||
| Alternaria | PH30445 (KP714378) | Alternaria sp. (KC771455) | 100 | 1 | 15 | |||
| PH30438 (KP714379) | Alternaria alternata (KM015489) | 100 | 2 | 5 | ||||
| PH30452 (KP714380) | Alternaria tenuissima (KF308886) | 100 | 2 | 2 | ||||
| PH30425 (KP714381) | Alternaria tenuissima (KF308886) | 100 | 1 | 1 | ||||
| PH30442 (KP714382) | Alternaria alternata (KM015489) | 100 | 1 | |||||
| Dictyosporium | PH30404 (KP714383) | Dictyosporium digitatum (DQ018089) | 98 | 1 | 1 | |||
| Phoma | PH30459 (KP714384) | Phoma draconis (GU237795) | 100 | 1 | 3 | |||
| PH30391 (KP714385) | Phoma radicina (JQ676200) | 99 | 2 | |||||
| Sordariales | ||||||||
| Chaetomium | PH30461 (KP714386) | Chaetomium globosum (KJ728838) | 100 | 1 | 1 | |||
| Humicola | PH30417 (KP714387) | Humicola fuscoatra (JN031580) | 99 | 1 | 1 | |||
| Thielavia | PH30430 (KP714388) | Thielavia arenaria (JX535015) | 99 | 1 | 1 | |||
| Incertae sedis | ||||||||
| Arthrinium | PH30448 (KP714390) | Arthrinium arundinis (KF850624) | 99 | 2 | 2 | |||
| Xylariales | ||||||||
| Pestalotiopsis | PH30451 (KP714391) | Pestalotiopsis vismiae (JX305715) | 100 | 2 | 4 | |||
| PH30414 (KP714392) | Pestalotiopsis uvicola (JN198506 ) | 100 | 2 | |||||
| Incertae sedis | PH30402 (KP714389) | Sordariomycetes sp. (JX244023) | 99 | 2 | 2 | |||
| ZYGOMYCETES | ||||||||
| Mucorales | ||||||||
| Mucor | PH30392 (KP714393) | Mucor hiemalis (JF299220) | 100 | 3 | 3 | |||
| No. of total isolates | 42 | 16 | 12 | 19 | 89 | |||
2.4. Phytopathogens used for antagonistic assays
A. panax, F. oxysporum, F. solani, Phoma herbarum, and Mycocentrospora were isolated from rotten root samples of P. notoginseng collected from Wenshan in August, 2012. The identification was performed by morphological and ITS sequencing methods. The pathogenicity was confirmed by experiments with detached roots of healthy notoginseng plants (unpublished data).
2.5. In vitro antagonistic activity against host phytopathogens
Well-grown slant culture of each fungus was used for liquid culture in potato dextrose broth medium (50 mL/250 mL flasks). The flasks were incubated at 28 ± 1°C on a shaker at 180 rpm (Fig. 1C) for 7 d. The culture broth was filtered through blotting paper and the supernatant was separated. The supernatant was extracted three times with ethyl acetate (EtOAc). The wet mycelium was extracted twice with acetone. After removal of acetone in a rotary vacuum, the aliquot residue was extracted three times with EtOAc. Combined the EtOAc extracts from supernatant and mycelium, the organic phase was dried over Na2SO4 (anhydrous). The extracts were then concentrated in a rotary vacuum. The crude extracts were stored at 4°C before usage. The inhibitory effect of the EtOAc extracts obtained from endophytic fungi was tested using the double-layer agar technique [34] (Fig. 1D). The crude extracts were dissolved in 1.0 mL ethanol as stock solution. A 100-μL volume of the ethanol extract suspension was pipetted into each hole. Test plates were incubated at 28 ± 2°C, and the diameters of the inhibition zones were measured after 24 h. The same volume of ethanol was used as the negative control.
3. Results
3.1. Phylogenetic analysis of endophytic fungi
A total of 89 endophytic fungi were isolated from the roots, stems, leaves, and seeds of healthy notoginseng plants. Forty-one morphotypes were recognizable on the basis of their morphological characteristics. One isolate of each morphotype was selected for classification by phylogenetic analysis. All sequences were BLAST searched in GenBank (NCBI database). The closest matches and the GeneBank accession numbers are listed in Table 1. Only isolate PH30454 revealed at least 98% similarity to known reported sequences. Among 89 endophytic fungi isolates, 86 belonged to phylum Ascomycota, and three to Zygomycota. All isolates were classified to 12 orders (Capnodiales, Chaetothyriales, Diaporthales, Eurotiales, Glomerellales, Helotiales, Hypocreales, Microascales, Mucorales, Pleosporales, Sordariales, Xylariales), 23 genera (Acremonium, Alternaria, Arthrinium, Aspergillus, Botryotinia, Chaetomium, Cladosporium, Colletotrichum, Dictyosporium, Fusarium, Humicola, Ilyonectria, Mucor, Myrothecium, Penicillium, Periconia, Pestalotiopsis, Phialophora, Phoma, Phomopsis, Plectosphaerella, Thielavia, and Trichoderma), in which isolate PH30448 belonging to genus Arthrinium was incertae sedis at order level and isolate PH30402 was an unknown fungus belonging to Sordariomycetes.
