Abstract
Background
Recently, protein from ginseng was studied and used for the treatment of several kinds of diseases. However, the effect of ginseng total protein (GTP) on proliferation and wound healing in fibroblast cells remains unclear.
Methods
In this study, cell viability was analyzed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Cell cycle distribution was analyzed by flow cytometer. The levels of transforming growth factor β1, vascular endothelial growth factor, and collagens were analyzed by enzyme-linked immunosorbent assay and immunofluorescence staining. The expressions of cyclin A, phosphorylation of extracellular signal-related kinase (p-ERK1/2), and ERK1/2 were analyzed by Western blotting.
Results
Our results showed that GTP promoted cell proliferation and increased the percentage of cells in S phase through the upregulation of cyclin A in NIH/3T3 cells. We also found that GTP induced the secretion of type I collagen, and promoted the expression of other factors that regulate the synthesis of collagen such as transforming growth factor β1 and vascular endothelial growth factor. In addition, the phosphorylation of ERK1/2 at Thr202/Tyr204 was also increased by GTP.
Conclusion
Our studies suggest that GTP promoted proliferation and secretion of collagen in NIH/3T3 cells by activating the ERK signal pathway, which shed light on a potential function of GTP in promoting wound healing.
Keywords: collagen secretion, ERK1/2, ginseng total protein, NIH/3T3 fibroblasts, proliferation
1. Introduction
Panax ginseng Meyer (ginseng), the most popular herbal medicine, has been widely used in Eastern Asia for more than 2,000 yr [1]. Ginseng has multiple active components, including ginsenosides, ginseng protein, and ginseng polysaccharides. It has previously been reported that ginseng has positive effects on human diseases, including atherosclerosis, postmenopausal disorder, liver dysfunction, cerebrovascular diseases, and cancers [1], [2], [3]. Of note, the purified ginsenosides or the extracts of ginseng root have beneficial effects on damaged skin. For example, red ginseng root extract protected skin from acute UVB irradiation [4], and ginsenoside Rb1 promotes the healing process of burn wounds by enhancing angiogenesis [5]. Recently, protein from ginseng was studied and used for treatment of several kinds of diseases [6], [7], [8], [9], [10]. However, the effect of ginseng total protein (GTP) on proliferation and wound healing in fibroblast cells remains unclear.
The recovery process of skin wounds involves complex biological mechanisms, which include inflammation, repair or proliferation, and tissue remodeling [11]. The proliferation of fibroblast cells within the wound site plays a key role in the formation of granulation tissue. Collagens that are synthesized by fibroblasts are extracellular matrix (ECM) fibrillar molecules in dermal connective tissue and comprise about 80% of the extracellular material, contributing to its strength and facilitating elasticity, tightening, and the cell integrity of the skin [12]. Therefore, fibroblast proliferation and its collagen synthesis capacity are very important for wound healing.
During wound healing, transforming growth factor β1 (TGF-β1) contributes to the fibrotic process by recruiting fibroblasts and stimulating their synthesis of collagens, proteoglycans, fibronectin, and other ECM components. Besides TGF-β1, vascular endothelial growth factor (VEGF) as an endothelial cell mitogen and inducer of vascular permeability is unique for its effects on multiple components of wound-healing cascade, including angiogenesis and collagen deposition [13]. Importantly, the mitogen-activated protein kinase/extracellular signal-related kinase (MAPK/ERK) pathway plays an important role in cell proliferation, cell cycle progression, and collagen biosynthesis [14], [15], [16].
In our study, the possible effects of GTP on wound healing were investigated in vitro. The results showed that GTP promoted proliferation and wound healing in fibroblast cells. We also found that GTP induced the secretion of type I collagen and increased the expression of related factors, such as TGF-β1 and VEGF. The wound-healing effect of GTP was partially mediated through the activation of the ERK pathway. Our data shed light on a potential function of GTP in promoting wound healing.
2. Materials and methods
2.1. Materials
Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), bFGF, penicillin, streptomycin, Dulbecco’s modified Eagle’s medium (DMEM; high glucose), and new bovine serum (NBS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Propidium iodide kit was acquired from BD Biosciences (San Diego, CA, USA). Mouse type I and III collagens, TGF-β1, and VEGF enzyme-linked immunosorbent assay (ELISA) kits were purchased from IBL (International GmbH, German). Rabbit primary antibodies against cyclin A, p-ERK 1/2 (Thr202/Tyr204), ERK1/2, TGF-β1, VEGF, and β-actin, and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other chemicals were of reagent grade.
2.2. GTP extraction, purification, and hydrolysis
Five-year-old ginseng plants grown in JingYu (Jilin Province, China) were collected and identified to fit the regulation of the Chinese Pharmacopoeia (2015). Fresh ginsengs (1 kg) were milled in liquid nitrogen and soaked in phosphate-buffered saline (PBS) for 4 h at 4°C. The supernatant from ginsengs was centrifuged at 8,500g and condensed with hollow-fiber membrane (30 kDa). Then the concentrating solution was separated and collected with Sephadex G50 to obtain GTP, the purity of which reached 90.2%. In addition, we hydrolyzed GTP by proteinase K (50 μg/mL) for 30 min at 37°C, and inactivated proteinase K for 1 h at 85°C. GTP and its hydrolysate were used for functional research analysis.
