Abstract
The occurrence and progression of hepatocellular carcinoma (HCC) are affected by complicated signal transduction factors. Our previous study identified Ikaros as a novel reactivated therapeutic target that acts as a transcriptional repressor and reactivates anticancer mechanisms in HCC therapy. Annexin A4 (ANXA4) is a member of the Annexin family that plays an essential role in several cancers, but it has not been investigated in HCC proliferation. Using cDNA microarrays, ANXA4 was shown to be associated with Ikaros in Ikaros-overexpressing cells. The aim of this work was to characterize the relationship between Ikaros and ANXA4 and the role of ANXA4 in HCC. The effect of Ikaros on ANXA4 was analyzed in HCC cell lines and HCC patient samples, and functional recovery experiments were performed between Ikaros and ANXA4. Furthermore, the effect of ANXA4 on cell proliferation in vitro was analyzed by MTT and colony formation assays in HCC cells. We used a subcutaneous xenograft model to elucidate the role of ANXA4 in vivo. We found that ANXA4 overexpression promotes HCC cell proliferation, but Ikaros can inhibit ANXA4 expression by repressing its promoter activity. Moreover, we demonstrated that downregulated expression of ANXA4 inhibited HCC cell proliferation and tumorigenesis in vitro and in vivo. Our findings indicate that ANXA4 may be a critical factor in HCC tumorigenesis. Ikaros is an attractive inhibitor of ANXA4 and may function as an anticancer agent in HCC.
Keywords: Annexin A4, Ikaros, proliferation, hepatocellular carcinoma
Introduction
Primary liver cancer is a common malignant tumor and ranks as the third most prevalent cancer worldwide. Remarkably, 90% of primary hepatic carcinomas are hepatocellular carcinomas (HCC), and due to its high prevalence, poor outcomes and low survival rate, HCC is the second most common cause of cancer-related deaths worldwide [1,2]. According to the World Cancer Report 2014, there were approximately 782,500 incident cases of HCC in 2012, and the five-year survival rate is below 5%. Because of the large number of patients with hepatitis and cirrhosis in China, HCC represents a serious disease burden in China. HCC patients are usually diagnosed at an advanced disease stage [3] characterized by multiple organ metastases and poor prognosis [4]; thus, early diagnosis is critical for treating HCC [5]. Although hepatitis virus infection is an important epidemiological cause of HCC, the mechanism of HCC development remains unknown [6]. It is therefore critically necessary to elucidate the pathogenesis of HCC and find efficient therapeutic targets at the molecular level [7].
The Ikaros gene encodes a transcription factor that contains a specific Kruppel-like zinc-finger structure [8]. As a tumor suppressor, Ikaros is clinically relevant to some hematological malignancies [9,10]. Moreover, in our previous study, we revealed that CD133+ HCC cells have characteristics of cancer stem-like cells, and Ikaros interacted with the transcription repressor CtBP as a complex and inhibited CD133 expression by directly binding to its promoter in HCC. Ikaros can regulate the biological activation of CD133+ HCC cells and inhibit CD133+ HCC cell proliferation; in addition, patients with higher Ikaros expression exhibited longer survival [11]. Thus, our results suggested that reactivation of Ikaros is a novel strategy for HCC treatment. Furthermore, we performed cDNA microarray analyses in Ikaros-overexpressing cells to investigate the downstream molecular mechanisms and identified that ANXA4 was significantly downregulated in Ikaros-overexpressing cells.
Annexins are members of a polygene family that maintain an evolutionarily conserved structure; they can bind with bivalent calcium ions, phospholipids and are located on the cell membrane [12-14]. Annexins consist of a variable N-terminal region, which is thought to be responsible for their extensive bioactivities, and a conserved C-terminal domain [15,16]. ANXA4 is a member of the Annexin family and was first detected in epithelial cells; it is reported to be associated with cell metabolic processes, such as cell cycle promotion, apoptosis inhibition, and anticoagulation [17]. With respect to cancer, we found that ANXA4 acts as an oncogene involved in promoting tumor cell proliferation, metastasis and drug resistance [18]. In the ovarian cancer cell lines OVTOKO and OVISE, ANXA4 knockdown significantly inhibits cell proliferation, increases cell sensitivity to carboplatin and suppresses migration and invasion [19]. Helicobacter pylori is a risk factor for gastric tumors [14]. Elevated ANXA4 and bivalent calcium ion levels were observed in gastric tumor cells infected with Helicobacter pylori [20,21].
ANXA4 can activate the PI3K/Akt signaling pathway and sensitize the hyaluronan-mediated motility receptor (RHAMM) and cyclin-dependent kinase 1 (CDK1), thus promoting tumorigenesis in breast cancer [15,22]. By triggering downstream signal, ANXA4 increases tumor cell proliferation and drug resistance in ovarian cancer [23,24]. ANXA4 overexpression activates the NF-κB pathway via transcriptionally activating p65 subunit and promotes tumorigenesis in gallbladder cancer (GBC) [25,26]. In this study, we examined the expression profile of ANXA4 in HCC and described the biochemical and genetic interactions between Ikaros and ANXA4, demonstrating that ANXA4 acts as a carcinogenic factor in HCC.
Materials and methods
Patients and tissue samples
Samples from thirty-seven patients diagnosed with HCC were obtained from the Qidong Liver Cancer Institute. Each patient sample consisted of cancer tissue and matched adjacent liver tissue. For all cases, pathological and clinical follow-up data were collected after obtaining ethical approval from the China Ethical Review Committee. HCC was diagnosed based on endocrine evaluations, clinical symptoms, and imaging examinations.