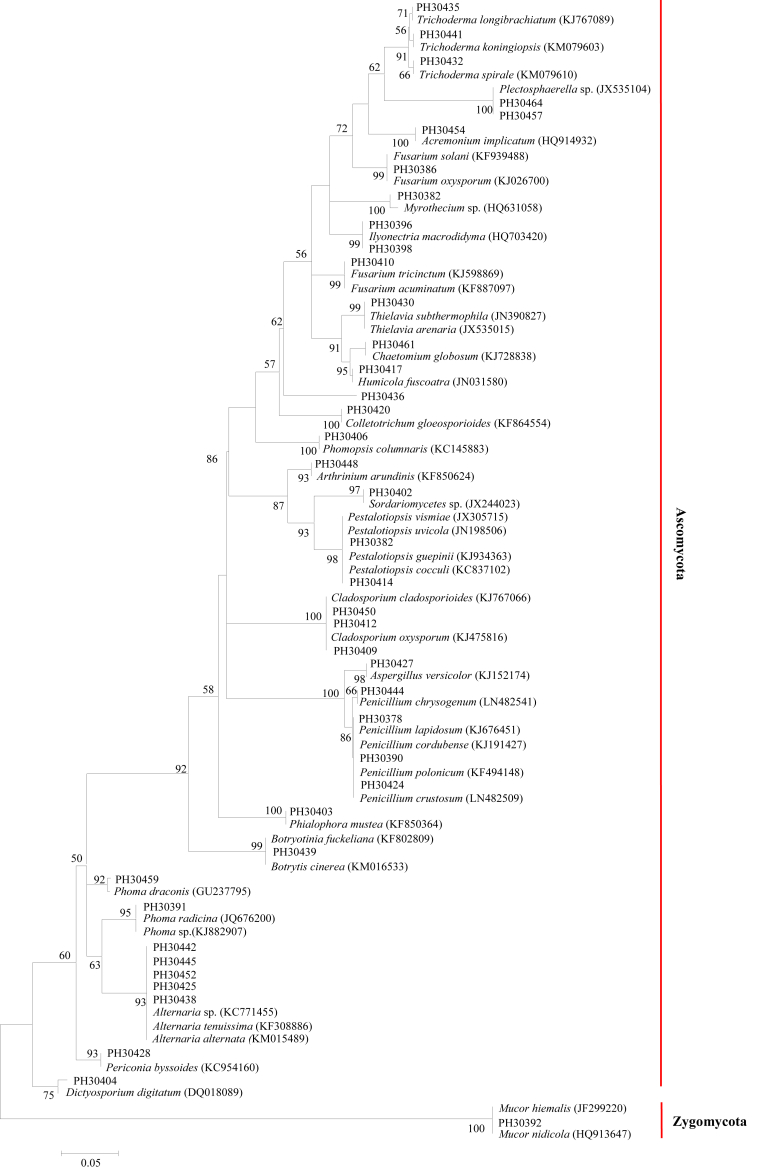
The phylogenetic tree (Fig. 2) revealed the relationship between the different species of fungal endophytes obtained from different parts of P. notoginseng. The most frequently occurring groups were Hypocreales with 26 isolates (29.2%) and Pleosporales with 19 isolates (21.3%). Hypocreales group included six genera: Acremonium, Fusarium, Ilyonectria, Myrothecium, Plectosphaerella, and Trichoderma. Pleosporales were integrated by three different genera: Alternaria, Dictyosporium, and Phoma. The groups Capnodiales, Chaetothyriales, Diaporthales, Glomerellales, Helotiales, Microascales, Mucorales, and Xylariales comprised only one taxon (genus) each (Table 1 and Fig. 2). The results suggested that there were diverse endophytic fungi harbored in the cultivated P. notoginseng.
Fig. 2.
Phylogenetic tree based on neighbor-joining analysis of the rDNA internal transcribed spacer sequences of the endophytic fungi obtained from different tissues of Panax notoginseng. Significant bootstrap values (> 50%) are indicated at the branching points. The tree has been drawn to scale (0.05).
3.2. Fungal distribution analysis
The number of isolates obtained from different tissues of P. notoginseng ranged from 12 to 42 for leaves and roots, respectively (Table 1). Forty-two isolates from roots were assigned to 16 genera and an unknown taxon, and the genera Fusarium, Cladosporium, and Penicillium were the dominant groups. Nineteen isolates from seeds belonged to nine genera. Eight genera fungi (16 isolates) were obtained from stems. The Alternaria genus was dominant in seeds and stems. The amount of endophytic fungi from leaves was the least, with only 12 isolates belonging to four genera. In addition, the distribution of endophytic fungi showed significant differences in various organs and showed obvious specificity. For example, the endophytic fungi belonging to Colletotrichum, Ilyonectria, and Mucor were only isolated from roots.
