Abstract
Background
The chemical constituents of Panax ginseng are changed by processing methods such as steaming or sun drying. In the present study, the chemical change of Panax ginseng induced by steaming was monitored in situ.
Methods
Samples were separated from the same ginseng root by incision during the steaming process, for in situ monitoring. Sampling was sequentially performed in three stages; FG (fresh ginseng) → SG (steamed ginseng) → RG (red ginseng) and 60 samples were prepared and freeze dried. The samples were then analyzed to determine 43 constituents among three stages of P. ginseng.
Results
The results showed that six malonyl-ginsenoside (Rg1, Rb1, Rb3, Rc, Rd, Rb2) and 15 amino acids were decreased in concentration during the steaming process. In contrast, ginsenoside-Rh1, 20(S)-Rg2, 20(S, R)-Rg3 and Maillard reaction product such as AF (arginine-fructose), AFG (arginine-fructose-glucose), and maltol were newly generated or their concentrations were increased.
Conclusion
This study elucidates the dynamic changes in the chemical components of P. ginseng when the steaming process was induced. These results are thought to be helpful for quality control and standardization of herbal drugs using P. ginseng and they also provide a scientific basis for pharmacological research of processed ginseng (Red ginseng).
Keywords: Chemical change, Maillard reaction products, malonyl-ginsenoside, steaming process, Panax ginseng
1. Introduction
Korean ginseng (Panax ginseng Meyer) has been considered as one of the most valuable medicinal herbs in oriental countries for over 2,000 years and is now widely used as an alternative medicine and health enhancing supplement [1]. Approximately 8,000 tons of ginseng is produced per year and it is consumed all around the world, especially in Asia, [2] because of its renowned pharmacological efficacies such as maintaining homeostasis, enhancing immune-system function, antidiabetic effects, and adjusting blood pressure [3].
In traditional oriental medicines, the processing methods of medicinal herbs play an important role in the application and usage. Generally, the main purpose of processing medicinal herbs is to transform the properties of the plants or their products to increase their pharmacological effects and reduce toxicity or side-effects. The processing methods of medicinal herbs involve special manipulations, such as toasting, steaming, cooking, and fermentation. Ginseng is mostly consumed after various types of processing. Fresh ginseng (nonprocessed ginseng) is rarely used, because it is easily decomposed due to high water content (i.e., 70–80%) and it may coexist with soil microorganisms. The most common types of processed ginseng used are white ginseng (WG) and red ginseng (RG). WG is produced by drying the fresh ginseng in sunlight, and RG is manufactured by steaming the fresh ginseng at 95–100°C for 2–3 h, then drying. Processing conditions have a great influence on the chemical constituents of ginseng, which is the reason for differences among the types of processed ginseng [4]. Therefore, many researchers have studied the chemical change of ginseng and especially constituents such as ginsenosides [5], [6], [7], [8], [9], [10], [11], phenolics [12], [13], and amino acids [14]. The research groups are always interested in the biological activity of ginseng and ginsenosides which have been generated during processing [15], [16], [17], [18]. Recently, chemometric tools, called “metabolomics” have been applied for metabolite profiling and to identify the complicated constituents of steaming-induced components and different types of ginseng [19], [20].
Although several studies have reported the chemical change of fresh and processed ginseng, there is currently limited sample preparation. In this report, in situ monitoring of chemical changes induced by steaming was performed. For the in situ analysis of chemical components in ginseng, samples were obtained from the same ginseng root after processing steps. Forty three components (ginsenosides, amino acids, free sugars, and some Maillard reaction products) were determined using various chromatographic techniques, such as ultra performance liquid chromatography photo diode array detector (UPLC-PDA), high performance liquid chromatography (HPLC) fluorescence detector, and high pressure ion chromatography pulsed amperometric detector.
2. Materials and methods
All the reagents used in this experiment were of extra pure grade. HPLC-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). All distilled water used in this experiment was purified by the Milli-Q gradient system (Millipore, Bedford, MA, USA) and the resistance value was measured as 18 MΩ prior to use.
2.1. Ginseng sample preparation
Ginseng samples (6-year-old aged P. ginseng) used in this experiment were obtained from the red ginseng manufacturing factory of Korea Ginseng Corporation (Buyeo, Chung-nam, Korea) as follows. First, each ginseng root sample was given a serial number to distinguish one from another. Before the steaming process, the washed fresh ginseng (FG) was given a longitudinal incision and one-third of the portion was separated and frozen at –80°C. The remaining two-thirds were steamed in a closed chamber at 98°C for 3 h. Immediately after steaming, the steamed ginseng (SG) was sliced in half and one portion was separated and kept frozen at –80°C. Finally, the remaining one-third piece of ginseng was dried in a chamber (65°C, 3 h) under daylight (for 13 d) to make RG. Frozen FG and SG samples were freeze-dried (Bondiro, PVTF010A, Ilshin Lab, Seoul, Korea) and RG was further dried in a dry-oven (60°C, 2 h; WiseVan, VS1202-D3, Daihan scientific, Seoul, Korea) and all samples were grinded to fine powder and stored at –20°C until used for analysis.
2.2. Analysis of ginsenosides
Ginsenoside Rg1, Re, Rf, Rh1, Rb1, Rc, Rb2, Rb3, Rd, 20(S)-Rg3, and 20(R)-Rg3 standards were purchased from Chromadex (Irvine, CA, USA) and ginsenoside 20(S)-Rg2, 20(R)-Rg2 were obtained from Ambo Institute (Seoul, Korea).
The sample was prepared in a similar manner as in our previous studies [21]. A half gram of ginseng powder was weighed in a centrifugal tube (15 mL, polypropylene single use; BioLogix Group, Jinan, Shandong, China) and shaken vigorously after the addition of 10 mL of 70% MeOH. Extraction was performed in an ultrasonic cleaner (60 Hz, Wiseclean; Daihan Scientific, Seoul, Korea) for 30 min. After ultrasonic extraction, centrifugal separation (Legand Mach 1.6R; Thermo, Frankfurt, Germany) was performed for 10 min at 3,000 rpm. The resulting supernatant solution was filtered (0.2 μm, Acrodisk; Gelman Sciences, Ann Arbor, MI, USA) and injected into the UPLC-PDA system (Waters Co., Milford, MA, USA).
