Abstract
Background
Korean Red Ginseng (KRG) is an ethnopharmacological plant that is traditionally used to improve the body’s immune functions and ameliorate the symptoms of various diseases. However, the antitumorigenic effects of KRG and its underlying molecular and cellular mechanisms are not fully understood in terms of its individual components. In this study, in vitro and in vivo antitumorigenic activities of KRG were explored in water extract (WE), saponin fraction (SF), and nonsaponin fraction (NSF).
Methods
In vitro antitumorigenic activities of WE, SF, and NSF of KRG were investigated in the C6 glioma cell line using cytotoxicity, migration, and proliferation assays. The underlying molecular mechanisms of KRG fractions were determined by examining the signaling cascades of apoptotic cell death by semiquantitative reverse transcriptase polymerase chain reaction and Western blot analysis. The in vivo antitumorigenic activities of WE, SF, and NSF were investigated in a xenograft mouse model.
Results
SF induced apoptotic death of C6 glioma cells and suppressed migration and proliferation of C6 glioma cells, whereas WE and NSF neither induced apoptosis nor suppressed migration of C6 glioma cells. SF downregulated the expression of the anti-apoptotic gene B-cell lymphoma-2 (Bcl-2) and upregulated the expression of the pro-apoptotic gene Bcl-2-associated X protein (BAX) in C6 glioma cells but had no effect on the expression of the p53 tumor-suppressor gene. Moreover, SF treatment resulted in activation of caspase-3 as evidenced by increased levels of cleaved caspase-3. Finally, WE, SF, and NSF exhibited in vivo antitumorigenic activities in the xenograft mouse model by suppressing the growth of grafted CT-26 carcinoma cells without decreasing the animal body weight.
Conclusion
These results suggest that WE, SF, and NSF of KRG are able to suppress tumor growth via different molecular and cellular mechanisms, including induction of apoptosis and activation of immune cells.
Keywords: antitumorigenic activity, Korean Red Ginseng, nonsaponin fraction, saponin fraction, xenograft mouse model
1. Introduction
Cancer is a group of diseases characterized by cells with uncontrolled growth and the potential to spread or invade into other nascent parts of the body and is a leading cause of death worldwide. Although conventional approaches, such as surgery, chemotherapy, and radiation therapy, are successful in treating certain types of cancers [1], the complex characteristics of cancer often limit the therapeutic efficacy of these conventional methods. Thus, there is an emerging need for alternative methods to improve the therapeutic efficacy of conventional treatment modalities [2]. Complementary and alternative medicine, including a wide range of both ancient and modern approaches, have recently gained significant attention for their potential to prevent and treat various diseases, including cancer [3], [4].
Herbal medicines have long been considered to be the major traditional form of therapy in many countries and are used for the prevention and treatment of a variety of diseases [5], [6]. Although many herbs are used for therapeutic purposes, ginseng is one of the most widely used herbal medicinal plants in the world [7], [8]. Panax ginseng Meyer, also known as Korean ginseng, is a perennial plant traditionally used as an herbal medicine to support vitality, promote a long and healthy life, act as a spirit supplementation, and to prevent and treat various diseases in far eastern Asian countries, such as Korea, China, and Japan [8], [9], [10]. Upon processing by steaming, fresh ginseng becomes dark red in color; thus, steamed ginseng is called red ginseng [11]. Red ginseng has been extensively studied and used to improve the condition of a variety of human diseases, and some previous studies have demonstrated that red ginseng has anticancer activity in many types of human malignant cancers [12], [13], [14], [15], [16], [17]. In addition, several other studies have suggested that red ginseng has superior anticancer activities compared to white ginseng [18], [19].
While anticancer activity of red ginseng has been reported, the specific molecular components of red ginseng responsible for these effects and their underlying molecular and cellular mechanisms of action are not yet fully understood. In this study, we obtained three fractions of Korean Red Ginseng (KRG), namely, water extract (WE), saponin fraction (SF), and nonsaponin fraction (NSF), and investigated their anticancer effects in vitro and in vivo using cultured C6 glioma cells and a xenograft mouse model.