3.3. Antagonism assay of fungal endophytes as potential biocontrol agents
All EtOAc crude extracts of 41 fungal endophyte strains were tested for antagonistic activities against five major fungal pathogens that cause root-rot disease of P. notoginseng (Table 2). Twenty-six of the 41 isolates (63.4%) exhibited activity against at least one of the pathogens tested. The highest frequency (16 isolates, 39.0%) was against the pathogen A. panax, followed by F. solani (15 isolates, 36.6 %), F. oxysporum (14 isolates, 34.1%), Phoma herbarum (12 isolates, 29.3%), and M. acerina (7 isolates, 17.1%), respectively. Five isolates, PH30409, PH30412, PH30414, PH30441, and PH30444, were found to inhibit all pathogens tested, implicating they have a broad spectrum of antagonistic activity. Isolates, PH30409, PH30414, PH30420, PH30424, and PH30441 exhibited relatively strong inhibitory effects against the pathogens F. oxysporum, F. solani, and Phoma herbarum. The broad spectrum or strong inhibitory activities of these strains against the host plant pathogens suggest that these endophytic fungi may be potential candidates for the production of bioactive compounds, and have the potential as biological control agents of root-rot disease of P. notoginseng. In other virulence tests with detached healthy notoginseng roots, isolates PH30409 and PH30412 were found to be able to cause root rot (unpublished data), although they showed antagonism to tested pathogens.
Table 2.
Endophytic fungal antagonists of root-rot disease pathogens
| Isolate No. | Antagonistic activity1),2) |
||||
|---|---|---|---|---|---|
| Alternaria panax, | Fusarium oxysporum | Fusarium solani | Phoma herbarum | Mycocentrospora acerina | |
| PH30378 | + | − | + | − | − |
| PH30382 | − | − | − | − | − |
| PH30386 | + | − | − | − | − |
| PH30390 | − | − | + | − | − |
| PH30391 | − | − | − | − | − |
| PH30392 | + | − | − | − | − |
| PH30396 | − | − | − | − | − |
| PH30398 | − | − | − | − | − |
| PH30402 | − | − | + | − | − |
| PH30403 | + | + | − | + | − |
| PH30404 | − | − | + | − | − |
| PH30406 | − | − | − | − | − |
| PH30409 | ++ | + | +++ | + | + |
| PH30410 | − | − | − | − | − |
| PH30412 | + | ++ | ++ | + | + |
| PH30414 | ++ | + | + | +++ | + |
| PH30417 | − | − | − | − | − |
| PH30420 | ++ | ++ | +++ | − | − |
| PH30424 | − | +++ | ++ | ++ | ++ |
| PH30425 | − | − | − | + | − |
| PH30427 | − | + | ++ | − | − |
| PH30428 | − | − | − | − | − |
| PH30430 | − | − | − | − | − |
| PH30432 | − | ++ | + | − | − |
| PH30435 | − | − | − | ++ | − |
| PH30436 | − | − | − | − | − |
| PH30438 | − | − | − | − | − |
| PH30439 | + | + | + | − | − |
| PH30441 | ++ | +++ | +++ | + | + |
| PH30442 | − | − | − | + | − |
| PH30444 | ++ | ++ | ++ | + | + |
| PH30445 | − | − | − | − | − |
| PH30448 | + | − | − | − | − |
| PH30450 | + | + | − | + | + |
| PH30451 | ++ | − | − | − | − |
| PH30452 | − | − | − | + | − |
| PH30454 | + | − | − | − | − |
| PH30457 | − | + | − | − | − |
| PH30459 | − | − | − | − | − |
| PH30461 | ++ | ++ | ++ | − | − |
| PH30464 | − | − | − | − | − |
Estimated by measuring the diameter of the clear zone of growth inhibition
Symbols: −, no activity; +, ++, and +++, weak activity, moderate activity, and strong activity, respectively
4. Discussion
Endophytic fungi have been considered as a promising source for the development of biological control agents (BCAs) against phytopathogens, because they exhibit the beneficial functions for host plants [24], [25], [26], [27], [35], [36], and are ubiquitous within almost every tissue type studied [37]. Even so, endophytic fungi are still a poorly investigated group of microorganisms [38], and their complex ecological functions remain to be extensively exploited. P. notoginseng, Panax ginseng, and Panax quinquefolius, have been widely used for medicinal plants all over the world. There are a few investigations of the endophytic fungi in Panax genus, but most of them have been focused on ginseng (P. ginseng) [29], [39], [40], [41], [42] and American ginseng (P. quinqefolius) [43]. P. notoginseng, an important Chinese medicine plant, has attracted increasing attention in medical and chemical fields because of its active ingredients, but little in ecological and other research areas because of its narrow distribution.