Malonyl (ma)-ginsenosides were analyzed by an indirect base-hydrolysis method as reported [22]. Acidic ginsenosides were hydrolyzed by adding 80 μL of 5% KOH to a portion (850 μL) of the ginseng extract. After 2 h, the solution was neutralized by adding 80 μL of 0.01 M KH2PO4 solution. The mixture was diluted with 850 μL of acetonitrile and injected into the UPLC-PDA system (Waters Co.).
The instrumental conditions of UPLC-PDA were as follows. The chromatographic separation was obtained by using an ACQUITY BEH C18 column (50 mm × 2.1 mm, 1.7 μm; Waters Co.) at 40°C. The binary gradient elution system consisted of: (A) 0.01 M KH2PO4 in water; and (B) acetonitrile. The separation was achieved using the following protocol: 0–0.5 min (15% B); 14.5 min (30% B); 15.5 min (32% B); 16.5 min (40% B); 17.0 min (55% B); 21.0 min (90% B); 25.0–27.0 min (15% B). The flow rate was set at 0.6 mL/min and the sample injection volume was 2.0 μL. The ginsenosides were determined at a UV wavelength of 203 nm using a photo diode array detector (Waters Co.).
2.3. Extraction of water soluble components
Sugar, amino-sugar, and maltol were extracted as follows. A 100 mg sample of ginseng powder was weighed in a centrifugal tube (15 mL, polypropylene-single use; BioLogix Group) and shaken vigorously after the addition of 10 mL of deionized water. Extraction was performed in an ultrasonic cleaner (60 Hz, Wiseclean) for 30 min. After ultrasonic extraction, centrifugal separation (Legand Mach 1.6R; Thermo) was performed for 10 min at 3000 rpm. The resulting supernatant solution was filtered (0.2 μm, Acrodisk; Gelman Sciences) and this filtrate was used as analytical solution for sugar, amino-sugar, amino acid, and maltol.
2.4. Analysis of sugar and amino-sugar
Glucose, fructose, maltose, and sucrose standard materials were purchased from Sigma-Aldrich (SUPELCO, Bellefonte, PA, USA). Arginyl-fructose (AF), arginyl-fructose-glucose (AFG) standard materials were obtained from Ambo Institute.
The sample solution was prepared by 10× dilution of water-soluble extraction filtrate. Chromatographic determinations were performed according to Joo et al [23] with some modifications. These components were determined using ICS-3000 high pressure ion chromatography and a pulsed amperometric detector with Au working electrode and Ag/AgCl reference electrode (Dionex, Sunnyvale, CA, USA). The chromatographic separation was obtained using a CarboPac PA-10 column (250 mm × 4 mm; Dionex, Sunnyvale, CA, USA) at 30°C. The gradient elution system consisted of: (A) 250 mM NaOH; and (B) water. The separation was achieved using the following protocol: 0–20 min (93% B); 30–35 min (50% B); 36–45 min (0% B); 46–60 min (93% B). The flow rate was set at 1.0 mL/min and the sample injection volume was 5.0 μL.
2.5. Analysis of amino acids
Simultaneous determination of 17 kinds of amino acids was performed using the AccQ·Fluor reagent kit (Waters Co.) and modified appropriately for the application of ginseng samples.
In this experiment the precolumn, derivatization method was used. Firstly 10 μL of 10× diluted filtrate was mixed with 70 μL AccQ·Fluor derivatization buffer and immediately mixed. Then 20 μL of AccQ·Fluor reagent was added to this solution and vortexed for 5 min. It was then allowed to stand for 2 min at room temperature, transferred to an auto-sampler vial and heated at 55°C for 10 min in a preheated heating block (HB-48, Wisetherm; Daihan Scientific).
The instrumental conditions of HPLC (model 2695; Waters Co.) were as follows. The chromatographic separation was obtained using a Discovery C18 column (250 mm × 4.6 mm, 5 μm, SUPELCO) at 37°C. The gradient elution system consisted of: (A) AccQ·Tag eluent (pH 5.0; Waters Co.); (B) acetonitrile; and (C) water. The separation was achieved as follows: 0–5 min (97% A, 3% B); 17 min (91% A, 9% B); 25 min (80% A, 20% B); 34–40 min (69% B, 31% C); 42–50 min (97% A, 3% B). The flow rate was set at 1.0 mL/min and the sample injection volume was 5.0 μL. The amino acids were determined using a fluorescence detector (model 2475; Waters Co.); the excitation and emission wavelengths were set at 250 nm and 395 nm, respectively, and gain was adjusted at 1.0.
2.6. Analysis of maltol
Standard material of maltol was purchased from Sigma-Aldrich (SUPELCO) and chromatographic analysis was modified according to Risner and Kiser [24].
Water soluble extraction filtrate (1 μl) was injected into the UPLC system (H-class; Waters Co.). The instrumental conditions of UPLC were as follows. The chromatographic separation was obtained using an ACQUITY BEH C18 column (50 mm × 2.1 mm, 1.7 μm; Waters Co.) and the column temperature was 30°C. The binary gradient elution system consisted of: (A) 0.1% phosphoric acid in water; and (B) 0.1% phosphoric acid in acetonitrile. The separation was achieved using the following protocol: 0–4 min (8% B); 8 min (7% B); 9–12 min (90% B); 13–15 min (8% B). The flow rate was set at 0.4 mL/min. The detection wavelength was 275 nm using a tunable UV detector (TUV; Waters Co.)
2.7. Statistical analysis
The statistical analysis was performed using statistical product and service solutions (SPSS version 20.0; IBM Inc., New York, USA) and all data are presented as mean ± standard deviation. In all group comparisons, analysis was based on the Duncan’s multiple range test and a value of p < 0.01 was considered significant.