Cell lines and culture conditions
The SMMC-7721 cell line was provided by the cell bank of the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). The Hep3B and PLC/PRF/5 HCC cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). The MHCC-LM3 and MHCC-97L cell lines were obtained from the Liver Cancer Institute, Zhongshan Hospital of Fudan University (Shanghai, China). The Huh7 cell line was obtained from the Riken Cell Bank (Tsukuba, Japan) as previously described [11]. The HCC-LY5 cell line was established in our laboratory. Confluent cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (FBS; Sigma, USA), and penicillin and streptomycin (100 μg/mL, Sigma-Aldrich, USA) at 37°C in 5% CO2.
Quantitative RT-PCR
According to the manufacturer’s protocol, total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and reverse transcription was performed using a Prime-Script RT Reagent Kit (TaKaRa, Shanghai, China). RNA concentration and purity were determined using an Agilent Technologies 2100 Bioanalyzer. The reaction conditions were 37°C for 15 min, 85°C for 5 s, and 4°C for 5 min. Quantitative real-time polymerase chain reaction (qRT-PCR) were performed using a 7500 system (Thermo Scientific, MA, USA). The qRT-PCR primer sequences were as follows: ANXA4-F: 5’-ACCAGCAGCAATATGGACGG-3’; ANXA4-R: 5’-TTCGGTTCCGGGAACAGAG-3’; Ikaros-F: 5’-ATGGGCGTGCCTGTGAAATGA-3’; Ikaros-R: 5’-GCCGTTCTCCAGTGTGGCTTCTT-3’.
Plasmid constructs
First, we amplified the human ANXA4 gene coding sequence and inserted it into the empty vector pWPXL at the BamHI and EcoRI sites. We cloned the ANXA4 promoter sequence from -1078 bp to +37 bp and deleted the regions from -900, -750, -500, and -300 bp to +37 bp to generate truncated promoter plasmids. A plasmid containing a mutated DNA binding site (-1043 bp to -1031 bp) was also created. All the promoter sequences were inserted into pGL3-enhancer at the BglII and MluI sites.
Western blot analyses
Proteins were extracted from cell lines or tissue lysates and lysed in Tissue Protein Extraction Reagent (Thermo Scientific, MA, USA) with 1 × phosphatase inhibitor cocktail and 1 × protease inhibitor cocktail (Roche) for 5 min on ice, followed by centrifugation (12,000 rpm, 4°C, 15 min). Soluble proteins in the supernatant were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, USA). Nonspecific binding was blocked by incubating the membranes in 5% nonfat milk for 1 h at room temperature. The membranes were then incubated overnight at 4°C with either an anti-ANXA4 monoclonal antibody (1:200, MAB-4146, R&D) or an anti-Ikaros polyclonal antibody (1:100, SC-13039, Santa Cruz). After washing in phosphate-buffered saline with 1% Tween 20 (PBST), the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit secondary antibodies (1:3000; Sigma-Aldrich) at room temperature for 2 h and then washed again in PBST. Western blots were visualized using an enhanced chemiluminescence (ECL) detection reagent with an ECL kit (Thermo Scientific). The monoclonal anti-β-actin-peroxidase antibody (1:2,0000, A3854, Sigma-Aldrich) was used to visualize the amount of protein loaded as an internal loading control.
Cell proliferation assay
For the colony formation assay, cells were trypsinized, counted, and seeded at a density of three thousand cells per well in 6-well plates. During colony growth, the culture medium was replaced every 3 days, and two weeks later, 10% formaldehyde was added for 30 min at 37°C. Next, the cells were stained with Giemsa solution and washed, and the number of cell colonies was determined.
For the MTT assay, thiazolyl blue tetrazolium bromide (MTT) (M5655) was purchased from Sigma-Aldrich. A total of two thousand cells per well were seeded into 96-well plates. After 24 h of incubation, 10 µL of MTT solution (5 mg/mL) was added to the medium in each well, followed by 4 h of incubation at 37°C. Then, the culture medium was removed, and 150 μL of MTT solvent (DMSO) was added to the plate. The optical density (OD) values were read at 570 nm for the cell proliferation assay.
Short hairpin RNA-mediated gene knockdown assay
To generate stable ANXA4 knockdown cell lines, we transfected cells with three short hairpin RNAs (shRNAs) targeting ANXA4 mRNA (shANXA4-1, shANXA4-2, shANXA4-3) and a negative control nonspecific shRNA (shNC) synthesized by GenePharma (Shanghai, China). ANXA4 knockdown was confirmed by qRT-PCR and western blot. The shRNA sequences targeting ANXA4 were as follows: shANXA4-1F: CGCGTccccCCGATGAAGACGCCATTATttcaagagaATAATGGCGTCTTCATCGGtttttGGAAAT; shANXA4-1R: cgatTTCCaaaaaCCGATGAAGACGCCATTATtctcttgaaATAATGGCGTCTTCATCGGGGGGA; shANXA4-2F: CGCGTccccGGATATCACAGAAGGATATttcaagagaATATCCTTCTGTGATATCCtttttGGAAAT; shANXA4-2R: cgatTTCCaaaaaGGATATCACAGAAGGATATtctcttgaaATATCCTTCTGTGATATCCGGGGA; shANXA4-3F: CGCGTccccGAGGAACAAATCTGCATATttcaagagaATATGCAGATTTGTTCCTCtttttGGAAAT; shANXA4-3R: cgatTTCCaaaaaGAGGAACAAATCTGCATATtctcttgaaATATGCAGATTTGTTCCTCGGGGA.
Tumor xenograft models
For the in vivo tumor proliferation assay, we established a subcutaneous HCC model. Six-week-old BALB/c (nu/nu) male mice were raised in a specific pathogen-free laboratory. The mice were randomly divided into four groups and injected with 2 × 106 MHCC-LM3, 3 × 106 Hep3B cells or stable ANXA4 knockdown versions of these cell lines. Four weeks after HCC cell inoculation, all animals were euthanized. The tumor tissues were weighed and fixed with 10% neutral formalin.