In the present study, the diversity, tissue distribution, and biocontrol potential of cultivable endophytic fungi harbored in P. notoginseng plants were described for the first time. We proposed that the endophytic fungi would exert certain functions to help the host survive in plantation against disastrous root-rot diseases. In the survived and healthy notoginseng plants, the harbored fungal endophytes may give some clue to find effective approaches to control root rot, caused by complex pathogens. Thus, in order to screen for the most promising endophytes, we evaluated the potential of all the endophytic isolates as BCAs by challenging five major root-rot pathogens of the host plant, A. panax, F. oxysporum, F. solani, Phoma herbarum, and M. acerina, whose pathogenicity was confirmed in our previous work. Among the isolates, crude extracts of PH30409 and PH30441 showed strong inhibition on all tested phytopathogens, implicating that the two isolates have the potential to produce active compounds. Other isolates showed selective activity against one or two pathogens. According to the viewpoint by Zhang et al [36], the mechanisms involved in biocontrol of endophytic fungi against phytopathogens include antibiosis, competition for nutrients and space, induction of defense response, and mycoparasitism. The endophytic isolates can be used to screen their antagonistic activity by fermenting in different media in further work. Other mechanisms should also be investigated in the development of BCAs. For instance, Trichoderma spp. widely used in agriculture as biofungicides, have shown many ways against phytopathogens, such as producing active compounds, secreting hydrolytic enzymes, and competing for nutrients [44]. In this study, isolates belonging to Trichoderma were also obtained and tested as active endophytic strains. Endphytic fungus Penicillium chrysogenum showed effects on various plant pathogens [45], [46], [47]. García et al [48] reported that the isolate EEZ10 of Penicillium chrysogenum from soil inhibited the growth of Verticillium dahlia in vitro, and is a possible biological control agent for Verticillium disease [48]. The sequence of isolate PH30444 was 100% similar to that of Penicillium chrysogenum, and its crude extract also exhibited inhibition against all tested host phytopathogens. Recently, we found that some endophytic isolates can inhibit pathogens of notoginseng root-rot diseases by mycoparasitism and producing active secondary metabolites and volatile organic compounds [49], [50], [51], [52], [53], [54]. These findings showed that endophytic fungi from P. notoginseng are potential BCAs for the host plant.
The culture-based study and molecular identification showed that the total fungal diversity was relatively high, and the overwhelming majority belonged to Ascomycota (96.6% of the total number of isolates). Only a single Zygomycota group was obtained. This finding is consistent with a previous study in Cannabis sativa L. [26]. In 12 morphologically distinct groups, Hypocreales (29.2%) and Pleosporales (21.3%) were dominant orders among the endophytic fungi of different tissues of P. notoginseng. This result was similar to the previous studies in many medicinal plants [26], [55]. The endophytic fungi associated with P. notoginseng comprised a number of dominant groups, such as Alternaria, Fusarium, and Penicillium. All these genera have been previously isolated as endophytes, not only from other Panax species [29], [39], [40], [42], but also from a wide range of plant hosts in the temperate and tropical zones [26], [56], [57]. Although, genera Alternaria, Cladosporium, Colletotrichum, and Fusarium were found as endophytes in all three Panax species (P. ginseng, P. notoginseng, and P. quinquefolium) [29], [39], [40], [41], [42], [43], other genera Arthrinium, Botryotinia, Chaetomium, Dictyosporium, Humicola, Ilyonectria, Mucor, Periconia, Pestalotiopsis, and Thielavia, have not been obtained from other Panax plants, suggesting that P. notoginseng harbors specific and diverse fungi due to the unique soil, climatic conditions of Wenshan region and the approaches taken in agro-management.
Some isolated taxa showed preference for specific tissues types. Previous studies in other plants have also indicated that endophytes exhibit tissues specificity [58], [59], [60]. As expected, tissue specificity of fungal endophytes was demonstrated in our study. Particular endophytes were only found in one tissue, which suggests that certain species survive within the specific chemistry or texture of different tissues [57]. The diversity and species richness of endophytic fungi were higher in the underground part (roots) than that in the above-ground parts (stems, leaves, and seeds). Some similar results have been reported in the previous studies [55], [57]. The possible reasons are that soil-borne fungi are typically more prevalent and diversified than those that infect aerial plant tissues [55], and there are abundant nutriments in roots of P. notoginseng that are suitable for the growth of fungi. The relative abundance of each fungus identified in P. notoginseng reflects an unequal distribution of isolate richness among species. Similar results have been found in other plants, such as Stellera chamaejasme and Taxus globose [55], [57].
Plant–endophytic fungus interactions have been extensively investigated. Most of the symbionts may have helped for host plants. It has also been confirmed that the pathogenic–endophytic lifestyles of some fungi are interchangeable [61], [62]. These phenomena are caused by many factors, such as environment changes, chemical inducers, and molecular and genetic bases [63], [64]. Groups of fungal phytopathogens also contain large numbers of endophytic taxa. In this study, isolates PH30409 and PH30412 caused root-rot symptoms in experimental infection through detached healthy roots of P. notoginseng (unpublished data). Interestingly, crude extracts of them showed antagonistic activities against the tested phytopathogens, and more experimental infections to confirm the pathogenicity of Cladosporium sp. for P. notoginseng in plantation are underway. It is also interesting that some species in Alternaria and Fusarium from soil are pathogenic fungi for P. notoginseng, and these genera were dominant groups in the endophytic isolates. In the cultivation soil, Fusarium was the most isolated genus [65]. Endophytes should originate from the environment in which their host plants live. Therefore, these genera have more opportunities to invade and colonize in the tissues, and evolve as endophytes. Their evolution should be investigated in the further studies.