3. Results
3.1. Method validation of analytical methods
The specificity of individual analytic components was confirmed by demonstrating the sufficient separation of the substance present in the sample matrix. As shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4, chromatograms in each stage of ginseng preparation compared with those of the standard solution were sufficient to confirm the specificity of the analytic component. In other words, appropriate separation was defined as adequate resolution between the analytic components, the impurity and placebo peaks did not need not be separated from each other [25].
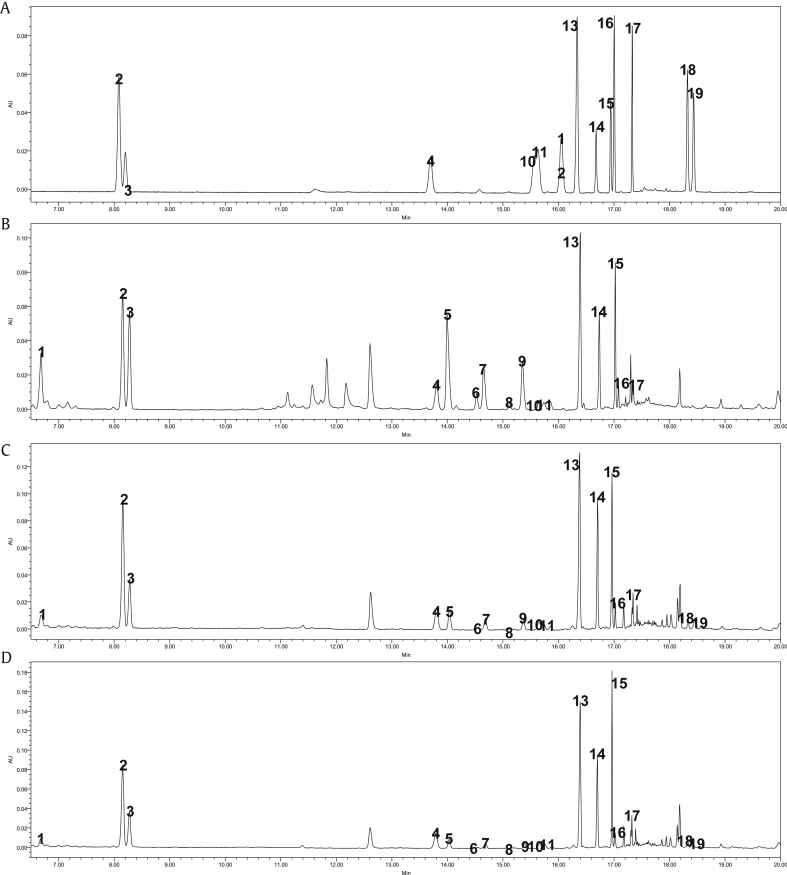
Fig. 1.
Representative UPLC chromatogram of ginsenosides in various P. ginseng samples. (A) Ginsenoside standards. (B) Fresh ginseng. (C) Steamed ginseng. (D) Red ginseng; 1; Ma-Rg1, 2; Rg1, 3; Re, 4; Rf, 5; Ma-Rb1, 6; Ma-Rb3, 7; Ma-Rc, 8; M-Rd, 9; Ma-Rb2, 10; Rh1, 11; 20(S)-Rg2, 12; 20(R)-Rg2, 13; Rb1, 14; Rc, 15; Rb2, 16; Rb3, 17; Rd, 18; 20(S)-Rg3, 19; 20(R)-Rg3.
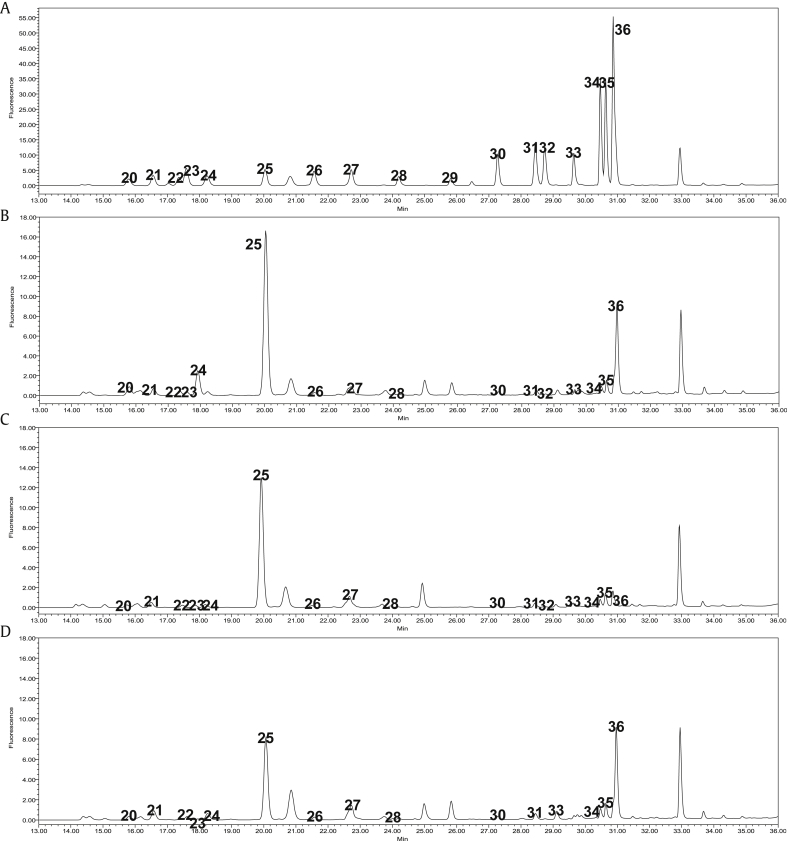
Fig. 2.