Luciferase reporter assay
Cells were cultured in DMEM and, after reaching approximately 90% confluence, transfected with the corresponding reporter plasmids and the pRL-TK reporter construct. After 48 h, a Dual-Luciferase Reporter Assay Kit (Promega, USA) was used to measure the Renilla and firefly luciferase activity.
Immunohistochemistry (IHC)
Human primary HCC tissue sections were probed with an anti-ANXA4 polyclonal antibody (HPA007393) (1:80, Sigma-Aldrich), and then an HRP-conjugated secondary antibody was applied. The HCC tissue microarray preparation and IHC were performed according to our previously described procedures [27].
Statistical analysis
We used SPSS 13.0 software to analyze the statistical data in this study, and the data are expressed as the mean ± standard deviation (SD). Student’s t test was used to analyze differences between two groups. P < 0.05 was considered statistically significant.
Results
ANXA4 is negatively associated with the expression of Ikaros and is downregulated by Ikaros
Ikaros is a transcriptional inhibitor and a novel reactivated therapeutic target. It can also repress HCC proliferation and reactivate anticancer mechanisms in HCC therapy. To investigate the molecular mechanisms underlying the inhibitory effect of Ikaros on HCC cell proliferation and downstream differentially expressed genes, we performed cDNA microarray analyses. The ANXA4 gene was dramatically differentially expressed in Ikaros-overexpressing cells compared with the control group and was therefore selected for further investigation in HCC.
We first detected the endogenous expression of Ikaros and ANXA4 in eight HCC cell lines. Ikaros was expressed at a low level in Hep3B, Huh7, and PLC/PRF/5 cells, while ANXA4 expression was high (Figure 1A). We then established Ikaros-overexpressing HCC cell lines and confirmed that ANXA4 was downregulated at both the mRNA and protein levels in stable Ikaros-overexpressing HCC cells (Figure 1B). We corroborated our in vitro data with an investigation in HCC patients (Figure 1C). The negative relationship between Ikaros and ANXA4 in HCC cell lines and human primary HCC tissues led us to hypothesize that ANXA4 is a target of Ikaros.
Figure 1.
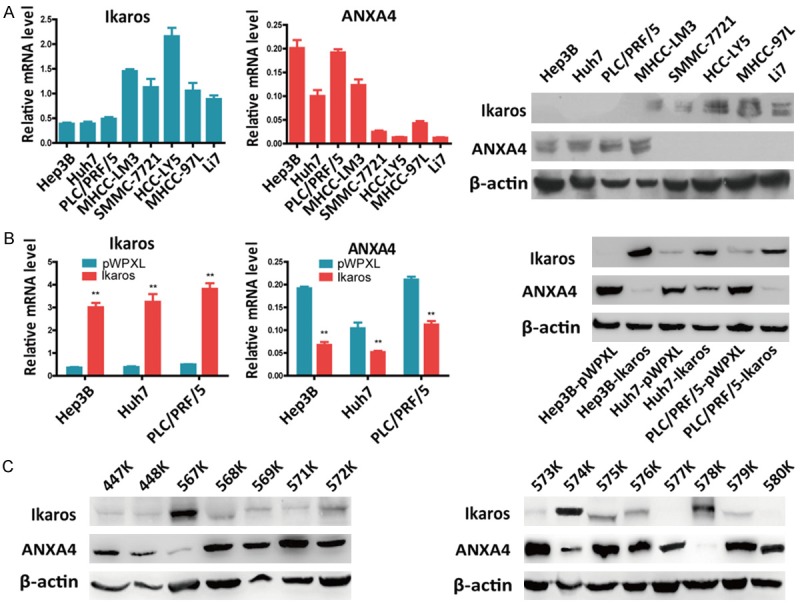
Ikaros represses ANXA4 expression in HCC. A. Analysis of Ikaros and ANXA4 mRNA and protein levels in HCC cell lines. B. ANXA4 expression was detected by qRT-PCR and western blot when Ikaros was stably overexpressed in HCC cells. C. The relationship between Ikaros and ANXA4 in HCC tissues was evaluated by western blot, indicating that the expression of Ikaros protein is negatively associated with ANXA4. *P < 0.05, **P < 0.01.
ANXA4 is upregulated in human primary HCC tissues
We analyzed ANXA4 expression in primary HCC patient tissues (K), and compared with matched normal liver tissues (N), ANXA4 protein was overexpressed in 15/24 (62%) of the HCC tissues, whereas it was downregulated in 3/24 (13%) cases, and no difference was observed in 6/24 (25%) of the HCC tissues (Figure 2A, 2B). We also confirmed the above results by analyzing the protein expression of ANXA4 in 59 human primary HCC tissues using IHC, which demonstrated that 58% (34/59) of the HCC tissues displayed high positive staining, 27% (16/59) exhibited moderate staining, and only 15% (9/59) showed low staining (Figure 2C). Thus, the abnormal upregulation of ANXA4 in HCC tissues led us to explore its function in HCC.
Figure 2.