2. Materials and methods
2.1. Materials
WE, NSF, and SF of KRG were provided by the Korea Ginseng Cooperation (Daejeon, Korea). C6 glioma and CT-26 carcinoma cells were purchased from American Type Culture Collection (Manassas, VA, USA). Dulbecco's Modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), streptomycin, penicillin, and L-glutamine were purchased from Gibco (Grand Island, NY, USA). Male Balb/c mice (age, 6–8 wk; weight, 17–21 g) were purchased from Orient Bio (Gyeonggi, Korea). Annexin V-FITC, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), propidium iodide (PI), and staurosporine were purchased from Sigma Chemical Co. (St Louis, MO, USA). TRI reagent was purchased from Molecular Research Center Inc. (Cincinnati, OH, USA). MuLV reverse transcriptase was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Polymerase chain reaction (PCR) premix and the primers used for semiquantitative reverse transcription PCR (RT-PCR) were obtained from Bioneer Inc. (Daejeon, Korea). The antibodies used in this study were purchased from Cell Signaling Technology (Beverly, MA, USA). The enhanced chemiluminescence system was purchased from AbFrontier (Seoul, Korea).
2.2. Mice
Male Balb/c mice (age, 6–8 wk; weight, 17–21 g) were maintained in mouse cages under standard conditions. Water and food (Samyang, Daejeon, Korea) were supplied ad libitum. Studies were performed according to the guidelines established by the Institutional Animal Care and Use Committee at Sungkyunkwan University, Suwon, Korea.
2.3. Cell culture
C6 glioma and CT-26 carcinoma cells were cultured in DMEM containing 10% heat-inactivated FBS, glutamine, and antibiotics (penicillin and streptomycin) at 37°C in a 5% CO2-humidified incubator. Cell culture media was refreshed every 2 d, and the cells were maintained by splitting twice a week.
2.4. Cell morphology changes
The morphology of C6 glioma cells treated with WE, SF, and NSF for 12 h was analyzed by imaging with an inverted phase contrast microscope equipped with a video camera (COUH, CA, USA).
2.5. Confocal microscopy—Hoechst staining
Confocal microscopic analysis of nuclei was performed using C6 glioma cells, as previously described [20].
2.6. Cell death assay by flow cytometry analysis
C6 glioma cell death was analyzed by Annexin V and PI staining [21]. C6 glioma cells treated with WE, SF, and NSF for 24 h were washed thrice with staining buffer (2% rabbit serum and 1% sodium azide in PBS), followed by incubation with FITC-labeled Annexin V and PI for 45 min on ice. After washing the cells thrice with staining buffer, C6 glioma cell death was determined using a FACScan flow cytometer (Beckton-Dikinson, CA, USA).
2.7. Cell migration assay
Migration assays for C6 glioma cells were performed, as previously described [22]. Briefly, C6 glioma cells were grown to a confluent monolayer in 12-well cell culture plates, and a wound was generated by scraping the monolayer of cells using a p200 pipette tip. Phase contrast images were acquired with an inverted microscope (Olympus, Tokyo, Japan).
2.8. Determination of in vitro tumorigenic responses by proliferation assay
Tumorigenic responses of C6 glioma cells were determined by measuring cell proliferation, as previously described [22], [23]. Briefly, C6 glioma cells were treated with WE, SF, and NSF for 72 h, and cell viability was determined every 24 h by conventional MTT assay.
2.9. mRNA analysis by semiquantitative RT-PCR
C6 glioma cells were treated with WE, NSF, and SF for 6 h, and total RNA was isolated from the cells with TRI reagent, according to the manufacturer’s instructions. cDNA was synthesized from 1 μg of total RNA using MuLV reverse transcriptase, according to the manufacturer’s instruction, and semiquantitative RT-PCR reactions were conducted using primers specific for Bcl-2, BAX, p53, and β-actin, as previously reported [24]. The primer sequences used in this study are listed in Table 1.
Table 1.