2.3. Cell culture
Mouse NIH/3T3 fibroblast cells, obtained from the American Type Culture Collection (Manassas, VA, USA), were grown in DMEM medium supplemented with 10% (v/v) NBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, and cultured at 37°C in a humidified incubator containing 5% CO2.
2.4. Cell viability assay
NIH/3T3 cells was seeded in 96-well plates and treated with different concentrations of GTP and its hydrolysate for 24 h. For viability analysis, MTT solution (0.5 mg/mL) was added to each well, and incubated for 4 h at 37°C. After the incubation, the formazan crystals were dissolved in 100 μL DMSO, and optical density was measured on a microplate reader at 490 nm [17].
2.5. Cell cycle analysis
After the treatment with GTP for 24 h, cells were washed with PBS, fixed with 70% ethanol at –20°C overnight, and incubated with RNase (100 mg/mL; Sigma-Aldrich, St. Louis, MO, USA) for 5 min. After the addition of propidium iodide (50 μg/mL in PBS), DNA content was analyzed using a FACS Calibur flow cytometer (BD Biosciences).
2.6. ELISA for collagen and growth factors
NIH/3T3 cells were plated into each well of 24-well plates and cultured for 24 h. Then, DMEM containing different concentrations of GTP or 50 ng/mL bFGF was added, and the cells were cultured for 48 h. The content of type I and III collagens in the supernatant and intracellular TGF-β1 and VEGF levels were measured using commercially available ELISA kits according to the manufacturer’s protocol.
2.7. Wound healing assay
Wound healing assay was performed as previously reported [18] with some modifications. Fibroblasts kept in serum-free medium for 24 h were wounded with a plastic micropipette tip with a large orifice. After washing, medium was replaced by control medium with GTP or bFGF. Photographs of the wounded area were taken after 12 h or 24 h by a cell imaging multifunctional test system Cytation 3 (Bio-Tek Instruments, Winooski, VT, USA).
2.8. Western blotting
Western blot analysis was performed as previously reported [19], with some modifications. Briefly, cells were lysed with Triton X-100-based lysis buffer for 30 min on ice and centrifuged at 10,000g for 10 min at 4°C. Protein concentration was determined using the Bradford method. Equal amounts of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12%) and transferred to a PVDF membrane (GE Healthcare, PA, USA). Membranes were blocked with 5% (w/v) nonfat milk for 1 h and incubated with the following primary antibodies: cyclin A, p-ERK1/2, ERK1/2, and β-actin overnight, at 4°C. After the incubation with secondary antibody for 1 h at room temperature, proteins were detected using Enhanced Chemiluminescence Western blotting Detection System Plus (GE Healthcare).
2.9. Immunofluorescence staining
After the treatment with GTP, cells were fixed with 3% formaldehyde in PBS, permeabilized with 70% ethanol, and incubated with anti-TGF-β1, VEGF, and p-ERK1/2 antibodies in a microscopy buffer (2% bovine serum albumin and 0.1% Triton X-100 in PBS), followed by fluorescein isothiocynate conjugated secondary antibodies. The nucleus was stained with DAPI (4′,6-diamidino-2-phenylindole). Image acquisition and postprocessing were performed with a cell imaging multifunctional test system Cytation 3.
2.10. Statistical analysis
All values are shown as the mean ± standard deviations from three different experiments. Statistical comparisons between controls and treated experimental groups were performed using Student t test. Statistical evaluation was performed using GraphPad prism, version 5.0 (Graphpad Software, San Diego, CA, USA). We considered p < 0.05 to be statistically significant.
3. Results
3.1. GTP, but not protein hydrolysate, promotes NIH/3T3 cells proliferation
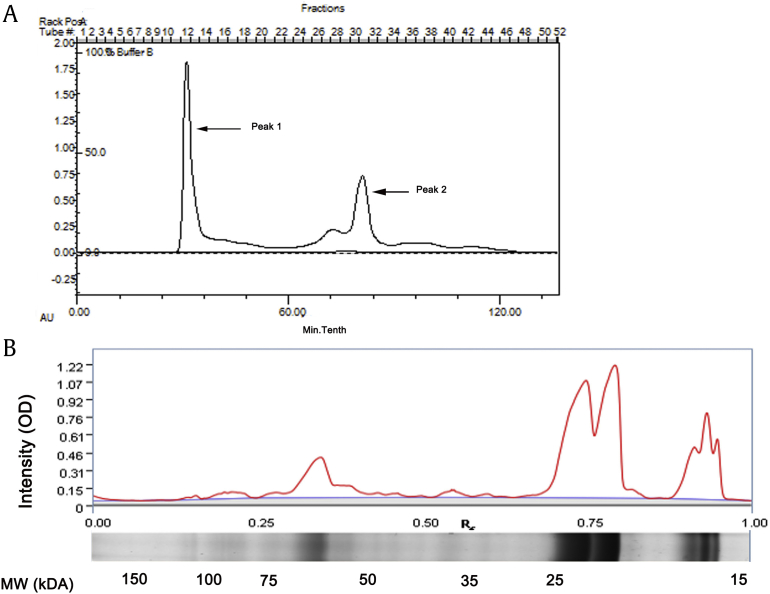
To investigate the function of GTP in NIH/3T3 cells function, specifically cell proliferation and collagen secretion, we first purified GTP and identified the purity and content using gel chromatograms (Fig. 1A) and SDS-PAGE (Fig. 1B) analysis. In Fig. 1A, peak 1 is GTP, and peak 2 may be nucleic acid. GTP was composed of protein subunits with molecular weights of about 16 kDa, 22 kDa, and 68 kDa, of which the concentration of the 22-kDa protein subunit was the highest.