Nowadays, the notoginseng supply mainly relies on intensive field cultivation under artificial shade structures with fertile and moist soils as the wild-type notoginseng disappeared many years ago [66]. The long shade cultivation, pesticide spray, and chemical fertilizers are widely used in cultivation of notoginseng. To achieve higher yield, the frequencies of pesticide and fertilizer use increase more as the product price increases. These agricultural managements improve the productivity to some degree, but also cause other problems, such as being toxic to humans, animals, and other crops, and development of fungicide-tolerant pathogen strains [67]. At the same time, they affect the structures and functions of microorganisms in bulk soil, rhizosphere soil, and endophytes. The harmful effects should be investigated for further reasonable agro-applications in notoginseng planting.
Taken together, our findings revealed that there are diverse endophytic fungi harbored in different tissues of P. notoginseng planted in Southwest China. The fungal endophytes serve as great promise not only as BCAs against the known and emerging phytopathogens of P. notoginseng, but also as a resource of biologically active novel secondary metabolites [68]. Three endophytic fungal isolates, PH30444, PH30414, and PH30441, are potential BCAs against A. panax, F. oxysporum, F. solani, Phoma herbarum, and M. acerina, and possibly other pathogens. Further studies are required to clarify the biocontrol active metabolites and the control efficiency of the potential endophytic isolates in both pot and field experiments. A full understanding of endophytic community structure, dynamics, and functions, and the shaping factors will be helpful in selecting pesticide, fertilizer, and other approaches for notoginseng planting.
Conflicts of interest
The authors declare that there are no conflicts of interests.
Acknowledgments
This work was partly supported by the grants from the National Natural Science Foundation of China (Nos. 31100009 and 41361075), Yunnan Natural Science Foundation (No. 2013FA015), and Foundation of Yunnan Educational Committee (No. ZD2013008).
References
- 1.He H.M., Cui L. Chinese Medical Science Press; Beijing: 2010. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- 2.Yang W.Z., Hu Y., Wu W.Y., Ye M., Guo D.A. Saponins in the genus Panax L. (Araliaeae): a systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Han L.F., Sakah K.J., Wu Z.Z., Liu L.L., Agyemang K. Bioactive protopanaxtriol saponins isolated from the roots of Panax notoginseng (Burk.) F. H. Chen. Molecules. 2013;18:10352–10366. doi: 10.3390/molecules180910352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X.T., Zhao Y.J., Jiang C.F., Zhang H.Q., Yu A.M. Determination of amino acids in Panax notoginseng by microwave hydrolysis and derivatization coupled with capillary zone electrophoresis detection. Chem Res Chin Univ. 2013;29:434–438. [Google Scholar]
- 5.Jia D., Deng Y., Gao J., Liu X., Chu J., Shu Y. Neuroprotective effect of Panax notoginseng polysaccharides against focal cerebral ischemia reperfusion injury in rats. Int J Biol Macromol. 2014;63:177–180. doi: 10.1016/j.ijbiomac.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Gao B., Huang L., Liu H., Wu H., Zhang E., Yang L., Wu X., Wang Z. Platelet P2Y12 receptors are involved in the haemostatic effect of notoginsenoside Ft1, a saponin isolated from Panax notoginseng. Brit J Pharmacol. 2014;171:214–223. doi: 10.1111/bph.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q., Wang X., Cui J., Wang P., Xiong M., Jia C., Liu L., Ning B., Li L., Wang W. Bidirectional regulation of angiogenesis and miR-18a expression by PNS in the mouse model of tumor complicated by myocardial ischemia. BMC Complement Altern Med. 2014;14:183. doi: 10.1186/1472-6882-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y., Feng Z., You C., Jin Y., Hu X., Wang X., Han C. In vitro evaluation of Panax notoginseng Rg1 released from collagen/chitosan-gelatin microsphere scaffolds for angiogenesis. BioMed Eng Online. 2013;12:134. doi: 10.1186/1475-925X-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q., Jiang L., Xu C., Luo D., Zeng C., Liu P., Yue M., Liu Y., Hu X., Hu H. Ginsenoside Rg1 inhibits platelet activation and arterial thrombosis. Thromb Res. 2014;133:57–65. doi: 10.1016/j.thromres.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Meng X., Wang M., Wang X., Sun G., Ye J., Xu H., Sun X. Suppression of NADPH oxidase- and mitochondrion-derived superoxide by notoginsenoside R1 protects against cerebral ischemia–reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf 2 pathways. Free Radical Res. 2014;48:823–838. doi: 10.3109/10715762.2014.911853. [DOI] [PubMed] [Google Scholar]