Primer sequences for semiquantitative reverse transcription PCR
| Name | Sequence (5′ to 3′) | |
|---|---|---|
| Bcl-2 | F | CACCCCTGGCATCTTCTCCTT |
| R | CACAATCCTCCCCCAGTTCACC | |
| BAX | F | ATGGCTGGGGAGACACCTGAG |
| R | CTAGCAAAGTAGAAAAGGGCAAC | |
| p53 | F | CTCTGTCATCTTCCGTCCCTTC |
| R | AGGACAGGCACAAACACGAAC | |
| â-actin | F | CGATATGGAGAAGATTTGGCACC |
| R | TACGACCAGAGGCATACAGGGAC |
BAX, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; PCR, polymerase chain reaction; F, Forward; R, Reverse.
2.10. Preparation of whole cell and nuclear lysates for Western blot analysis
C6 glioma cells were treated with WE, NSF, SF, and staurosporine for 24 h, and whole cell lysates were prepared, as previously described [24]. Western blot analysis was performed, as previously reported [24], using antibodies specific for pro-caspase-3, cleaved caspase-3, Bcl-2, and β-actin.
2.11. Determination of in vivo tumorigenic responses by xenograft mouse model
CT-26 cells were grown to near confluency in 100-mm petri dishes. The cells were then trypsinized and gently mixed in a sterile beaker with a stir-bar. The cells were counted with a hemocytometer and diluted with DMEM to a final concentration of 10,000 cells/0.1 mL. The lower posterior trunk of mice was shaved, and 10,000 CT-26 cells in 0.1 mL of media were injected subcutaneously using a 27-gauge needle. The length and width of tumors were recorded using a digital caliper, and the volume of each tumor was determined using the following formula: volume = length (largest measurement)/2 × (width)2. Tumor size and body weight of the mice were measured every 3 d starting on Day 6 after injection of CT-26 cells. Mice were sacrificed on Day 18 after obtaining final measurements of tumor volumes and body weights.
2.12. Statistical analysis
All data in this paper are presented as the mean ± standard deviation of an experiment performed with six or three replicates. For statistical comparisons, these results were analyzed using Kruskal–Wallis/Mann–Whitney U tests. A p value < 0.05 was considered statistically significant. All statistical tests were performed using the computer program SPSS version 22.0 (2013; IBM Corp., Armonk, NY, USA).
3. Results and discussion
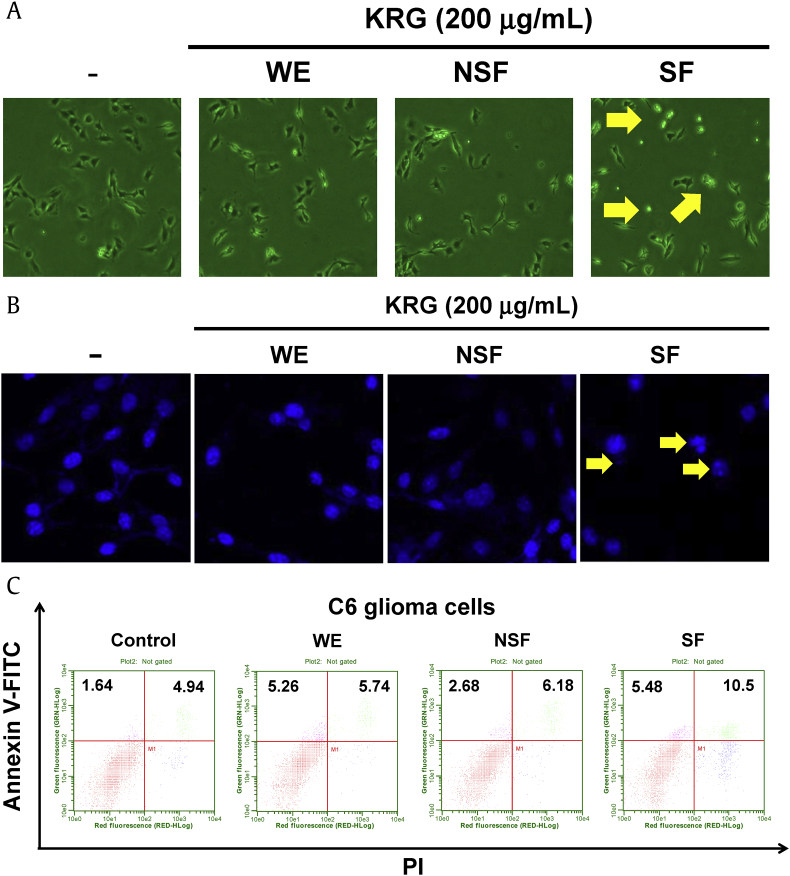
In this study, the role of KRG-derived fractions (WE, SF, and NSF) in antitumorigenic response was explored both in vitro and in vivo using C6 glioma cells and a xenograft mouse model. First, the cytotoxic effects of WE, SF, and NSF were investigated. C6 glioma cells were treated with WE, SF, or NSF, and their morphologies were observed. As shown in Fig. 1A, the morphology of C6 glioma cells treated with SF was shrunken, and the cells appeared dead, while the morphologies of C6 glioma cells treated with WE or NSF remained unchanged. Although SF altered the morphology of C6 glioma cells, a change in cell morphology is not necessarily indicative of cell death. Other representative characteristics of cell death are nuclear fragmentation and chromatin condensation. Therefore, to examine whether SF induced nuclear fragmentation