Fig. 1.
Purification and identification of ginseng total protein (GTP). (A) Gel chromatogram of ginseng protein, the left peak represent GTP, the right peak was nucleic acid impurity. (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of ginseng total protein.
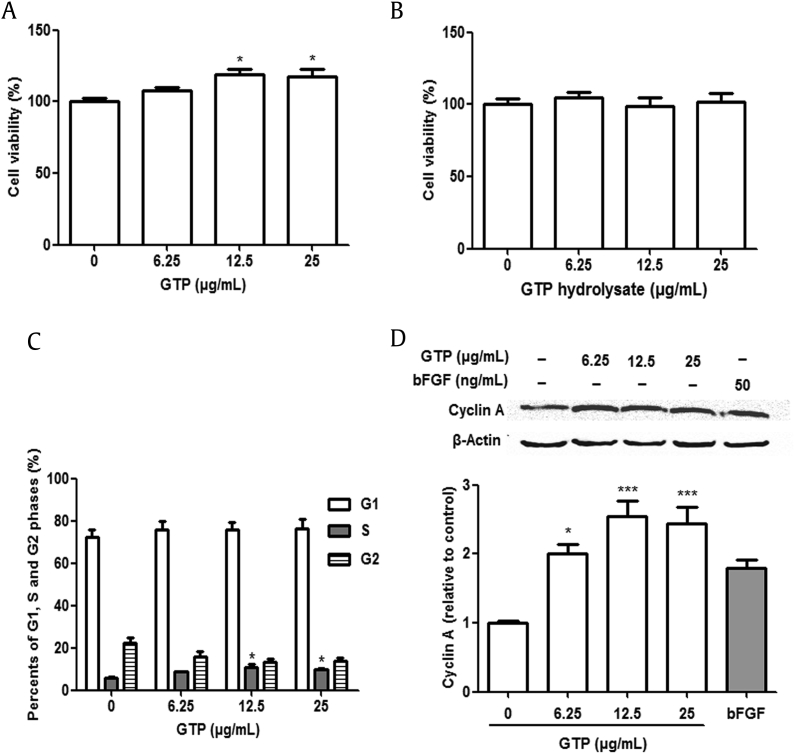
To further test our rationale that GTP had an important role in cell biological functions, we also observed the effect of hydrolyzed GTP by proteinase K on cell biological functions. Through a series of experiments, we found that cell viability was significantly enhanced with GTP compared with control (Fig. 2A). However, we did not find any influence of GTP hydrolysate on the viability of NIH/3T3 cells (Fig. 2B). These results indicated that the effect of promoting NIH/3T3 cell proliferation was attributed to GTP, but not to GTP hydrolysate.
Fig. 2.
Effects of GTP on proliferation and cell cycle distribution of NIH/3T3 cells. Cell viability was assessed after 24 h of treatment with GTP and its hydrolysate, as described in Materials and methods. (A) GTP. (B) GTP hydrolysate. (C) GTP accelerated the percentage of NIH/3T3 cells in S phase and G2 phase. (D) Western blotting analysis for the expression of cyclin A in NIH/3T3 cells after 48 h at different concentrations of GTP (6.25 μg/mL, 12.5 μg/mL, and 25 μg/mL). Values represent the mean ± SD of three independent experiments (significant at *p < 0.05 and **p < 0.01 against respective controls). GTP, ginseng total protein; SD, standard deviation.
We further tested the effect of GTP on cell cycle distribution using flow cytometric analysis. After the treatment of GTP, the percentage of NIH/3T3 cells in S phase was increased, whereas the proportion of cells in G2 phase was decreased compared with the control. At the concentration of 12.5 μg/mL GTP, the percentage of cells in the S phase was 1.9-fold higher than that of control. These results demonstrated that GTP could promote cell cycle progression by increasing the percentage of cells in the S phase (Fig. 2C).
Cyclin A is the main regulator of S phase progression; therefore, we examined the effect of GTP on the expression of cyclin A. Western blot analysis showed that GTP remarkably increased cyclin A, which was better than bFGF, as a positive control (Fig. 2D). The results suggest that GTP promoted cell cycle progression through the upregulation of cyclin A.