Representative HPLC-FLD chromatogram of amino acids in various P. ginseng samples. (A) Amino acid standards. (B) Fresh ginseng. (C) Steamed ginseng. (D) Red ginseng; 20; asparagines, 21; serine, 22; glutamine, 23; glycine, 24; histidine, 25; arginine, 26; threonine, 27; alanine, 28; proline, 29; cystine, 30; tyrosine, 31; valine, 32; methionine, 33; lysine, 34; isoleucine; 35; leucine, 36; phenylalanine.
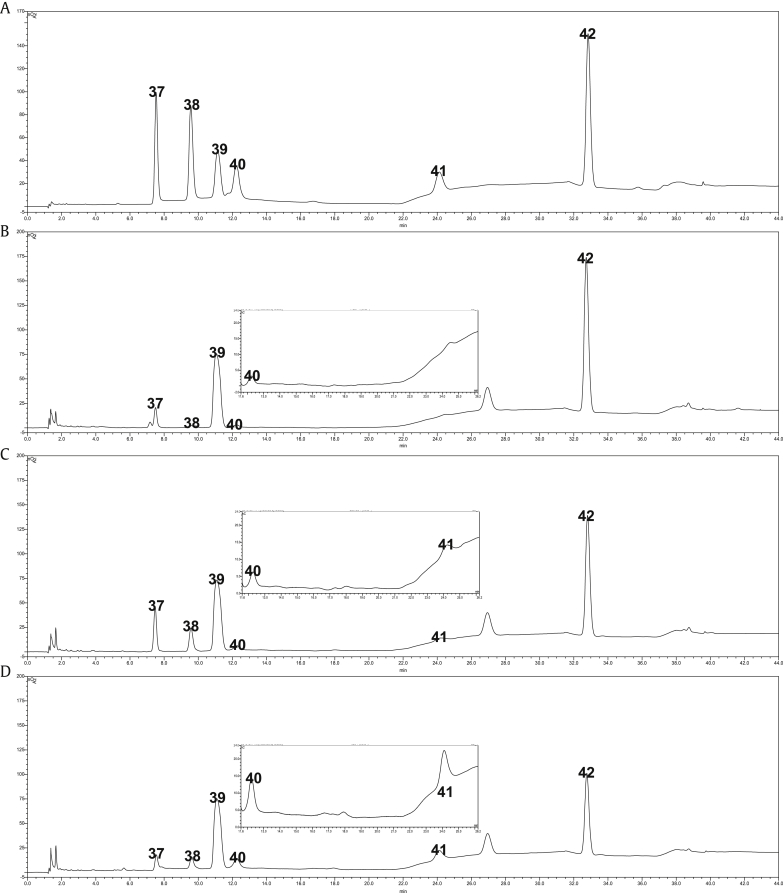
Fig. 3.
Representative high pressure ion chromatography pulsed amperometric detector HPIC-PAD chromatogram of sugar and amino-sugar in various P. ginseng samples. (A) Standard materials. (B) Fresh ginseng. (C) Steamed ginseng. (D) Red ginseng. 37; glucose, 38; fructose, 39; sucrose, 40; AF, 41; AFG, 42; maltose.
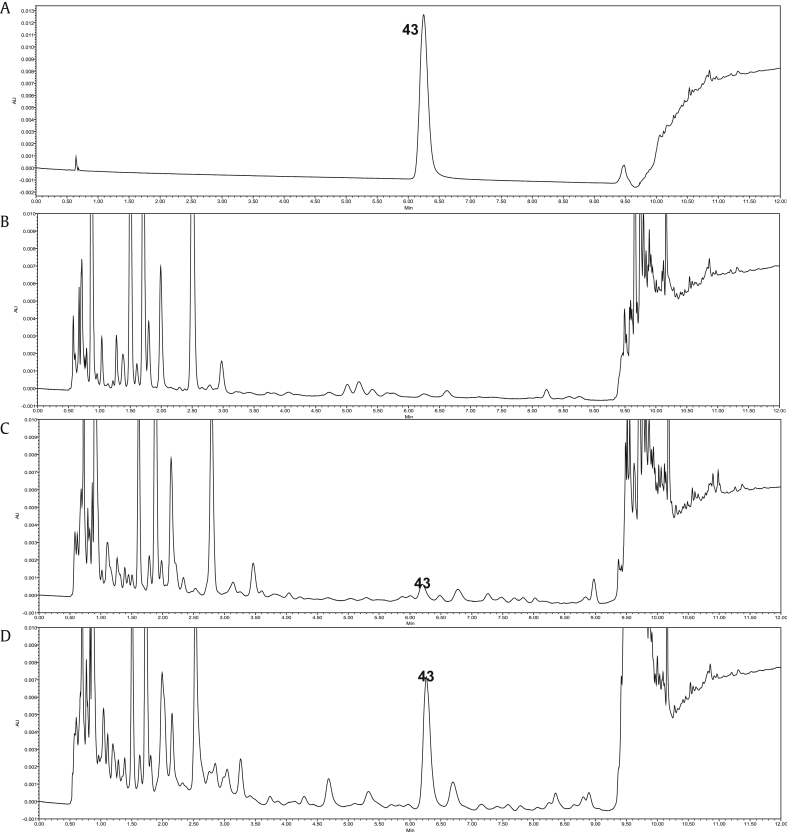
Fig. 4.
Representative UPLC-TUV chromatogram of maltol in various P. ginseng samples. (A) Standard material. (B) Fresh ginseng. (C) Steamed ginseng. (D) Red ginseng; 43; maltol.
A linear dependence of the signal and the analyte concentration is the most convenient indicator of sample quality or purity and is widely used in pharmaceutical analysis. The standard solutions of each analyte were injected into the chromatographic instruments and the calibration curves were plotted as the peak area versus the amount of each analyte. The linearity was evaluated by linear regression analysis, which is calculated by the least squares regression method. All components in this experiment showed R2 values > 0.999 as a result of linear regression.