and chromatin condensation, C6 glioma cells treated with SF were subjected to Hoechst staining. As shown in Fig. 1B, treatment of C6 glioma cells with SF resulted in nuclear fragmentation and chromatin condensation, while no changes in nuclear morphology were observed for C6 glioma cells treated with WE or NSF. To further examine cell death, flow cytometry analysis was performed in cells treated with KRG extracts and subjected to Annexin V/PI staining. Early apoptotic cell death (Annexin V+/PI–) was induced in C6 glioma cells treated with WE and SF, while late apoptotic cell death (Annexin V+/PI+) was markedly induced in C6 glioma cells treated with SF alone (Fig. 1C). On its own, NSF induced only weak early and late apoptosis compared to control (Fig. 1C). These results indicate that SF, but not WE and NSF, is responsible for the cytotoxic effect of KRG in C6 glioma cells and is mediated by induction of apoptosis.
Fig. 1.
Effects of WE, NSF, and SF of KRG on C6 glioma cell death. (A) C6 glioma cells were treated with either WE, NSF, or SF (200 μg/mL) for 12 h and then imaged for analysis of cell morphologies. Images of cells were obtained using an inverted phase contrast microscope equipped with a video camera. (B) C6 glioma cells were treated with either WE, NSF, or SF (200 μg/mL) for 24 h, and the nuclear morphology of cells was analyzed by Hoechst staining using a confocal microscope. (C) C6 glioma cells were treated with either WE, NSF, or SF (200 μg/mL) for 24 h, and the death of C6 glioma cells was analyzed by Annexin V and PI staining using a flow cytometer. Arrows indicate dead cells. KRG, Korean Red Ginseng; NSF, nonsaponin fraction; PI, propidium iodide; SF, saponin fraction; WE, water extract.
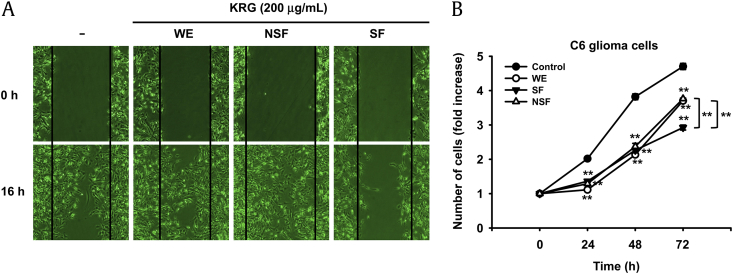
Increased cell migration and uncontrolled proliferation are representative characteristics of malignant tumor cells. Thus, we were next interested in determining the effects of WE, SF, and NSF on cell migration and proliferation. To this end, C6 glioma cells were treated with WE, SF, or NSF, and cell migration was determined by cell migration assay. As expected, SF dramatically suppressed the migratory activity of C6 glioma cells, while neither WE nor NSF suppressed C6 glioma cell migration (Fig. 2A). The antiproliferative effects of WE, SF, and NSF were also examined by treating C6 glioma cells and measuring the effect on proliferation by MTT assay. Interestingly, all three compounds markedly suppressed the proliferative activity of C6 glioma cells, and the antiproliferative effects of WE, SF, and NSF were comparable up to 48 h (Fig. 2B). However, SF had better antiproliferative effects than WE and NSF beyond 48 h (Fig. 2B). These results demonstrate that although WE and NSF inhibited the proliferation of C6 glioma cells, only SF significantly suppressed both migration and proliferation of C6 glioma cells. Moreover, the finding that WE and NSF suppressed only the proliferation but not the migration of C6 glioma cells suggests that WE, SF, and NSF have different molecular mechanisms of antitumorigenic responses.