3.2. GTP increased secretion of type I collagen and promoted wound healing in NIH/3T3 cells
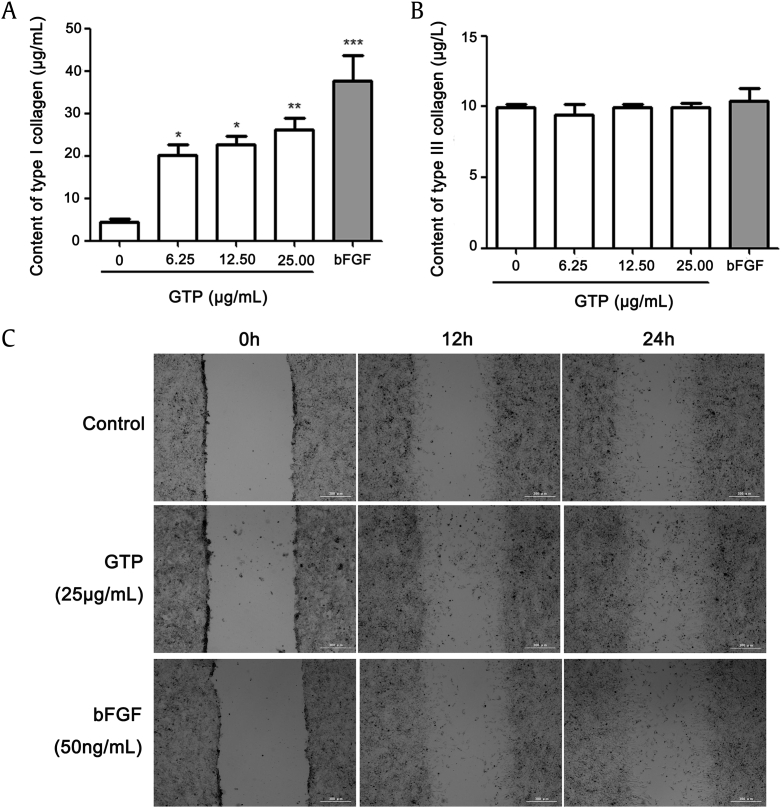
Collagens, ECM proteins, are the main regulators in the formation of granulation and tissue reconstruction; therefore, they play a vital role in tissue repair [20], [21]. We examined the effects of GTP on collagen secretion by measuring the level of type I and type III collagens using ELISA kits. As shown in Fig. 3A and B, GTP significantly increased the secretion of type I collagen in a dose-dependent manner, but exerted no change on the secretion of type III collagen. To further examine the effect of GTP on wound healing in fibroblasts, NIH/3T3 cells were scratched by plastic micropipette and then cultured for 12 h or 24 h under non-, GTP-, or bFGF-treated conditions. As shown in Fig. 3C, the wound-healing abilities of fibroblasts were markedly increased by GTP and bFGF compared to nontreated conditions. These data suggest that GTP promoted wound healing by increasing the secretion of type I collagen.
Fig. 3.
Effects of GTP on the secretion of type I collagen and wound healing in NIH/3T3 cells. Level of collagen was measured in cellular supernatant after 48 h of GTP treatment at different concentrations (6.25 μg/mL, 12.5 μg/mL, and 25 μg/mL) and bFGF (50 ng/mL) treatment. (A) Type I collagen. (B) Type III collagen. (C) Cell were scratched with plastic micropipette and cultured with control medium with GTP or bFGF for 12 h or 24 h. Photographs of the wounded area were taken. Results are expressed as means ± SEM (n = 3); *p < 0.05, **p < 0.01 versus control. bFGF, basic fibroblast growth factor; GTP, ginseng total protein; SEM, standard error of the mean.
3.3. Effects of GTP on expressions of TGF-β1 and VEGF in NIH/3T3 cells
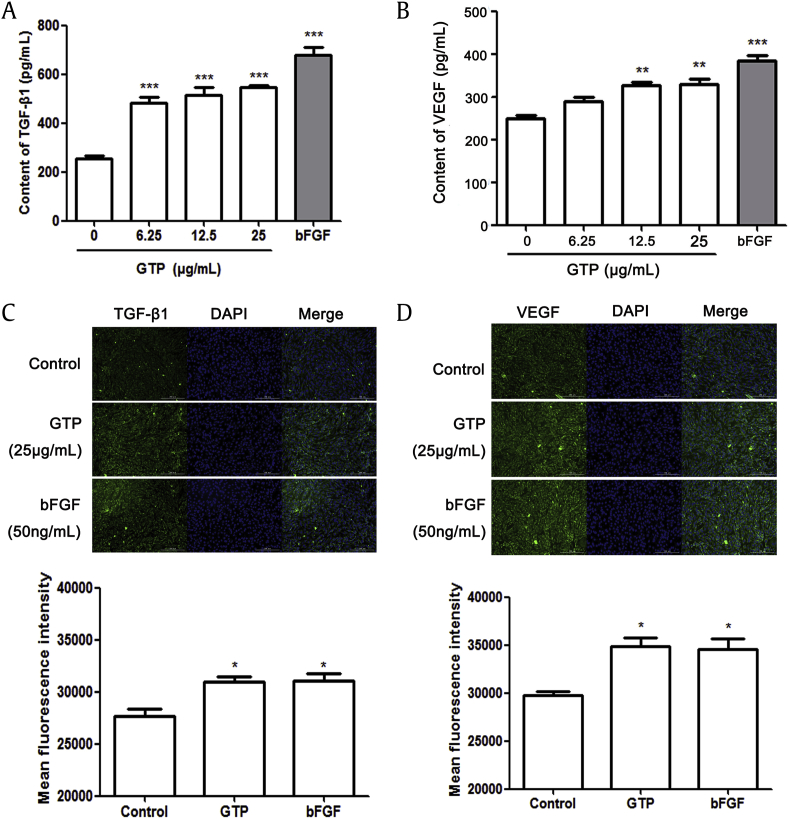
It has been reported that the synthesis of collagen was regulated by TGF-β1 and VEGF [22], [23], [24], [25]. Therefore, the effect of GTP on the expression of TGF-β1 and VEGF was observed and analyzed by ELISA kit and immunofluorescence staining. As shown in Fig. 4A and B, ELISA analysis showed that GTP treatment led to a significant increase in the expression of TGF-β1 and VEGF. Moreover, similar results on the expression of TGF-β1 and VEGF induced by GTP were observed via fluorescence staining, which was similar to cells stimulated by bFGF (Fig. 4C and D). These data indicated that GTP induced the secretion of type I collagen by increasing the expression of TGF-β1 and VEGF.