The precision of individual analytical methods were determined by relative standard deviations (RSDs) of three level three repeated tests. Powdered ginseng samples were extracted and analyzed as described in the materials and methods section. The result showed that at RSD ≤ 3%, most of the analytes showed good results but sucrose and AFG showed higher RSD values of 6.82% and 6.28%, respectively. The accuracy of the analytical methods was tested by spiking experiments for recovery investigations. The recovery values of the analytes in this experiment ranged from 88% to 111%.
The above validation results show that the analytical methods used in this experiment were confirmed to have high accuracy with reproducible techniques.
3.2. Analytical results of chromatographic determination
The chemical change during the steaming process was monitored by quantitative determination of ginseng components such as ginsenosides, sugar, amino-sugar, amino acids, and maltol. The analytical results of these compounds are summarized in Table 1, Table 2. Chromatograms of these analytes in FG, SG, and RG are shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4. As presented in Table 1, Table 2, the change of component concentration during the steaming process (FG→SG→RG) could be easily identified. For example, ma-ginsenosides were decreased during the steaming process because they are thermally unstable [26]. A decreasing pattern of ma-ginsenosides could be seen in step-by-step sample chromatograms (Fig. 1). The peaks of ma-ginsnoside (Peak 1, 5–9) were dramatically decreased in SG and RG samples, but only small amount of these compounds existed in SG and RG. Thus ma-ginsenoside could not be utilized as a marker substance of FG or WG alone. As suggested by Kite et al [27], the ratio of malonylated to nonmalonylated ginsenosides was used as an indicator. In contrast, ginsenoside Rg3 generated during the steaming process was not observed in FG samples.
Table 1.
The concentration of ginsenosides in various P. ginseng samples (mg/g, mean ± SD, n = 20)
| Peak | Sample name | FG | SG | RG |
|---|---|---|---|---|
| 1 | Ma-Rg1 | 1.020 ± 0.416 | 0.437 ± 0.169 | 0.527 ± 0.234 |
| 2 | Rg1 | 3.272 ± 1.823 | 2.919 ± 1.726 | 2.927 ± 1.612 |
| 3 | Re | 2.450 ± 1.100 | 1.846 ± 0.851 | 1.715 ± 0.705 |
| 4 | Rf | 0.728 ± 0.357 | 0.692 ± 0.345 | 0.713 ± 0.331 |
| 5 | Ma-Rb1 | 4.009 ± 2.175 | 2.080 ± 1.072 | 1.748 ± 0.806 |
| 6 | Ma-Rb3 | 0.308 ± 0.113 | 0.146 ± 0.064 | 0.112 ± 0.051 |
| 7 | Ma-Rc | 1.263 ± 0.535 | 0.654 ± 0.265 | 0.523 ± 0.389 |
| 8 | Ma-Rd | 0.210 ± 0.169 | 0.118 ± 0.059 | 0.091 ± 0.042 |
| 9 | Ma-Rb2 | 1.983 ± 0.838 | 0.979 ± 0.420 | 0.818 ± 0.393 |
| 10 | Rh1 | 0.153 ± 0.063 | 0.196 ± 0.084 | 0.216 ± 0.099 |
| 11 | 20(S)-Rg2 | 0.230 ± 0.086 | 0.284 ± 0.109 | 0.244 ± 0.105 |
| 12 | 20(R)-Rg2 | ND | ND | ND |
| 13 | Rb1 | 4.647 ± 2.619 | 4.981 ± 2.897 | 5.196 ± 2.680 |
| 14 | Rc | 1.512 ± 0.689 | 1.539 ± 0.628 | 1.635 ± 0.717 |
| 15 | Rb2 | 2.103 ± 0.987 | 2.267 ± 0.966 | 2.314 ± 1.046 |
| 16 | Rb3 | 0.334 ± 0.157 | 0.344 ± 0.147 | 0.355 ± 0.157 |
| 17 | Rd | 0.217 ± 0.126 | 0.189 ± 0.105 | 0.191 ± 0.094 |
| 18 | 20(S)-Rg3 | N.D. | 0.130 ± 0.067 | 0.139 ± 0.059 |
| 19 | 20(R)-Rg3 | N.D. | 0.160 ± 0.080 | 0.151 ± 0.062 |
Ma, malonyl; ND, not detected; SD, standard deviation.
Table 2.
The concentration of amino acids and water soluble components in various P. ginseng samples (mg/g, mean ± SD, n = 20)
| Peak | Sample name | FG | SG | RG |
|---|---|---|---|---|
| 20 | Asparagine | 2.109 ± 0.454 | 1.108 ± 0.482 | 0.970 ± 0.362 |
| 21 | Serine | 1.287 ± 1.371 | 0.658 ± 0.237 | 0.560 ± 0.209 |
| 22 | Glutamine | 0.803 ± 0.401 | 0.008 ± 0.036 | 0.011 ± 0.051 |
| 23 | Glycine | 0.207 ± 0.112 | 0.158 ± 0.060 | 0.106 ± 0.043 |
| 24 | Histidine | 1.172 ± 1.191 | 0.504 ± 0.207 | 0.594 ± 0.346 |
| 25 | Arginine | 21.44 ± 5.78 | 23.98 ± 6.21 | 13.88 ± 5.10 |
| 26 | Threonine | 0.359 ± 0.270 | 0.267 ± 0.107 | 0.177 ± 0.067 |
| 27 | Alanine | 0.739 ± 0.462 | 1.142 ± 0.335 | 0.908 ± 0.163 |
| 28 | Proline | 0.113 ± 0.140 | 0.020 ± 0.050 | 0.044 ± 0.063 |
| 29 | Cystine | ND | ND | ND |
| 30 | Tyrosine | 0.390 ± 0.321 | 0.380 ± 0.278 | 0.285 ± 0.195 |
| 31 | Valine | 0.227 ± 0.128 | 0.178 ± 0.059 | 0.139 ± 0.038 |
| 32 | Methionine | 0.028 ± 0.025 | 0.010 ± 0.017 | ND |
| 33 | Lysine | 0.585 ± 0.318 | 0.557 ± 0.335 | 0.195 ± 0.119 |
| 34 | Isoleucine | 0.177 ± 0.093 | 0.169 ± 0.059 | 0.126 ± 0.038 |
| 35 | Leucine | 0.255 ± 0.101 | 0.262 ± 0.056 | 0.186 ± 0.041 |
| 36 | Phenylalanine | 1.562 ± 0.189 | 0.339 ± 0.152 | 0.758 ± 0.603 |
| 37 | Glucose | 1.356 ± 0.744 | 4.881 ± 0.895 | 3.045 ± 0.739 |
| 38 | Fructose | 1.962 ± 1.726 | 4.269 ± 2.491 | 4.789 ± 2.292 |
| 39 | Sucrose | 111.3 ± 14.8 | 122.6 ± 15.6 | 122.1 ± 11.4 |
| 40 | AF | 3.999 ± 3.242 | 7.614 ± 4.845 | 22.722 ± 10.230 |
| 41 | AFG | ND | 25.966 ± 6.812 | 65.896 ± 13.354 |
| 42 | Maltose | 5.468 ± 6.406 | 97.813 ± 28.057 | 58.736 ± 24.338 |
| 43 | Maltol | ND | 0.011 ± 0.004 | 0.165 ± 0.040 |
AF, arginine-fructose; AFG, arginine-fructose-glucose; FG, fresh ginseng; ND, not detected; RG, red ginseng; SD, standard deviation; SG, steamed ginseng; WG, white ginseng.