Fig. 2.
Effects of WE, NSF, and SF of KRG on migration and proliferation of C6 glioma cells. (A) C6 glioma cells were scratched as described in Materials and methods section and treated with either WE, NSF, or SF (200 μg/mL) for 16 h. The migration patterns of treated cells were imaged using an inverted microscope. (B) C6 glioma cells were treated with either WE, NSF, or SF (100 μg/mL) for the indicated times, and viable cells were quantified by MTT assay. **p < 0.01 compared to control. KRG, Korean Red Ginseng; NSF, nonsaponin fraction; SF, saponin fraction; WE, water extract.
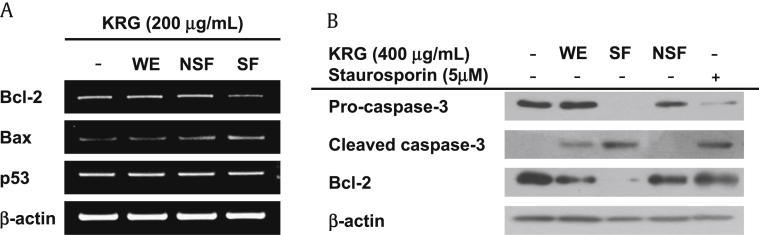
Apoptosis is a complex process of programmed cell death that occurs in multicellular organisms. As described above, apoptosis can be characterized by changes in cell morphology including cell shrinkage, nuclear fragmentation, chromatin condensation, chromosomal DNA fragmentation, and ultimately cell death. The biochemical and molecular processes involved in apoptosis are tightly regulated by a variety of genes, including mitochondrial Bcl-2 family proteins, caspases, and tumor-suppressor genes. Numerous anti-apoptotic genes have been identified, including Bcl-2, Bcl-xL, and Bcl-w, as well as pro-apoptotic genes, including BAX, BAD, BAK, and BOK. Considering that SF induced apoptotic cell death (Fig. 1), we next investigated the molecular mechanisms of SF-mediated apoptotic cell death at the transcriptional level using C6 glioma cells. Specifically, C6 glioma cells were treated with WE, SF, or NSF, and the levels of expression of the anti-apoptotic gene Bcl-2 and pro-apoptotic gene BAX were examined. As shown in Fig. 3A, SF downregulated the mRNA expression of Bcl-2 in C6 glioma cells, while WE and NSF had no effect on Bcl-2 expression. On the other hand, the mRNA expression of BAX was slightly upregulated by SF in C6 glioma cells, while WE and NSF did not have an effect on BAX expression. (Fig. 3A). To examine whether tumor-suppressor genes play a role in SF-mediated apoptotic cell death, the mRNA expression of the representative tumor-suppressor gene p53 was examined in the C6 glioma cells treated with SF. As shown in Fig. 3A, although the mRNA expression of mitochondrial Bcl-2 family genes was altered by SF, the mRNA expression of p53 was not changed by treatment with WE, SF, or NSF. Taken together, these results indicate that SF-induced cell death and suppression of cell proliferation is mediated through the induction of apoptotic signaling rather than modulation of tumor-suppressor gene expression.
Fig. 3.