Fig. 4.
Effects of GTP on the expression of TGF-β1 and VEGF in NIH/3T3 cells. Levels of TGF-β1 and VEGF were measured in NIH/3T3 cells after 48 h of treatment using different concentrations of GTP (6.25 μg/mL, 12.5 μg/mL, and 25 μg/mL) and bFGF (50 ng/mL): (A) TGF-β1. (B) VEGF. The expression of TGF-β1 and VEGF was determined by immunofluorescence staining. Cells were fixed and stained with anti-TGF-β1 or VEGF (green) and nucleus was stained by DAPI (blue): (C) TGF-β1. (D) VEGF. The fluorescence intensity for TGF-β1 and VEGF is also shown. Results are expressed as means ± SEM (n = 3); *p < 0.05, **p < 0.01 versus control. bFGF, ; DAPI, 4′,6-diamidino-2-phenylindole; GTP, ginseng total protein; SEM, standard error of the mean; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
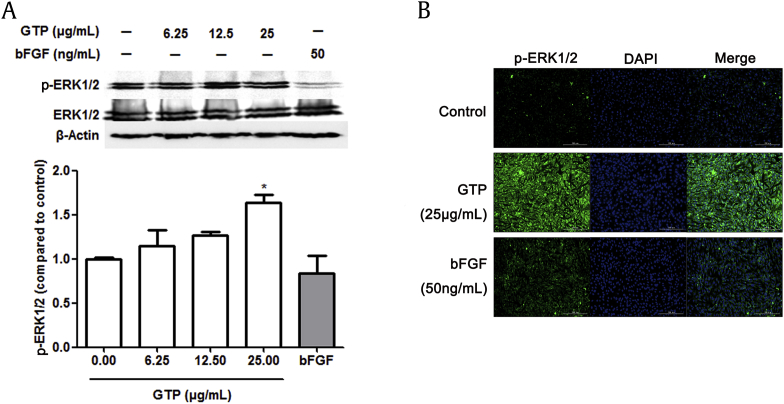
3.4. Effects of GTP on phosphorylation of ERK1/2 in NIH/3T3 cells
Our experimental results described above have shown that GTP enhanced cell proliferation and promoted the secretion of type I collagen in NIH/3T3 cells. MAPK/ERK signaling is an important pathway that affects cell proliferation, and can be activated by growth factors such as TGF-β1 [26], [27], [28]. Because our data showed that GTP induced the expression of TGF-β1, we therefore examined the effect of GTP on ERK signaling pathway by Western blotting and immunofluorescence staining. As shown in Fig. 5A and B, GTP induced an obvious increase in the phosphorylation of ERK1/2 at the concentration of 25 μg/mL. However, bFGF had no effect on the phosphorylation of ERK1/2 in NIH/3T3cells. These results indicated that GTP promoted the phosphorylation of ERK1/2 at Thr202/Tyr204 in NIH/3T3 cells.
Fig. 5.
Effects of GTP on the phosphorylation of ERK1/2 in NIH/3T3 cells. (A) Protein expression of p-ERK1/2 and ERK1/2 was analyzed by Western blotting in NIH/3T3 cells after 48 h of treatment using different concentrations of GTP (6.25 μg/mL, 12.5 μg/mL, and 25 μg/mL) and bFGF (50 ng/mL). (B) Expression of p-ERK1/2 at Thr202/Tyr204 was determined by immunofluorescence staining. Cells were fixed and stained with anti-p-ERK1/2 (green) and nucleus was stained by DAPI (blue). Results are expressed as means ± SEM (n = 3); *p < 0.05, **p < 0.01 versus control. bFGF, ; DAPI, 4′,6-diamidino-2-phenylindole; GTP, ginseng total protein; p-ERK1/2, phospho-extracellular signal-related kinase 1/2; SEM, standard error of the mean; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
4. Discussion
In the present study, we demonstrated for the first time the potential function of GTP in promoting cell proliferation and inducing the secretion of collagen I through the activation of ERK signaling pathway in fibroblast cells, which suggests GTP as a new natural product for promoting wound healing.
Optimum wound healing requires a well-orchestrated integration of complex biological and molecular events, including cell migration, proliferation, ECM deposition, and remodeling. Therefore, the activities of key cells are essential to mediate successful wound healing. Fibroblasts are the single most important cell type as they have numerous functions, including production of collagen, growth factors, antioxidants, and a balance of matrix-producing proteins and protease enzymes. Fibroblasts also play an essential role in initiating tissue remodeling during wound recovery.
Proliferation of fibroblast is one of the main manifestations in the process of wound healing [29], which is a normal physiological function obtained from the process of biological evolution. An important characteristic of cell proliferation is the upregulation of cyclin A and an increase of cells in the S phase of the DNA cell cycle [30]. S phase is responsible for DNA replication, and cyclin A plays an important role in the accurate separation of chromosome [31], driving G1 phase into S phase and enhancing cell proliferation. In our research, GTP increased the expression of cyclin A and the cell ratio of S phase, thus enhancing the proliferation of NIH/3T3 cells. Our results indicate that GTP may possess functions of wound repair.