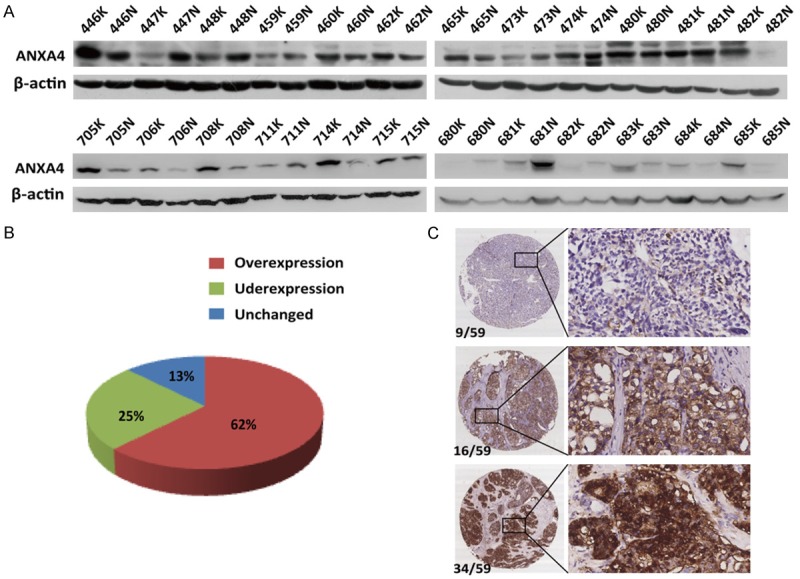
ANXA4 is upregulated in human primary HCC tissues. A, B. The protein level of ANXA4 was typically upregulated (62%, 15/24) in primary HCC patient tissues (K) compared with matched noncancerous liver tissue (N). ANXA4 was downregulated in 3/24 (13%) cases, and no difference was observed in 6/24 (25%) of the HCC tissues. C. Representative images of IHC analysis of ANXA4 expression in HCC tissues, in which 58% (34/59) of the HCC tissues displayed high positive staining, 27% (16/59) showed moderate staining, and only 15% (9/59) exhibited low staining. Original magnification 40 × (left) and 200 × (right).
ANXA4 promotes HCC cell proliferation
To determine the function of ANXA4 in HCC, we detected the endogenous expression of ANXA4 mRNA and protein in thirteen HCC cell lines by qRT-PCR and western blot (Figure 3A). In the HCC cell lines Hep3B, Huh7, PLC/PRF/5, and MHCC-LM3, ANXA4 was upregulated, whereas it was barely detectable in SMMC-7721, HCC-LY5, MHCC-97L and Li7 cells. We cloned the complete coding sequence of ANXA4 into a lentiviral vector and transfected it into cells to establish the following HCC cell lines: SMMC-7721-ANXA4, HCC-LY5-ANXA4, MHCC-97L-ANXA4, and Li7-ANXA4. The empty vector pWPXL was used as a control (Figure 3B). MTT and colony formation assays were performed to investigate the effect of ANXA4 on proliferation, and we found that ANXA4 promotes HCC cell growth in vitro (Figure 3C, 3D).
Figure 3.
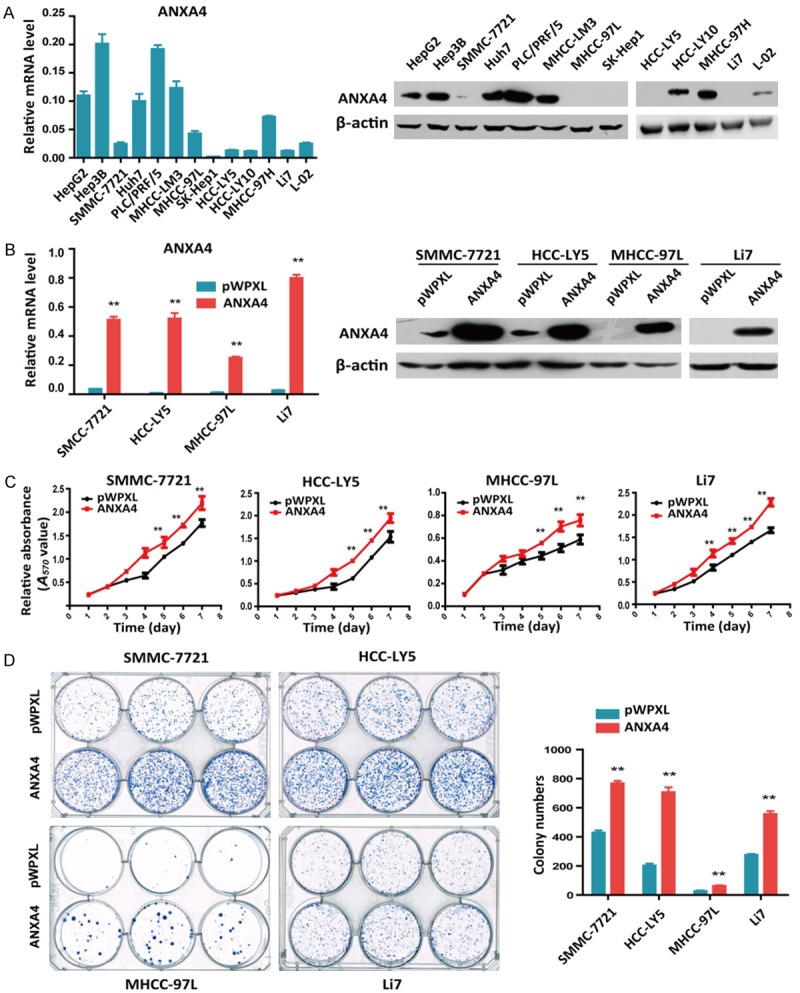
Overexpression of ANXA4 in HCC cells promotes proliferation in vitro. A. qRT-PCR analysis of ANXA4 expression in HCC cell lines (left), and western blot analysis of ANXA4 expression in HCC cell lines (right). B. qRT-PCR analysis of ANXA4 expression in HCC cells stably transfected with ANXA4 or control plasmids (left), and western blot analysis of ANXA4 protein expression in HCC cells stably transfected with ANXA4 or control plasmids (right). C. Cell growth was analyzed at different time points by MTT assay. D. A colony formation assay was performed in HCC cells stably transfected with ANXA4 or control plasmids (left). The results of the statistical analysis are shown on the right. *P < 0.05, **P < 0.01.