In the case of amino acids, all were decreased during the steaming process, which is in agreement with previous studies [14]. Another specific compound of RG, such as AFG and maltol was dramatically increased during the processing time as reported [23], [28]. These phenomena were also confirmed visually in step-by-step chromatograms (Fig. 3, Fig. 4). Additionally, these Maillard reaction products were generated much more in the drying period (SG→RG) than the steaming period (FG→SG).
4. Discussion
It was difficult to understand the tendencies for increase or decrease in components that were not mentioned in previous research because of interferences caused by the large value of standard deviation in analytical results (Table 1, Table 2). Thus normalization of analytical data should be performed to determine more accurate compositional changes.
All the analytical results used in this article consisted of one set with three-stage data, due to the use of in situ sampling. Thus, analytical results of the individual components are connected to each other with one data set (FG, SG, and RG were made up of the same ginseng which have the same serial number). In order to normalize the analytical results, they were arranged by serial number and data was divided according to the maximum value in each data set. As a result, all analytical results were obtained with values between 0 and 1. The use of normalized data meant the statistical analysis was more meaningful because it minimized the interpretative error caused by deviations in analytical results between samples. The normalized data and results of statistical analysis are presented in Table 3, Table 4 (superscript numbers represent a statistically significant difference).
Table 3.
The normalized data and result of statistical analysis for ginsenosides contents (mean ± SD, n = 20)
| Sample name | FG | SG | RG |
|---|---|---|---|
| Ma-Rg1 | 0.985 ± 0.066 1) | 0.435 ± 0.112 2) | 0.529 ± 0.173 2) |
| Rg1 | 0.931 ± 0.120 1) | 0.834 ± 0.158 1) | 0.853 ± 0.160 1) |
| Re | 0.981 ± 0.045 1) | 0.765 ± 0.209 2) | 0.712 ± 0.144 2) |
| Rf | 0.899 ± 0.122 1) | 0.862 ± 0.151 1) | 0.888 ± 0.117 1) |
| Ma-Rb1 | 1.000 ± 0.000 1) | 0.543 ± 0.199 2) | 0.464 ± 0.146 2) |
| Ma-Rb3 | 0.997 ± 0.015 1) | 0.489 ± 0.199 2) | 0.367 ± 0.117 3) |
| Ma-Rc | 1.0 1) | 0.547 ± 0.200 2) | 0.485 ± 0.145 2) |
| Ma-Rd | 0.876 ± 0.272 1) | 0.638 ± 0.331 2) | 0.460 ± 0.201 2) |
| Ma-Rb2 | 1.000 ± 0.000 1) | 0.510 ± 0.192 2) | 0.415 ± 0.143 2) |
| Rh1 | 0.713 ± 0.192 1) | 0.872 ± 0.131 2) | 0.950 ± 0.094 2) |
| 20(S)-Rg2 | 0.763 ± 0.163 1) | 0.927 ± 0.120 2) | 0.801 ± 0.168 1,2) |
| Rb1 | 0.808 ± 0.167 1) | 0.870 ± 0.183 1) | 0.907 ± 0.114 1) |
| Rc | 0.812 ± 0.170 1) | 0.845 ± 0.195 1) | 0.876 ± 0.109 1) |
| Rb2 | 0.802 ± 0.182 1) | 0.877 ± 0.191 1) | 0.876 ± 0.106 1) |
| Rb3 | 0.820 ± 0.172 1) | 0.863 ± 0.197 1) | 0.873 ± 0.108 1) |
| Rd | 0.836 ± 0.218 1) | 0.753 ± 0.264 1) | 0.746 ± 0.160 1) |
| 20(S)-Rg3 | 0.0 1) | 0.866 ± 0.166 2) | 0.945 ± 0.106 2) |
| 20(R)-Rg3 | 0.0 1) | 0.911 ± 0.141 2) | 0.888 ± 0.131 2) |
1,2,3) Values with different superscript numbers within the same raw sample are significantly different (p<0.01).
FG, fresh ginseng; Ma, malonyl; RG, red ginseng; SD, standard deviation; SG, steamed ginseng.
Table 4.