Human skin fibroblasts play key roles in wound healing by inducing the secretion of collagens and cytokines [32], [33]. Collagens secreted by fibroblasts play an important role in wound healing. Once at the wound site, collagen III is quick to produce, with the early matrix acting as a barrier to pathogens and to loss of serum and fluids. This is later degraded by proteases and remodeled by the fibroblasts to be replaced by collagen I, which has a much higher tensile strength, but takes longer to deposit [34]. In our study, GTP significantly increased the secretion of collagen I, but induced no change in the expression of collagen III in NIH/3T3 cells treated with GTP for 48 h. So, we speculated that this phenomenon about the secretion of different collagens induced by GTP was dependent on the time that fibroblast cells were treated, which would be investigated in the future.
TGF-β1 regulates various cell functions such as proliferation, differentiation, apoptosis, cell adhesion, cell motility, and production of ECM [35]. Expression of TGF-β1 was upregulated when cells were treated by GTP (Fig. 4A and C), which led to significant increase of collagen I. Then, collagen bundles are typically formed and mutually cross-link, which strengthens the flexibility of the cells [36]. VEGF could supply the necessary nutrients to the tissue, which is the main determinant of tissue repair [37], [38]. In this study, we demonstrated that GTP promotes the synthesis of VEGF (Fig. 4B and D), which is involved in the process of angiogenesis, providing nutrition for tissue metabolism.
MAPK/ERK is a crucial signal transduction pathway, which is stimulated and activated by growth factors. The MAPK/ERK pathway is a well-conserved three-tiered kinase cascade, which is Raf-MEK1/2-ERK1/2, and then phosphorylated ERK enters the nucleus through translocation, and combines with downstream substrates, initiating transcription and translation [39]. MAPK/ERK participates in cell cycle regulation and proliferation [40]. It has also been reported that activated ERK can initiate transcription of genes coding for proteins involved in DNA replication, accelerating the transformation of G1 phase to S phase [41], thus promoting cell proliferation. In addition, ERK1/2 signaling can also regulate the progress of collagen biosynthesis [42]. In this study, we found that GTP enhanced the intracellular level of p-ERK1/2, thus activating the MAPK/ERK signal pathway. This suggested the possibility that GTP may increase cell proliferation and collagen synthesis in NIH/3T3 cells through the activation of the MAPK/ERK signal pathway.
In summary, our findings provide a better understanding of the function of GTP in the wound healing process and raise the possibility of therapeutic usage of GTP in wound healing.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This research was financially supported by two foundations of National Natural Science (No. 81274038 and No81373932.), a Jilin Nature Science Foundation Project (No. 20150101079JC), Science and Technology Development Plan of Jilin Province (No. 20160307027YY), Science and Technology Development Plan of Jilin City (No. 20156410), the Young and Middle-Aged Technological Innovation Leading Personnel and Team Project of Jilin Province (No. 20140519016JH).
Contributor Information
Daqing Zhao, Email: zhaodaqing1963@163.com.
Liwei Sun, Email: Sunnylilwei@163.com.
References
- 1.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. PubMed PMID: 10571242. [DOI] [PubMed] [Google Scholar]
- 2.Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol. 1996;23:728–732. doi: 10.1111/j.1440-1681.1996.tb01767.x. PubMed PMID: 8886498. [DOI] [PubMed] [Google Scholar]
- 3.Nag S.A., Qin J.J., Wang W., Wang M.H., Wang H., Zhang R. Ginsenosides as anticancer agents: in vitro and in vivo activities, structure–activity relationships, and molecular mechanisms of action. Front Pharmacol. 2012;3:25. doi: 10.3389/fphar.2012.00025. PubMed PMID: 22403544; PubMed Central PMCID: PMC3289390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y.G., Sumiyoshi M., Kawahira K., Sakanaka M., Kimura Y. Effects of Red Ginseng extract on ultraviolet B-irradiated skin change in C57BL mice. Phytother Res. 2008;22:1423–1427. doi: 10.1002/ptr.2339. PubMed PMID: 18803235. [DOI] [PubMed] [Google Scholar]
- 5.Kimura Y., Sumiyoshi M., Kawahira K., Sakanaka M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br J Pharmacol. 2006;148:860–870. doi: 10.1038/sj.bjp.0706794. PubMed PMID: 16770323; PubMed Central PMCID: PMC1617068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. PubMed PMID: 25788049. [DOI] [PubMed] [Google Scholar]