Knockdown of ANXA4 suppresses HCC cell proliferation in vitro and in vivo
Next, we designed three shRNA lentiviral vectors to specifically knock down endogenous ANXA4 expression in MHCC-LM3, Huh7, PLC/PRF/5, and Hep3B cells (Figure 4A). Cell proliferation assays showed that ANXA4 knockdown had a significant effect on HCC cell proliferation in vitro (Figure 4B, 4C). Furthermore, to translate our in vitro data to a more human-like setting, we analyzed the in vivo tumorigenesis of human HCC cells. The in vivo tumorigenic role of ANXA4 was assessed using subcutaneous xenograft models. In mice injected with the HCC cell line MHCC-LM3, the tumor weights indicated that ANXA4 knockdown significantly suppressed HCC tumorigenesis (n = 11, Figure 5A-C). We observed the same subcutaneous tumorigenesis result in mice injected with the HCC cell line Hep3B (n = 7, Figure 5D-F). Thus, these findings indicated that ANXA4 knockdown decreased the tumorigenic potential in a subcutaneous xenograft model and that ANXA4 is crucial for HCC proliferation.
Figure 4.
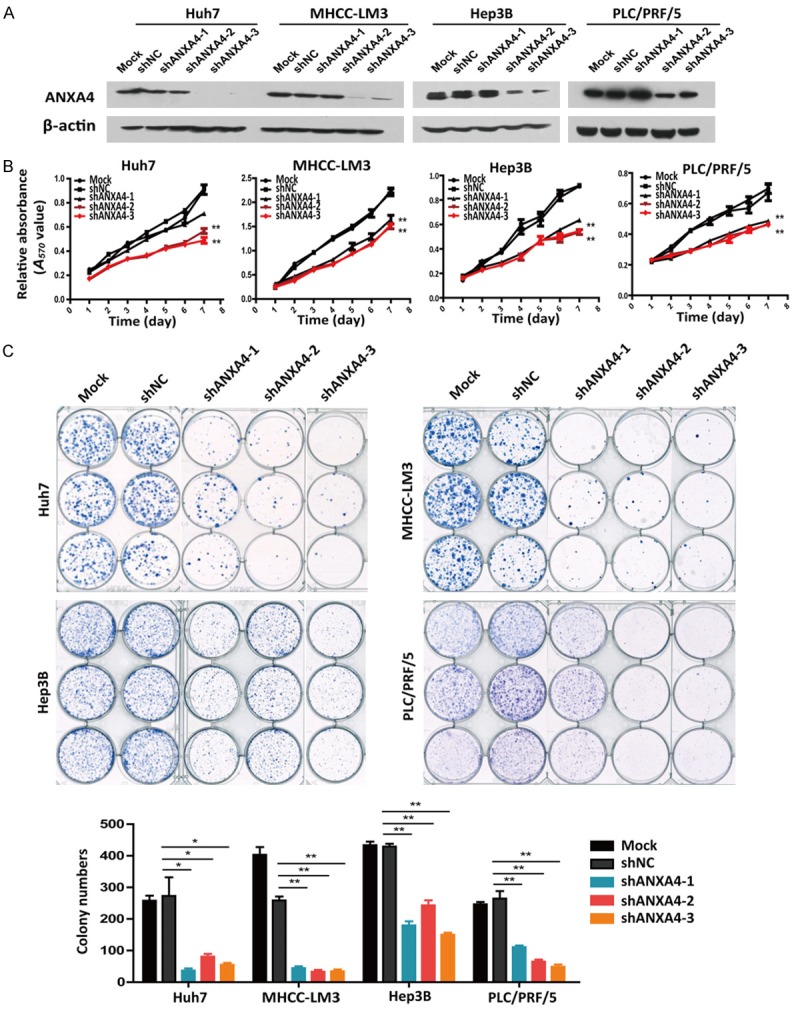
Knockdown of ANXA4 inhibits cell proliferation in vitro. A. Western blot analysis of ANXA4 expression in four shANXA4-HCC cell lines. B. Cell growth was analyzed at different time points by MTT assay. C. A colony formation assay was performed in 4 HCC cell lines stably transfected with shANXA4 or control plasmids (shNC) (images). The results of the statistical analysis are shown in a bar graph. *P < 0.05, **P < 0.01.
Figure 5.
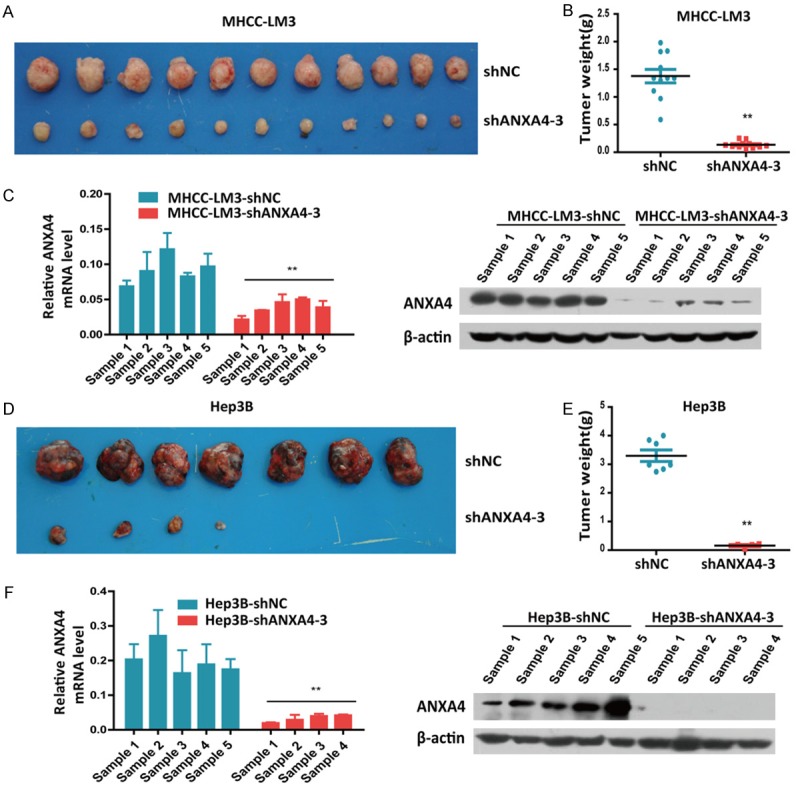
ANXA4 silencing suppresses HCC tumorigenesis in vivo. (A) MHCC-LM3 cells stably transfected with shANXA4-3 were subcutaneously injected into nude mice; shNC was used as a control, and the tumors were removed from the nude mice after 4 weeks. (B) The weight of the xenograft tumors shown in (A) was quantified in (B). (C) qRT-PCR and western blot analysis of ANXA4 expression in the MHCC-LM3 cell-derived xenograft samples. (D-F) We observed the same subcutaneous tumorigenesis result in mice injected with Hep3B HCC cells stably transfected with shANXA4-3. *P < 0.05, **P < 0.01.