- 11.Zhou N., Tang Y., Keep R.F., Ma X., Xiang J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine. 2014;21:1189–1195. doi: 10.1016/j.phymed.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan S., Li Z., Li H., Arancio O., Zhang W. Notoginsenoside R1 increases neuronal excitability and ameliorates synaptic and memory dysfunction following amyloid elevation. Sci Rep. 2014;4:6352. doi: 10.1038/srep06352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng X.S., Zhou X.S., Luo F.C., Jia J.J., Qi L., Yang Z.X., Zhang W., Bai J. Comparative analysis of the neuroprotective effects of ginsenosides Rg1 and Rb1 extracted from Panax notoginseng against cerebral ischemia. Can J Physiol Pharmacol. 2014;92:102–108. doi: 10.1139/cjpp-2013-0274. [DOI] [PubMed] [Google Scholar]
- 14.Wang P., Cui J., Du X., Yang Q., Jia C., Xiong M., Yu X., Li L., Wang W., Chen Y. Panax notoginseng saponins (PNS) inhibits breast cancer metastasis. J Ethnopharmacol. 2014;154:663–671. doi: 10.1016/j.jep.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Yang X., Xiong X., Wang H., Wang J. Protective effects of Panax notoginseng saponins on cardiovascular diseases: a comprehensive overview of experimental studies. Evid Based Complement Alternat Med. 2014;2014:204840. doi: 10.1155/2014/204840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao D., Guo Y., Li X., Li X., Li Z., Xue M., Ou Z., Liu M., Yang M., Liu S. An aqueous extract of Radix astragali, Angelica sinensis, and Panax notoginseng is effective in preventing diabetic retinopathy. Evid Based Complement Alternat Med. 2013;2013:578165. doi: 10.1155/2013/578165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uzayisenga R., Ayeka P.A., Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): a review. Phytother Res. 2014;28:510–516. doi: 10.1002/ptr.5026. [DOI] [PubMed] [Google Scholar]
- 18.Yang X., Xiong X., Wang H., Yang G., Wang J. Xuesaitong soft capsule (Chinese patent medicine) for the treatment of unstable angina pectoris: a meta-analysis and systematic review. Evid Based Complement Alternat Med. 2013;2013:948319. doi: 10.1155/2013/948319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Li Z., Zhao X., Liu W., Liu Y., Yang J., Li X., Fan X., Cheng Y. A network study of Chinese medicine xuesaitong injection to elucidate a complex of action with multicompound, multitarget, and multipathway. Evid Based Complement Alternat Med. 2013;2013:652373. doi: 10.1155/2013/652373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou X., Jin H., Guo L., Cui X., Xiao Y., Liu D., Huang L. Effects of balanced fertilization and soil amendment on growth and yield of Sanqi in continuous cropping. China J Chinese Materia Medica. 2012;37:1905–1911. [PubMed] [Google Scholar]
- 21.Miao Z.Q., Li S.D., Liu X.Z., Chen Y.J., Li Y.H., Wang Y., Guo R.J., Xia Z.Y., Zhang K.Q. The causal microorganisms of Panax notoginseng root rot disease. Scientia Agricultura Sinica. 2006;39:1371–1378. [Google Scholar]
- 22.Sun Y., Ke J., Ma N., Chen Z., Wang C., Cui X. Effects of root rot on saponin content in Panax notoginseng. J Chin Med Mat. 2004;27:79–80. [PubMed] [Google Scholar]
- 23.Miao C.P., Qiao X.G., Zheng Y.K., Chen Y.W., Xu L.H., Guan H.L., Zhao L.X. First report of Fusarium flocciferum causing root rot of Sanqi (Panax notoginseng) in Yunnan, China. Plant Disease. 2015;99:1650. [Google Scholar]
- 24.Hartley S.E., Eschen R., Horwood J.M., Gange A.C., Hill E.M. Infection by a foliar endophyte elicits novel arabidopside-based reactions in its host, Cirsium arvense. New Phytologist. 2015;205:816–827. doi: 10.1111/nph.13067. [DOI] [PubMed] [Google Scholar]
- 25.Johnson L.J., de Bonth A.C.M., Briggs L.R., Caradus J.R., Finch S.C., Fleetwood D.J., Fletcher L.R., Hume D.E., Johnson R.D., Popay A.J. The exploitation of epichloae endophytes for agricultural benefit. Fungal Divers. 2013;60:171–188. [Google Scholar]
- 26.Kusari P., Kusari S., Spiteller M., Kayser O. Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant-specific phytopathogens. Fungal Divers. 2013;60:137–151. [Google Scholar]
- 27.Mei C., Flinn B.S. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat Biotechnol. 2010;4:81–95. doi: 10.2174/187220810790069523. [DOI] [PubMed] [Google Scholar]
- 28.Ma L., Cao Y.H., Cheng M.H., Huang Y., Mo M.H., Wang Y., Yang J.Z., Yang F.X. Phylogenetic diversity of bacterial endophytes of Panax notoginseng with antagonistic characteristics towards pathogens of root-rot disease complex. Antonie van Leeuwenhoek. 2013;103:299–312. doi: 10.1007/s10482-012-9810-3. [DOI] [PubMed] [Google Scholar]