- 7.Li H., Kang T., Qi B., Kong L., Jiao Y., Cao Y. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of d-galactose/AlCl3 inducing rats model of Alzheimer's disease. J Ethnopharmacol. 2016;179:162–169. doi: 10.1016/j.jep.2015.12.020. PubMed PMID: 26721223. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Song J., Zhang J., Wang T., Yan Y., Tao Z. Ginseng protein reverses amyloid beta peptide and H2O2 cytotoxicity in neurons, and ameliorates cognitive impairment in AD rats induced by a combination of d-galactose and AlCl3. Phytother Res. 2017;31:284–295. doi: 10.1002/ptr.5747. PubMed PMID: 27981642. [DOI] [PubMed] [Google Scholar]
- 9.Ng T.B., Wang H. Panaxagin, a new protein from Chinese ginseng possesses anti-fungal, anti-viral, translation-inhibiting and ribonuclease activities. Life Sci. 2001;68:739–749. doi: 10.1016/s0024-3205(00)00970-x. PubMed PMID: 11205866. [DOI] [PubMed] [Google Scholar]
- 10.Qi B., Wang S., Wang Q., Zhang H., Bai X.Y. Characterization and immunostimulating effects on murine peritoneal macrophages of a novel protein isolated from Panax quinquefolius L. J Ethnopharmacol. 2016;193:700–705. doi: 10.1016/j.jep.2016.10.034. PubMed PMID: 27742408. [DOI] [PubMed] [Google Scholar]
- 11.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. PubMed PMID: 12843410. [DOI] [PubMed] [Google Scholar]
- 12.Gordon M.K., Hahn R.A. Collagens. Cell Tissue Res. 2010;339:247–257. doi: 10.1007/s00441-009-0844-4. PubMed PMID: 19693541; PubMed Central PMCID: PMC2997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao P., Kodra A., Tomic-Canic M., Golinko M.S., Ehrlich H.P., Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. PubMed PMID: 19027922; PubMed Central PMCID: PMC2728016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai C.F., Chaudhary L., Fausto A., Halstead L.R., Ory D.S., Avioli L.V. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–14450. doi: 10.1074/jbc.M010021200. PubMed PMID: 11278600. [DOI] [PubMed] [Google Scholar]
- 15.Juretic N., Santibanez J.F., Hurtado C., Martinez J. ERK 1,2 and p38 pathways are involved in the proliferative stimuli mediated by urokinase in osteoblastic SaOS-2 cell line. J Cell Biochem. 2001;83:92–98. doi: 10.1002/jcb.1211. PubMed PMID: 11500957. [DOI] [PubMed] [Google Scholar]
- 16.Craxton A., Shu G., Graves J.D., Saklatvala J., Krebs E.G., Clark E.A. p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. J Immunol. 1998;161:3225–3236. PubMed PMID: 9759836. [PubMed] [Google Scholar]
- 17.Hong N.Y., Cui Z.G., Kang H.K., Lee D.H., Lee Y.K., Park D.B. p-Synephrine stimulates glucose consumption via AMPK in L6 skeletal muscle cells. Biochem Biophys Res Commun. 2012;418:720–724. doi: 10.1016/j.bbrc.2012.01.085. PubMed PMID: 22306011. [DOI] [PubMed] [Google Scholar]
- 18.Yeom C.H., Lee G., Park J.H., Yu J., Park S., Yi S.Y. High dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J Transl Med. 2009;7:70. doi: 10.1186/1479-5876-7-70. PubMed PMID: 19671184; PubMed Central PMCID: PMC2732919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui R., Lu Q., Teng Y., Li K., Li N. Chitosan promoted the Corneal epithelial wound healing via activation of ERK pathway. Curr Eye Res. 2017;42(1):21–27. doi: 10.3109/02713683.2016.1145235. PubMed PMID: 27259381. [DOI] [PubMed] [Google Scholar]
- 20.Thornton M.J. Estrogens and aging skin. Dermato-endocrinology. 2013;5:264–270. doi: 10.4161/derm.23872. PubMed PMID: 24194966; PubMed Central PMCID: PMC3772914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdier-Sevrain S., Bonte F., Gilchrest B. Biology of estrogens in skin: implications for skin aging. Exp Dermatol. 2006;15:83–94. doi: 10.1111/j.1600-0625.2005.00377.x. PubMed PMID: 16433679. [DOI] [PubMed] [Google Scholar]
- 22.Liu G., Wang X., Sun X., Deng C., Atala A., Zhang Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials. 2013;34:8617–8629. doi: 10.1016/j.biomaterials.2013.07.077. PubMed PMID: 23932297. [DOI] [PubMed] [Google Scholar]
- 23.Rosano J.M., Cheheltani R., Wang B., Vora H., Kiani M.F., Crabbe D.L. Targeted delivery of VEGF after a myocardial infarction reduces collagen deposition and improves cardiac function. Cardiovasc Eng Technol. 2012;3:237–247. doi: 10.1007/s13239-012-0089-3. PubMed PMID: 22844388; PubMed Central PMCID: PMC3405981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagai N., Kumasaka N., Kawashima T., Kaji H., Nishizawa M., Abe T. Preparation and characterization of collagen microspheres for sustained release of VEGF. J Mater Sci Mater Med. 2010;21:1891–1898. doi: 10.1007/s10856-010-4054-0. PubMed PMID: 20232232. [DOI] [PubMed] [Google Scholar]
- 25.Borselli C., Ungaro F., Oliviero O., d'Angelo I., Quaglia F., La Rotonda M.I. Bioactivation of collagen matrices through sustained VEGF release from PLGA microspheres. J Biomed Mater Res A. 2010;92:94–102. doi: 10.1002/jbm.a.32332. PubMed PMID: 19165799. [DOI] [PubMed] [Google Scholar]