Ikaros inhibits the expression of ANXA4 by repressing its promoter activity
Ikaros is a crucial regulator in HCC suppression, and ANXA4 is negatively associated with the expression of Ikaros and is downregulated by Ikaros. To determine whether Ikaros downregulates ANXA4 via binding to the ANXA4 promoter, we analyzed all potential Ikaros binding sites along the ANXA4 promoter from -1.5 kb to +0.1 kb of the transcription start site using the PROMO and Gene Regulation databases. ANXA4 promoter recombinant expression plasmids were constructed, and the primary binding sites were determined to be located in the ANXA4 promoter region from -1078 bp to +37 bp (Figure 6A). Reporter assays showed that ANXA4 promoter activity was inhibited in HCC cells overexpressing Ikaros (Figure 6B).
Figure 6.
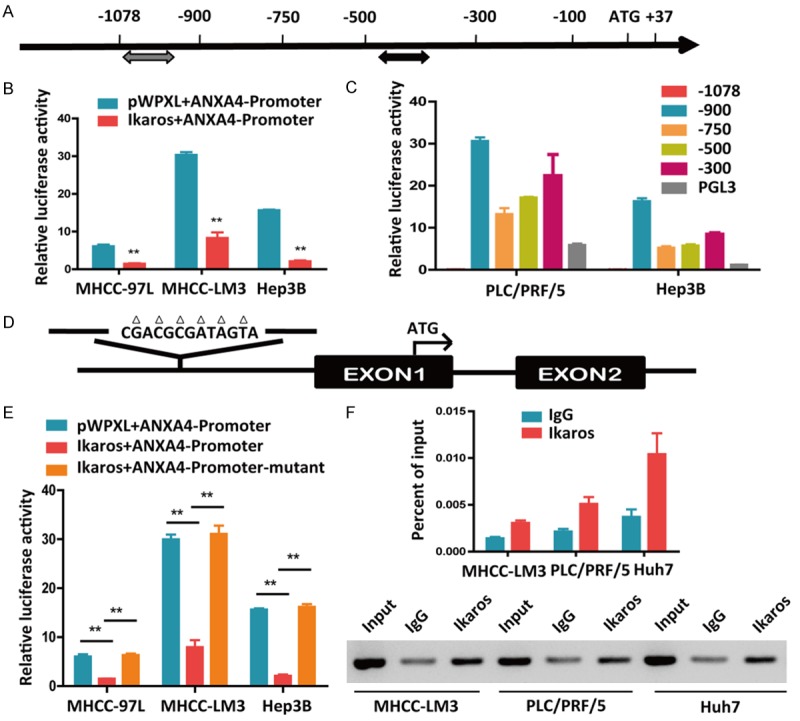
Ikaros directly binds to and represses the ANXA4 promoter in HCC cells. A. Identification of potential Ikaros binding sites in the ANXA4 promoter according to the PROMO and Gene Regulation databases. B. Activity of the ANXA4 promoter after transfection of Ikaros into HCC cells. C. The promoter activity of ANXA4 was detected after transfection with truncated ANXA4 promoter constructs. D. The mutated sequence is shown at the predicted Ikaros binding site on the ANXA4 promoter. E. The activities of the ANXA4 promoter and mutant promoter after transfection of HCC cells with Ikaros. F. The binding of Ikaros to the ANXA4 promoter in HCC cells was determined by chromatin immunoprecipitation, and qRT-PCR was used to analyze the ANXA4 promoter (top); agarose gel electrophoresis shows the crosslinking status (bottom). *P < 0.05, **P < 0.01.
The above results suggested that the promoter activity of ANXA4 was directly or indirectly downregulated by Ikaros. Next, we successively deleted sequences from the ANXA4 promoter and investigated the activity of these truncated promoters. The promoter activity was increased when the -1078/-900 bp sequences were deleted (Figure 6C). Based on this result, we hypothesized that this region may contain the binding site for the transcriptional repressor Ikaros. After analyzing the potential Ikaros binding sites using the Gene Regulation database, we constructed a plasmid containing a mutation in the potential binding site at -1043 bp to -1031 bp (Figure 6D). The reporter activity assay (Figure 6E) was performed, and a chromatin immunoprecipitation assay confirmed that Ikaros indeed bound this site on the ANXA4 promoter (Figure 6F).