Molecular mechanism of SF-mediated apoptotic death of C6 glioma cells. (A) C6 glioma cells were treated with either WE, NSF, or SF (200 μg/mL) for 6 h, and mRNA levels of Bcl-2, BAX, and p53 were determined by semiquantitative PCR. (B) C6 glioma cells were treated with either WE, NSF, SF (200 μg/mL), or staurosporine (5μM) for 24 h, and protein expression levels of pro-caspase-3, cleaved caspase-3, and Bcl-2 were determined by Western blot analysis. BAX, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; NSF, nonsaponin fraction; SF, saponin fraction; WE, water extract.–
The cysteine-aspartic acid protease (caspases) protein family plays a pivotal role in various forms of cell death, such as apoptosis, pyroptosis, and non-programmed death such as necrosis via their sequential activation [25], [26], [27]. During apoptotic cell death, two types of caspases are critically involved. The first type, the initiator caspases, includes caspase-8, -9, and -10, while the second type, the executioner caspases, includes caspase-3 [25], [26], [27], [28]. Activation of initiator caspases leads to a chain reaction culminating in the activation of executioner caspases, followed by degradation of over 600 cellular components that ultimately facilitate apoptotic cell death [28], [29], [30]. To further determine whether SF-mediated apoptotic death of C6 glioma cells was mediated through caspase activation, the activation of caspase-3 was examined in C6 glioma cells treated with SF. As expected, SF treatment induced the cleavage of pro-caspase-3 to active cleaved caspase-3 in C6 glioma cells (Fig. 3B). In addition, treatment with WE also slightly induced the activation of caspase-3, while NSF did not induce the activation of caspase-3 (Fig. 3B). The protein expression level of Bcl-2 was also examined in C6 glioma cells treated with SF. Consistent with our mRNA expression results, SF markedly reduced the expression of Bcl-2 in C6 glioma cells, while WE and NSF did not have significant suppressive effects on Bcl-2 expression (Fig. 3B). These results strongly suggest that SF induces apoptotic cell death of C6 glioma cells by activating caspase-mediated apoptotic signaling.
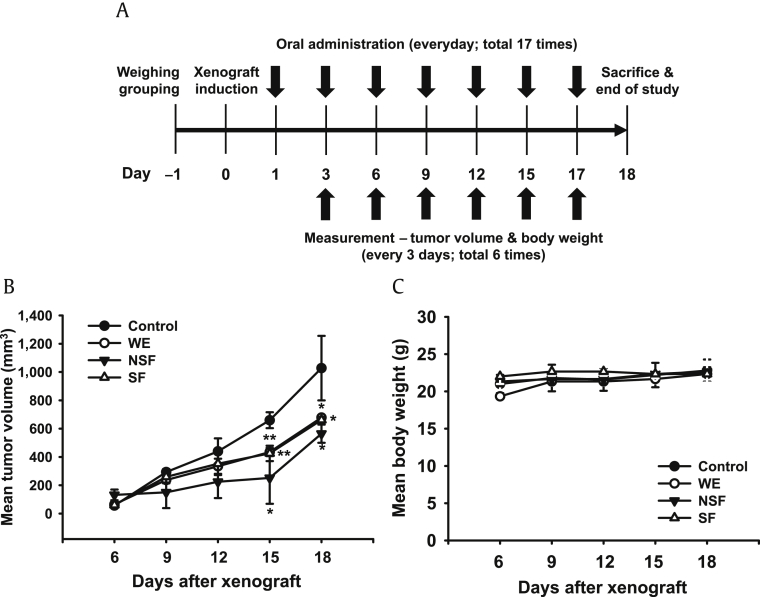
The in vitro studies described above supported the antitumorigenic effects of SF and provided insight into the molecular mechanisms of its antitumorigenic activity in C6 glioma cells. However, studies of the in vivo antitumorigenic effects of SF in animal models were considered essential to confirm the results of the present in vitro studies and to establish a basis for developing efficacious and safe anticancer therapeutics from SF. Therefore, the in vivo antitumorigenic effect of SF was next explored using a mouse xenograft model. A number of previous studies have demonstrated that oral administration of ginseng or its metabolites/derivatives provide a reasonable route for in vivo animal studies; thus, mice with CT-26 xenograft tumors were treated with oral WE, SF, or NSF, and their antitumorigenic effect were investigated according to the schedule depicted in Fig. 4A. WE, SF, and NSF significantly suppressed the growth of xenograft tumors in mice compared with control animals (Fig. 4B), while the body weight was comparable among groups (Fig. 4C). These in vivo results indicate that oral administration of WE, SF, and NSF produces antitumor activity without significant toxicity. Thus, SF could be a potential antitumorigenic agent to treat cancers with a good efficacy and low toxicity profile.
Fig. 4.