- 29.Park S.U., Lim H.S., Park K.C., Park Y.H., Bae H. Fungal endophytes from three cultivars of Panax ginseng Meyer cultivated in Korea. J Ginseng Res. 2012;36:107–113. doi: 10.5142/jgr.2012.36.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin S., Li J., Chen H.H., Zhao G.Z., Zhu W.Y., Jiang C.L., Xu L.H., Li W.J. Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl Environ Microbiol. 2009;75:6176–6186. doi: 10.1128/AEM.01034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim W.K., Mauthe W., Hausner G., Klassen G.R. Isolation of high molecular weight DNA and double-stranded RNAs from fungi. Can J Bot. 1990;68:1898–1902. [Google Scholar]
- 32.White T.J., Bruns T., Lee S., Taylor J. Protocols: a guide to methods and applications. PCR Academic Press; San Diego: 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [Google Scholar]
- 33.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos S.B., Carvalho C.M., Sillankorva S., Nicolau A., Ferreira E.C., Azeredo J. The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 2009;9:148. doi: 10.1186/1471-2180-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backman P.A., Sikora R.A. Endophytes: an emerging tool for biological control. Biol Control. 2008;46:1–3. [Google Scholar]
- 36.Zhang Q.H., Zhang J., Yang L., Zhang L., Jiang D.H., Chen W.D., Li G.Q. Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol Control. 2014;72:98–108. [Google Scholar]
- 37.Hyde K.D., Soytong K. The fungal endophyte dilemma. Fungal Divers. 2008;33:163–173. [Google Scholar]
- 38.Tejesvi M.V., Kajula M., Mattila S., Pirttilä A.M. Bioactivity and genetic diversity of endophytic fungi in Rhododendron tomentosum Harmaja. Fungal Divers. 2011;47:97–107. [Google Scholar]
- 39.Eo J.K., Choi M.S., Eom A.H. Diversity of endophytic fungi isolated from Korea ginseng leaves. Mycobiology. 2014;42:147–151. doi: 10.5941/MYCO.2014.42.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park Y.H., Kim Y.C., Park S.U., Lim H.S., Kim J.B., Cho B.K., Bae H. Age-dependent distribution of fungal endophytes in Panax ginseng roots cultivated in Korea. J Ginseng Res. 2012;36:327–333. doi: 10.5142/jgr.2012.36.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park Y.H., Lee S.G., Ahn D.J., Kwon T.R., Park S.U., Lim H.S., Bae H. Diversity of fungal endophytes in various tissues of Panax ginseng Meyer cultivated in Korea. J Ginseng Res. 2012;36:211–217. doi: 10.5142/jgr.2012.36.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu H., Yang H.Y., You X.L., Li Y.H. Diversity of endophytic fungi from roots of Panax ginseng and their saponin yield capacities. SpringerPlus. 2013;2:107. doi: 10.1186/2193-1801-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xing X., Guo S., Fu J. Biodiversity and distribution of endophytic fungi associated with Panax quinquefolium L. cultivated in a forest reserve. Symbiosis. 2010;51:161–166. [Google Scholar]
- 44.Keswani C., Mishra S., Sarma B.K., Singh S.P., Singh H.B. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Appl Microbiol Biotechnol. 2014;98:533–544. doi: 10.1007/s00253-013-5344-5. [DOI] [PubMed] [Google Scholar]
- 45.Böhm J., Hoff B., O’Gorman C.M., Wolfers S., Klix V., Binger D., Zadra I., Kürnsteiner H., Pöggeler S., Dyer P.S. Sexual reproduction and mating-type–mediated strain development in the penicillin-producing fungus Penicillium chrysogenum. Proc Natl Acad Sci USA. 2013;110:1476–1481. doi: 10.1073/pnas.1217943110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson A.J., Walters D.R., Marshall G. Evaluation of Penicillium chrysogenum and its antifungal extracts as potential biological control agents against Botrytis fabae on faba beans. Mycol Res. 1994;98:1117–1126. [Google Scholar]
- 47.Murali M., Sudisha J., Amruthesh K.N., Ito S.I., Shetty H.S. Rhizosphere fungus Penicillium chrysogenum promotes growth and induces defence-related genes and downy mildew disease resistance in pearl millet. Plant Biology. 2013;15:111–118. doi: 10.1111/j.1438-8677.2012.00617.x. [DOI] [PubMed] [Google Scholar]
- 48.García M., Arriagada C., García-Romera I., Ocampo J.A. Are plant cell wall hydrolysing enzymes of saprobe fungi implicated in the biological control of the Verticillium dahlia pathogenesis? Crop Protect. 2011;30:85–87. [Google Scholar]
- 49.Liu K., Yang Y., Miao C.P., Zheng Y.K., Chen J.L., Chen Y.W., Xu L.H., Guan H.L., Ding Z.T., Zhao L.X. Koningiopisins A-H, polyketides with synergistic antifungal activities from the endophytic fungus Trichoderma koningiopsis. Planta Medica. 2016;82:371–376. doi: 10.1055/s-0035-1558228. [DOI] [PubMed] [Google Scholar]