Reintroduction of ANXA4 impairs Ikaros-induced suppression of HCC cell proliferation
Transcription factors can bind to their target gene promoter region and regulate their expression. We demonstrated that stable overexpression of Ikaros suppressed the proliferation of HCC cells in vitro, whereas restored expression of ANXA4 antagonized the inhibition of the in vitro proliferation of HCC cells (Figure 7A-C). In this functional rescue experiment, reintroduction of ANXA4 reversed the Ikaros-induced repression of cell proliferation. Thus, ANXA4 can promote cell proliferation in HCC, and it is a downstream regulatory target of Ikaros.
Figure 7.
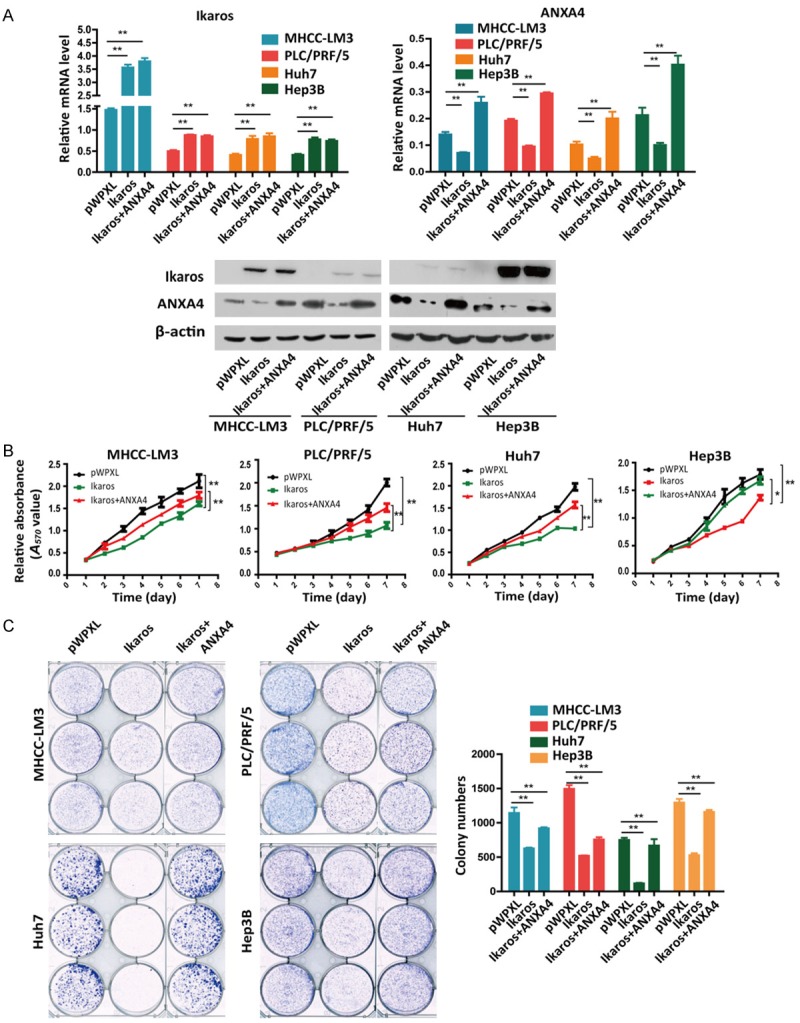
Reintroduction of ANXA4 impairs Ikaros-induced suppression of HCC cell proliferation. A. Western blot and qRT-PCR analyses of Ikaros or ANXA4 expression in Ikaros-transfected HCC cells and control cells. B, C. Cell growth at different time points was analyzed using MTT and colony formation assays. *P < 0.05, **P < 0.01.
Discussion
By inducing cell differentiation, the transcription factor Ikaros can suppress and control the development of leukemia, while mutations in Ikaros lead to decreased response to treatment and shorter long-term survival in leukemia patients [28,29]. Moreover, Ikaros protein expression can also be observed in liver tissue [30]. Downregulation of Ikaros expression is correlated with the malignant progression of acute lymphoblastic leukemia and 13 other types of cancer, including breast, skin, and ovarian cancers [19]. In a previous study, we determined that Ikaros induces the differentiation of CD133+ HCC cells and that Ikaros suppresses CD133 expression; thus, inhibition of the stemness property of CSCs is one functional role of Ikaros in HCC [11].
ANXA4 is a member of the Annexin family [31]. The human ANXA4 gene is located on chromosome 2p13, and the molecular mass of the ANXA4 protein is 35.9 kDa. Each Annexin consists of five α-helices and four Annexin domains [32]. By interacting with NF-κB p50, ANXA4 promotes the development of ovarian clear cell carcinoma [33]. According to lung cancer studies, fragile histidine triad (Fhit) gene mutations were detected in almost 80% of non-small cell lung cancer patients, and mutant Fhit increases paclitaxel resistance in lung cancer. ANXA4 is involved in the drug resistance mechanism, exhibiting a synergistic effect with Fhit [34]. Based on its importance in other cancers, we examined the function of ANXA4 in HCC cells.
ANXA4 has been shown to be essential for maintaining normal physiological cell conditions and is involved in the formation of membranes, membrane transport, signal transduction, and a series of calmodulin-dependent cell activities on the cell membrane surface, such as endocytosis and exocytosis [35]. In our study, we examined ANXA4 expression in primary HCC tissues. Compared with normal adjacent liver tissues, ANXA4 was frequently upregulated in HCC tissues. When we established stable Ikaros-expressing HCC cell lines, and found that ANXA4 was downregulated at both the mRNA and protein levels. Furthermore, our data also showed that ANXA4 promoted cell proliferation and contributed to the growth of HCC cells in vitro and in vivo. Thus, additional studies are needed to elucidate the molecular mechanism underlying the effects of ANXA4 on tumorigenesis in HCC and its different functions in different tumors.