In vivo antitumorigenic effects of WE, NSF, and SF in xenograft mice. (A) Schedule for evaluating the antitumorigenic effects of WE, NSF, and SF in a mouse xenograft mouse model. Mice were subcutaneously injected with 10,000 CT-26 cells in a volume of 0.1 mL. (B) Tumors volumes were determined using digital calipers every 3 d for 18 d. (C) Body weight was measured every 3 d for 18 d. *p < 0.05, **p < 0.01 compared with control cells.
In summary, the present results showed that SF of KRG exhibited antitumorigenic activity by inducing apoptotic signaling cascades in C6 glioma cells and suppressed the growth of tumor cells in a xenograft mouse model. Interestingly, although all three fractions (WE, SF, and NSF) significantly reduced the growth of tumor cells in mice without any toxicity (Figs. 4B and 4C), only SF exerted significant antitumorigenic activity in C6 glioma cells, as evidenced by induction of apoptotic death, suppression of migration, regulation of the expression of apoptosis-related genes, and activation of apoptotic caspase (Fig. 1, Fig. 2, Fig. 3). However, WE, SF, and NSF all inhibited the proliferation of C6 glioma cells (Fig. 2B). Together, these results strongly indicate that although WE, SF, and NSF all have antitumorigenic activities in vivo, the molecular and cellular mechanisms by which they inhibit tumor cells are different. Thus, further studies will be necessary to determine how WE, SF, and NSF modulate tumor cell responses at the molecular and cellular level. In conclusion, among WE, SF, and NSF of KRG, only SF exhibited in vitro and in vivo antitumor activity in C6 glioma glioma cells and a xenograft animal model. Thus, SF could be developed as an efficacious and safe drug to treat cancer.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
This study was supported by a grant from the Korean Ginseng Society (2015).
Contributor Information
Jong-Hoon Kim, Email: jhkim1@chonbuk.ac.kr.
Jae Youl Cho, Email: jaecho@skku.edu.
References
- 1.Rhee M.Y., Cho B., Kim K.I., Kim J., Kim M.K., Lee E.K., Kim H.J., Kim C.H. Blood pressure lowering effect of Korea ginseng derived ginseol K-g1. Am J Chin Med. 2014;42:605–618. doi: 10.1142/S0192415X14500396. [DOI] [PubMed] [Google Scholar]
- 2.Wang C.Z., Mehendale S.R., Yuan C.S. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leggett S., Koczwara B., Miller M. The impact of complementary and alternative medicines on cancer symptoms, treatment side effects, quality of life, and survival in women with breast cancer–a systematic review. Nutr Cancer. 2015;67:373–391. doi: 10.1080/01635581.2015.1004731. [DOI] [PubMed] [Google Scholar]
- 4.Han Y.S., Lee J.H., Lee S.H. Antitumor effects of fucoidan on human colon cancer cells via activation of Akt signaling. Biomol Ther (Seoul) 2015;23:225–232. doi: 10.4062/biomolther.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang-Lee M.K., Moss J., Yuan C.S. Herbal medicines and perioperative care. JAMA. 2001;286:208–216. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- 6.Kim M.S., Lee Y., Sung G.H., Kim J.H., Park J.G., Kim H.G., Baek K.S., Cho J.H., Han J., Lee K.H. Pro-apoptotic activity of 4-isopropyl-2-(1-phenylethyl) aniline isolated from Cordyceps bassiana. Biomol Ther (Seoul) 2015;23:367–373. doi: 10.4062/biomolther.2015.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan C.S., Wei G., Dey L., Karrison T., Nahlik L., Maleckar S., Kasza K., Ang-Lee M., Moss J. Brief communication: American ginseng reduces warfarin's effect in healthy patients: a randomized, controlled trial. Ann Intern Med. 2004;141:23–27. doi: 10.7326/0003-4819-141-1-200407060-00011. [DOI] [PubMed] [Google Scholar]