The normalized data and result of statistical analysis for amino acids and water soluble contents (mean ± SD, n = 20)
| Sample name | FG | SG | RG |
|---|---|---|---|
| Asparagine | 1.0 1) | 0.537 ± 0.207 2) | 0.479 ± 0.201 2) |
| Serine | 0.920 ± 0.146 1) | 0.710 ± 0.276 2) | 0.622 ± 0.296 2) |
| Glutamine | 1.0 1) | 0.015 ± 0.068 2) | 0.022 ± 0.098 2) |
| Glycine | 0.945 ± 0.112 1) | 0.778 ± 0.200 1) | 0.558 ± 0.267 2) |
| Histidine | 0.828 ± 0.240 1) | 0.550 ± 0.295 2) | 0.640 ± 0.349 1,2) |
| Arginine | 0.817 ± 0.185 1) | 0.908 ± 0.164 1) | 0.534 ± 0.203 2) |
| Threonine | 0.900 ± 0.165 1) | 0.803 ± 0.246 1) | 0.555 ± 0.230 2) |
| Alanine | 0.566 ± 0.232 1) | 0.926 ± 0.143 2) | 0.769 ± 0.192 2) |
| Proline | 0.495 ± 0.508 1) | 0.136 ± 0.337 1) | 0.288 ± 0.432 1) |
| Tyrosine | 0.828 ± 0.185 1,2) | 0.857 ± 0.231 1) | 0.658 ± 0.232 2) |
| Valine | 0.905 ± 0.152 1) | 0.797 ± 0.225 1,2) | 0.651 ± 0.246 2) |
| Methionine | 0.600 ± 0.503 1) | 0.227 ± 0.373 2) | 0.0 2) |
| Lysine | 0.897 ± 0.211 1) | 0.820 ± 0.212 1) | 0.318 ± 0.205 2) |
| Isoleucine | 0.845 ± 0.177 1) | 0.865 ± 0.187 1) | 0.668 ± 0.209 2) |
| Leucine | 0.845 ± 0.163 1) | 0.902 ± 0.151 1) | 0.657 ± 0.191 2) |
| Phenylalanine | 0.997 ± 0.010 1) | 0.211 ± 0.068 3) | 0.495 ± 0.408 2) |
| Glucose | 0.272 ± 0.129 1) | 1.0 3) | 0.639 ± 0.175 2) |
| Fructose | 0.348 ± 0.225 1) | 0.805 ± 0.180 2) | 0.943 ± 0.096 2) |
| Sucrose | 0.880 ± 0.106 1) | 0.964 ± 0.060 2) | 0.963 ± 0.038 2) |
| Maltose | 0.058 ± 0.060 1) | 1.000 ± 0.000 3) | 0.604 ± 0.154 2) |
| AF | 0.169 ± 0.106 1) | 0.323 ± 0.107 2) | 1.0 3) |
| AFG | 0.0 1) | 0.399 ± 0.098 2) | 1.0 3) |
| Maltol | 0.000 ± 0.000 1) | 0.068 ± 0.020 2) | 1.0 3) |
1,2,3) Values with different superscript numbers within the same raw sample are significantly different (p<0.01).
FG, fresh ginseng; RG, red ginseng; SD, standard deviation; SG, steamed ginseng.
As discussed in the previous section, six ma-ginsenosides were decreased during the steaming process. As shown in Table 3, there is a change in neutral ginsenosides such as Re, Rh1, 20(S)-Rg2, 20(S, R)-Rg3. The concentration of ginsenoside Re was slightly decreased and others were slightly enhanced or generated during the steaming process. In the case of amino acids, the concentrations of fifteen amino acids were decreased during the steaming process but proline did not show a statistical significance. Some Maillard reaction products such as AF, AFG, and maltol were generated or enhanced in concentration during the steaming process. Two patterns of changes were observed in the concentration of free sugars and the fructose and sucrose were increased in their concentrations. Concentrations of glucose and maltose were increased in the steaming period and decreased in the drying period, with the result that their final concentration was increased.
In the present study, in situ monitoring of 43 components in P. ginseng during the steaming process was performed. The concentration of ma-ginsenosides and amino acids were decreased during the steaming process. In contrast, Rh1, 20(S)-Rg2, 20(S, R)-Rg3, and Maillard reaction products were either generated or increased in their concentrations. Our study elucidates the dynamic changes of components when the steaming process is induced. These results throw a light on the changes in the chemical constituents of ginseng root due to the steaming process that, to our knowledge, can be helpful in the future for commercial production of ginseng supplements with special chemical formulations for a variety of bodily ailments.
Conflicts of interest
All authors declare no conflicts of interest.