- 26.Berzal S., Gonzalez-Guerrero C., Rayego-Mateos S., Ucero A., Ocana-Salceda C., Egido J. TNF-related weak inducer of apoptosis (TWEAK) regulates junctional proteins in tubular epithelial cells via canonical NF-kappaB pathway and ERK activation. J Cell Physiol. 2015;230:1580–1593. doi: 10.1002/jcp.24905. PubMed PMID: 25536182. [DOI] [PubMed] [Google Scholar]
- 27.Chang H.H., Chang M.C., Wu I.H., Huang G.F., Huang W.L., Wang Y.L. Role of ALK5/Smad2/3 and MEK1/ERK signaling in transforming growth factor beta 1-modulated growth, collagen turnover, and differentiation of stem cells from apical papilla of human tooth. J Endod. 2015;41:1272–1280. doi: 10.1016/j.joen.2015.03.022. PubMed PMID: 26001858. [DOI] [PubMed] [Google Scholar]
- 28.Cheng S., Guo J., Yang Q., Han L. Crk-like adapter protein is required for TGF-beta-induced AKT and ERK-signaling pathway in epithelial ovarian carcinomas. Tumour Biol. 2015;36:915–919. doi: 10.1007/s13277-014-2724-0. PubMed PMID: 25307974. [DOI] [PubMed] [Google Scholar]
- 29.Muthusubramaniam L., Zaitseva T., Paukshto M., Martin G., Desai T. Effect of collagen nanotopography on keloid fibroblast proliferation and matrix synthesis: implications for dermal wound healing. Tissue Eng Part A. 2014;20:2728–2736. doi: 10.1089/ten.tea.2013.0539. PubMed PMID: 24724556; PubMed Central PMCID: PMC4195479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabeche L., Compton D.A. Cyclin A regulates kinetochore microtubules to promote faithful chromosome segregation. Nature. 2013;502:110–113. doi: 10.1038/nature12507. PubMed PMID: 24013174; PubMed Central PMCID: PMC3791168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahlin C., Zhou W., Holmqvist M., Holmberg L., Nilsson C., Jirstrom K. Cyclin A is a proliferative marker with good prognostic value in node-negative breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2501–2506. doi: 10.1158/1055-9965.EPI-09-0169. PubMed PMID: 19706846. [DOI] [PubMed] [Google Scholar]
- 32.Spiekstra S.W., Breetveld M., Rustemeyer T., Scheper R.J., Gibbs S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Regen. 2007;15:708–717. doi: 10.1111/j.1524-475X.2007.00280.x. PubMed PMID: 17971017. [DOI] [PubMed] [Google Scholar]
- 33.Hunt T.K., Burke J., Barbul A., Gimbel M.L. Wound healing. Science. 1999;284:1775. doi: 10.1126/science.284.5421.1773d. PubMed PMID: 10391796. [DOI] [PubMed] [Google Scholar]
- 34.Witte M.B., Barbul A. General principles of wound healing. Surgical Clin North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. PubMed PMID: 9194878. [DOI] [PubMed] [Google Scholar]
- 35.Kane C.J., Hebda P.A., Mansbridge J.N., Hanawalt P.C. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991;148:157–173. doi: 10.1002/jcp.1041480119. PubMed PMID: 1907288. [DOI] [PubMed] [Google Scholar]
- 36.Oliver N., Sternlicht M., Gerritsen K., Goldschmeding R. Could aging human skin use a connective tissue growth factor boost to increase collagen content? J Invest Dermatol. 2010;130:338–341. doi: 10.1038/jid.2009.331. PubMed PMID: 20081886. [DOI] [PubMed] [Google Scholar]
- 37.Figueiredo A., Cordeiro A.L., Tomada N., Tomada I., Rodrigues A., Gouveia A. Real-time PCR study of Ang1, Ang2, Tie-2, VEGF, and KDR expression in human erectile tissue during aging. J Sexual Med. 2011;8:1341–1351. doi: 10.1111/j.1743-6109.2010.02116.x. PubMed PMID: 21091880. [DOI] [PubMed] [Google Scholar]
- 38.Muche A., Bigl M., Arendt T., Schliebs R. Expression of vascular endothelial growth factor (VEGF) mRNA, VEGF receptor 2 (Flk-1) mRNA, and of VEGF co-receptor neuropilin (Nrp)-1 mRNA in brain tissue of aging Tg2576 mice by in situ hybridization. Int J Dev Neurosci. 2015;43:25–34. doi: 10.1016/j.ijdevneu.2015.03.003. PubMed PMID: 25797338. [DOI] [PubMed] [Google Scholar]
- 39.Sebolt-Leopold J.S., Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. PubMed PMID: 15573115. [DOI] [PubMed] [Google Scholar]
- 40.Chambard J.C., Lefloch R., Pouyssegur J., Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. PubMed PMID: 17188374. [DOI] [PubMed] [Google Scholar]
- 41.Tamemoto H., Kadowaki T., Tobe K., Ueki K., Izumi T., Chatani Y. Biphasic activation of two mitogen-activated protein kinases during the cell cycle in mammalian cells. J Biol Chem. 1992;267:20293–20297. PubMed PMID: 1400347. [PubMed] [Google Scholar]
- 42.Szoka L., Karna E., Palka J. The mechanism of oxythiamine-induced collagen biosynthesis in cultured fibroblasts. Mol Cell Biochem. 2015;403:51–60. doi: 10.1007/s11010-015-2336-z. PubMed PMID: 25626895; PubMed Central PMCID: PMC4383821. [DOI] [PMC free article] [PubMed] [Google Scholar]