- 8.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasani-Ranjbar S., Nayebi N., Larijani B., Abdollahi M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 2009;15:3073–3085. doi: 10.3748/wjg.15.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae J.K., Kim Y.J., Chae H.S., Kim D.Y., Choi H.S., Chin Y.W., Choi Y.H. Korean red ginseng extract enhances paclitaxel distribution to mammary tumors and its oral bioavailability by P-glycoprotein inhibition. Xenobiotica. 2016;17:1–10. doi: 10.1080/00498254.2016.1182233. [DOI] [PubMed] [Google Scholar]
- 13.Lee S., Park J.M., Jeong M., Han Y.M., Go E.J., Ko W.J., Cho J.Y., Kwon C.I., Hahm K.B. Korean red ginseng ameliorated experimental pancreatitis through the inhibition of hydrogen sulfide in mice. Pancreatology. 2016;16:326–336. doi: 10.1016/j.pan.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Wang C.Z., Anderson S., Du W., He T.C., Yuan C.S. Red ginseng and cancer treatment. Chin J Nat Med. 2016;14:7–16. doi: 10.3724/SP.J.1009.2016.00007. [DOI] [PubMed] [Google Scholar]
- 15.Jung S.Y., Kim C., Kim W.S., Lee S.G., Lee J.H., Shim B.S., Kim S.H., Ahn K.S. Korean red ginseng extract enhances the anticancer effects of Imatinib mesylate through abrogation p38 and STAT5 activation in KBM-5 cells. Phytother Res. 2015;29:1062–1072. doi: 10.1002/ptr.5347. [DOI] [PubMed] [Google Scholar]
- 16.Kim H., Hong M.K., Choi H., Moon H.S., Lee H.J. Chemopreventive effects of Korean red ginseng extract on rat hepatocarcinogenesis. J Cancer. 2015;6:1–8. doi: 10.7150/jca.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin X., Che D.B., Zhang Z.H., Yan H.M., Jia Z.Y., Jia X.B. Ginseng consumption and risk of cancer: a meta-analysis. J Ginseng Res. 2016;40:269–277. doi: 10.1016/j.jgr.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun T.K. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523-524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 19.Sun S., Qi L.W., Du G.J., Mehendale S.R., Wang C.Z., Yuan C.S. Red notoginseng: higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chem. 2011;125:1299–1305. doi: 10.1016/j.foodchem.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J.Y., Lee Y.G., Kim M.Y., Byeon S.E., Rhee M.H., Park J., Katz D.R., Chain B.M., Cho J.Y. Src-mediated regulation of inflammatory responses by actin polymerization. Biochem Pharmacol. 2010;79:431–443. doi: 10.1016/j.bcp.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Kim S.N., Lee H.J., Jeon M.S., Yi T., Song S.U. Galectin-9 is involved in immunosuppression mediated by human bone marrow-derived clonal mesenchymal stem cells. Immune Netw. 2015;15:241–251. doi: 10.4110/in.2015.15.5.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi Y.S., Baek K.S., Cho J.Y. L1 cell adhesion molecule induces melanoma cell motility by activation of mitogen-activated protein kinase pathways. Pharmazie. 2014;69:461–467. [PubMed] [Google Scholar]
- 23.Won K.J., Park S.W., Lee S., Kong I.K., Chae J.I., Kim B., Lee E.J., Kim D.K. A new triggering receptor expressed on myeloid cells (TREM) family member, TLT-6, is involved in activation and proliferation of macrophages. Immune Netw. 2015;15:232–240. doi: 10.4110/in.2015.15.5.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong D., Yi Y.S., Sung G.H., Yang W.S., Park J.G., Yoon K., Yoon D.H., Song C., Lee Y., Rhee M.H. Anti-inflammatory activities and mechanisms of Artemisia asiatica ethanol extract. J Ethnopharmacol. 2014;152:487–496. doi: 10.1016/j.jep.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Shalini S., Dorstyn L., Dawar S., Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodsell D.S. The molecular perspective: caspases. Oncologist. 2000;5:435–436. doi: 10.1634/theoncologist.5-5-435. [DOI] [PubMed] [Google Scholar]
- 27.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y. Caspase activation: revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Sollberger G., Strittmatter G.E., Garstkiewicz M., Sand J., Beer H.D. Caspase-1: the inflammasome and beyond. Innate Immun. 2014;20:115–125. doi: 10.1177/1753425913484374. [DOI] [PubMed] [Google Scholar]
- 30.Riedl S.J., Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]