References
- 1.Soldati F. Panax ginseng; Standardization and Biological Activity. In: Cutler S.J., Cutler H.G., editors. Biologically active natural products. CRC Press; New York: 2000. pp. 209–232. [Google Scholar]
- 2.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 4.Christensen L.P. Ginsenoside: Chemistry, Biosynthesis, Analysis and Potential Health Effects. Adv Food Nutr Res. 2008;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 5.Lau A.J., Woo S.O., Koh H.L. Analysis of saponins in raw and steamed Panax notoginseng using high-performance liquid chromatography with diode array detection. J Chromatogr A. 2003;1011:77–87. doi: 10.1016/s0021-9673(03)01135-x. [DOI] [PubMed] [Google Scholar]
- 6.Du X.W., Wills R.B.H., Stuart D.L. Changes in neutral and malonyl ginsenosides in American ginseng (Panax quinquefolium) during drying, storage and ethanolic extraction. Food Chem. 2004;86:155–159. [Google Scholar]
- 7.Lau A.J., Seo B.H., Woo S.O., Koh H.L. High-performance liquid chromatographic method with quantitative comparisons of whole chromatogram of raw and steamed Panax notiginseng. J Chromatogr A. 2004;1057:141–149. doi: 10.1016/j.chroma.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 8.Wang D., Liao P.Y., Zhu H.T., Chen K.K., Xu M., Zhang Y.J., Yang C.R. The processing of Panax notoginseng and the transformation of its saponin components. Food Chem. 2012;132:1808–1813. [Google Scholar]
- 9.Sun B.S., Xu M.Y., Li Z., Wang Y.B., Sung C.K. UPLC-Q-TOF-MS/MS analysis for steaming times-dependent profiling of steamed Panax quinquefolius and its ginsenosides transformations induced by repetitious steaming. J Ginseng Res. 2012;36:277–290. doi: 10.5142/jgr.2012.36.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu C., Xu S., Li X., Yan J., Liu L. Profiling the ginsenosides of three ginseng products by LC-Q-Tof/MS. J Food Sci. 2013;78:653–659. doi: 10.1111/1750-3841.12102. [DOI] [PubMed] [Google Scholar]
- 11.Xie Y.Y., Luo D., Cheng Y.J., Ma J.F., Wang Y.M., Liang Q.L., Luo G.A. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from Panax ginseng by means of HPLC-ESI-MS/MS based multicomponent quantification fingerprint. J Agric Food Chem. 2012;60:8213–8224. doi: 10.1021/jf301116x. [DOI] [PubMed] [Google Scholar]
- 12.Jung M.Y., Jeon B.S., Bock J.Y. Free, esterified, and insoluble-bound phenolic acids in white and red Korean ginsengs (Panax ginseng C.A. Meyer) Food Chem. 2002;79:105–111. [Google Scholar]
- 13.Chung I.M., Kim J.W., Seguin P., Jun Y.M., Kim S.H. Ginsenosides and phenolics in fresh and processed Korean ginseng (Panax ginseng C.A. Meyer): Effects of cultivation location, year, and storage period. Food Chem. 2012;130:73–83. [Google Scholar]
- 14.Cho E.J., Piao X.L., Jang M.H., Baek S.H., Kim H.Y., Kang K.S., Kwon S.W., Park J.H. The effect of steaming on the free amino acid contents and antioxidant activity of Panax ginseng. Food Chem. 2008;107:876–882. [Google Scholar]
- 15.Park J.I., Han S.B., Kim J.M., Piao L., Kwon S.W., Lim N.Y., Kang T.L., Park M.K., Park J.H. Four new acetylated ginsenosides from processed ginseng (Sun Ginseng) Arch Pharm Res. 2002;25:837–841. doi: 10.1007/BF02977001. [DOI] [PubMed] [Google Scholar]
- 16.Kang K.S., Yamabe N., Kim H.Y., Okamoto T., Sei Y., Yokozawa T. Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J Ethnopharmacol. 2007;113:225–232. doi: 10.1016/j.jep.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y.J., Kim H.Y., Kang K.S., Lee J.G., Yokozawa T., Park J.H. The chemical and hydroxyl radical scavenging activity changes of ginsenoside-Rb1 by heat processing. Bioorg Med Chem Lett. 2008;18:4515–4520. doi: 10.1016/j.bmcl.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 18.Sun S., Wang C.Z., Tong R., Li X.L., Fishbein A., Wang Q., He T.C., Du W., Yuan C.S. Effects of steaming the root of Panax notoginseng on chemical composition and anticancer activities. Food Chem. 2010;118:307–314. [Google Scholar]
- 19.Toh D.F., New L.S., Koh H.L. Chan Eric CY. Ultra-high performance liquid chromatography/time-of-flight mass spectrometry (UHPLC/TOFMS) for time-dependent profiling of raw and steamed Panax notoginseng. J Pharm Biomed Anal. 2010;52:43–50. doi: 10.1016/j.jpba.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H.M., Li S.L., Zhang H., Wang Y., Zhao Z.L., Chen S.L. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J Pharm Biomed Anal. 2012;62:258–273. doi: 10.1016/j.jpba.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Park H.W., In G., Lee M.W., Kim S.Y., Kim K.T., Cho B.G., Han G.H., Chang I.M. Simultaneous determination of 30 ginsenosides in Panax ginseng preparations using ultra performance liquid chromatography. J Ginseng Res. 2013;37:457–467. doi: 10.5142/jgr.2013.37.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Court W.A., Hendel J.G., Elmi J. Reversed-phase high-performance liquid chromatographic determination of ginsenosides of Panax quinquefolium. J Chromatogr A. 1996;755:11–17. [Google Scholar]
- 23.Joo K.M., Park C.W., Jeong H.J., Lee S.J., Chang I.S. Simultaneous determination of two amadori compounds in Korean red ginseng (Panax ginseng) extracts and rat plasma by high-performance anion-exchange chromatography with pulsed amperometric detection. J Chromatogr B. 2008;865:159–166. doi: 10.1016/j.jchromb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Risner C.H., Kiser M.J. High-performance liquid chromatography procedure for the determination of flavor enhancers in consumer chocolate products and artificial flavors. J Sci Food Agric. 2008;88:1423–1430. [Google Scholar]
- 25.Ermer J. Validation in pharmaceutical analysis. Part I: An integrated approach. J Pharm Biomed Anal. 2001;24:755–767. doi: 10.1016/s0731-7085(00)00530-6. [DOI] [PubMed] [Google Scholar]
- 26.Kitagawa I., Taniyama T., Yoshikawa M., Ikenishi Y., Nakagawa Y. Chemical studies on crude drug processing. VI. Chemical structures of malonyl-ginsenosides Rb1, Rb2, Rc, and Rd isolated from the root of Panax ginseng C.A. Meyer. Chem Pharm Bull. 1989;37:2961–2970. [Google Scholar]
- 27.Kite G.C., Howes M.-J.R., Leon C.J., Simmonds M.S.J. Liquid chromatography/mass spectrometry of malonyl-ginsenosides in the authentication of ginseng. Rapid Commun Mass Spectrom. 2003;17:238–244. doi: 10.1002/rcm.899. [DOI] [PubMed] [Google Scholar]
- 28.Du Q.-Q., Liu S.-Y., Xu R.-F., Li M., Song F.-R., Liu Z.-Q. Studies on structures and activities of initial Maillard reaction products by electrospray ionization mass spectrometry combined with liquid chromatography in processing of red ginseng. Food Chem. 2012;135:832–838. doi: 10.1016/j.foodchem.2012.04.126. [DOI] [PubMed] [Google Scholar]