- 50.Liu K., Yang Y.B., Chen J.L., Miao C.P., Wang Q., Zhou H., Chen Y.W., Li Y.Q., Ding Z.T., Zhao L.X. Koninginins N-Q, polyketides from the endophytic fungus Trichoderma koningiopsis harbored in Panax notoginseng. Nat Prod Bioprospect. 2016;6:49–55. doi: 10.1007/s13659-015-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J.L., Sun S.Z., Miao C.P., Wu K., Chen Y.W., Xu L.H., Guan H.L., Zhao L.X. Endophytic Trichoderma gamsii YIM PH30019: a promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J Ginseng Res. 2016;40:315–324. doi: 10.1016/j.jgr.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J.L., Liu K., Miao C.P., Sun S.Z., Chen Y.W., Xu L.H., Guan H.L., Zhao L.X. Salt tolerance of endophytic Trichoderma koningiopsis YIM PH30002 and its volatile organic compounds (VOCs) allelopathic activity against phytopathogens associated with Panax notoginseng. Ann Microbiol. 2016;66:981–990. [Google Scholar]
- 53.Yang Y., Yang F., Miao C., Liu K., Li Q., Qin S., Zhao L., Ding Z. Antifungal metabolites from the rhizospheric Penicillium sp. YIM PH30003 associated with Panax notoginseng. Phytochem Lett. 2015;11:249–253. [Google Scholar]
- 54.Li W., Yang X., Yang Y., Duang R., Chen G., Li X., Li Q., Qin S., Li S., Zhao L. Anti-phytopathogen, multi-target acetylcholinesterase inhibitory and antioxidant activities of metabolites from endophytic Chaetomium globosum. Nat Prod Res. 2016 doi: 10.1080/14786419.2015.1129328. [DOI] [PubMed] [Google Scholar]
- 55.Jin H., Yan Z., Liu Q., Yang X., Chen J., Qin B. Diversity and dynamics of fungal endophytes in leaves, stems and roots of Stellera chamaejasme L. in northwestern China. Antonie van Leeuwenhoek. 2013;104:949–963. doi: 10.1007/s10482-013-0014-2. [DOI] [PubMed] [Google Scholar]
- 56.Gazis R., Chaverri P. Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol. 2010;3:240–254. [Google Scholar]
- 57.Rivera-Orduña F.N., Suarez-Sanchez R.A., Flores-Bustamante Z.R., Gracida-Rodriguez J.N., Flores-Cotera L.B. Diversity of endophytic fungi of Taxus globosa (Mexican yew) Fungal Divers. 2011;47:65–74. [Google Scholar]
- 58.Errasti A., Carmaran C., Novas V. Diversity and significance of fungal endophytes from living stems of naturalized trees from Argentina. Fungal Divers. 2010;41:29–40. [Google Scholar]
- 59.Wearn J.A., Sutton B.C., Morley N.J., Gange A.C. Species and organ specificity of fungal endophytes in herbaceous grassland plants. J Ecol. 2012;100:1085–1092. [Google Scholar]
- 60.Sánchez Márquez S., Bills G.F., Domínguez Acuña L., Zabalgogeazcoa I. Endophytic mycobiota of leaves and roots of the grass Holcus lanatus. Fungal Divers. 2010;41:115–123. [Google Scholar]
- 61.Álvarez-Loayza P., White J.F., Jr, Torres M.S., Balslev H., Kristiansen T., Svenning J.C., Gil N. Light converts endosymbiotic fungus to pathogen, influencing seedling survival and niche-space filling of a common tropical tree, Iriartea deltoidea. PloS One. 2011;6:e16386. doi: 10.1371/journal.pone.0016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lana T.G., Azevedo J.L., Pomella A.W., Monteiro R.T., Silva C.B., Araújo W.L. Endophytic and pathogenic isolates of the cacao fungal pathogen Moniliophthora perniciosa (Tricholomataceae) are indistinguishable based on genetic and physiological analysis. Gen Mol Res. 2011;10:326–334. doi: 10.4238/vol10-1gmr895. [DOI] [PubMed] [Google Scholar]
- 63.Eaton C.J., Cox M.P., Scott B. What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci. 2011;180:190–195. doi: 10.1016/j.plantsci.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Rai M., Agarkar G. Plant-fungal interactions: what triggers the fungi to switch among lifestyles? Crit Rev Microbiol. 2016;42:428–438. doi: 10.3109/1040841X.2014.958052. [DOI] [PubMed] [Google Scholar]
- 65.Miao C.P., Mi Q.L., Qiao X.G., Zheng Y.K., Chen Y.W., Xu L.H., Guan H.L., Zhao L.X. Rhizospheric fungi of Panax notoginseng: diversity and antagonism to host phytopathogens. J Ginseng Res. 2016;40:127–134. doi: 10.1016/j.jgr.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou S.L., Xiong G.M., Li Z.Y., Wen J. Loss of genetic diversity of domesticated Panax notoginseng F H Chen as evidenced by ITS Sequence and AFLP polymorphism: a comparative study with P. stipuleanatus H T Tsai et K M Feng. J Integr Plant Biol. 2005;47:107–115. [Google Scholar]
- 67.Haas D., Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 68.Kusari S., Hertweck C., Spiteller M. Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem Biol. 2012;19:792–798. doi: 10.1016/j.chembiol.2012.06.004. [DOI] [PubMed] [Google Scholar]