Upon further examination, we found that Ikaros inhibited the expression of ANXA4 by repressing its promoter activity. To identify potential Ikaros binding sites along the ANXA4 promoter, we searched the PROMO and Gene Regulation databases and then successively deleted these predicted binding site sequences from the ANXA4 promoter. Next, we investigated the activity of these truncated promoters. We found that the promoter activity was increased when the -1078/-900-bp sequences were deleted. We then constructed a plasmid containing a mutant version of the potential binding site between -1043 bp and -1031 bp, and a reporter assay confirmed that this site was the binding site for Ikaros on the ANXA4 promoter.
In summary, we demonstrated that Ikaros acts as a transcriptional repressor of ANXA4 in HCC, and our data indicate that ANXA4 inhibition may be a potential strategy for anticarcinogenic therapy of HCC.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (81472726), the National Key Program for Basic Research of China (973) (2015CB553905), the Key Discipline and Specialty Foundation of Shanghai Municipal Commission of Health and Family Planning, the National KeySci-Tech Special Project of China (2013ZX10002-011), and the SKLORG Research foundation (91-15-03).
Disclosure of conflict of interest
None.
Abbreviations
- HCC
hepatocellular carcinoma
- RHAMM
hyaluronan-mediated motility receptor
- CDK1
cyclin-dependent kinase 1
- ALL
acute lymphoblastic leukemia
- Fhit
fragile histidine traid
References
- 1.Hernanda PY, Chen K, Das AM, Sideras K, Wang W, Li J, Cao W, Bots SJ, Kodach LL, de Man RA, Ijzermans JN, Janssen HL, Stubbs AP, Sprengers D, Bruno MJ, Metselaar HJ, ten Hagen TL, Kwekkeboom J, Peppelenbosch MP, Pan Q. SMAD4 exerts a tumor-promoting role in hepatocellular carcinoma. Oncogene. 2015;34:5055–5068. doi: 10.1038/onc.2014.425. [DOI] [PubMed] [Google Scholar]
- 2.Trojan J, Zangos S, Schnitzbauer AA. Diagnostics and treatment of hepatocellular carcinoma in 2016: standards and developments. Visc Med. 2016;32:116–120. doi: 10.1159/000445730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CC, Kim KH, Lau LF. The matricellular protein CCN1 suppresses hepatocarcinogenesis by inhibiting compensatory proliferation. Oncogene. 2016;35:1314–1323. doi: 10.1038/onc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L, Tong X, Zhang S, Yin F, Li X, Wei H, Li C, Guo Y, Zhao J. ASPP2 suppresses stem cell-like characteristics and chemoresistance by inhibiting the Src/FAK/Snail axis in hepatocellular carcinoma. Tumour Biol. 2016;37:13669–13677. doi: 10.1007/s13277-016-5246-0. [DOI] [PubMed] [Google Scholar]
- 5.Testino G, Leone S, Patussi V, Scafato E, Borro P. Hepatocellular carcinoma: diagnosis and proposal of treatment. Minerva Med. 2016;107:413–426. [PubMed] [Google Scholar]
- 6.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 8.Capece D, Zazzeroni F, Mancarelli MM, Verzella D, Fischietti M, Di TA, Maccarone R, Plebani S, Di IM, Gulino A. A novel, non-canonical splice variant of the Ikaros gene is aberrantly expressed in B-cell lymphoproliferative disorders. PLoS One. 2013;8:e68080. doi: 10.1371/journal.pone.0068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jager R, Gisslinger H, Passamonti F, Rumi E, Berg T, Gisslinger B, Pietra D, Harutyunyan A, Klampfl T, Olcaydu D, Cazzola M, Kralovics R. Deletions of the transcription factor Ikaros in myeloproliferative neoplasms. Leukemia. 2010;24:1290–1298. doi: 10.1038/leu.2010.99. [DOI] [PubMed] [Google Scholar]
- 10.Singh H, Medina KL, Pongubala JM. Contingent gene regulatory networks and B cell fate specification. Proc Natl Acad Sci U S A. 2005;102:4949–4953. doi: 10.1073/pnas.0500480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Li H, Ge C, Li M, Zhao FY, Hou HL, Zhu MX, Tian H, Zhang LX, Chen TY, Jiang GP, Xie HY, Cui Y, Yao M, Li JJ. Inhibitory effects of transcription factor Ikaros on the expression of liver cancer stem cell marker CD133 in hepatocellular carcinoma. Oncotarget. 2014;5:10621–10635. doi: 10.18632/oncotarget.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benz J, Hofmann A. Annexins: from structure to function. Biol Chem. 1997;378:177–183. [PubMed] [Google Scholar]
- 13.Mirsaeidi M, Gidfar S, Vu A, Schraufnagel D. Annexins family: insights into their functions and potential role in pathogenesis of sarcoidosis. J Transl Med. 2016;14:89. doi: 10.1186/s12967-016-0843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei B, Guo C, Liu S, Sun MZ. Annexin A4 and cancer. Clin Chim Acta. 2015;447:72–78. doi: 10.1016/j.cca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Lin LL, Huang HC, Juan HF. Revealing the molecular mechanism of gastric cancer marker annexin A4 in cancer cell proliferation using exon arrays. PLoS One. 2012;7:e44615. doi: 10.1371/journal.pone.0044615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 17.Heinick A, Husser X, Himmler K, Kirchhefer U, Nunes F, Schulte JS, Seidl MD, Rolfes C, Dedman JR, Kaetzel MA, Gerke V, Schmitz W, Muller FU. Annexin A4 is a novel direct regulator of adenylyl cyclase type 5. FASEB J. 2015;29:3773–3787. doi: 10.1096/fj.14-269837. [DOI] [PubMed] [Google Scholar]
- 18.Han EK, Tahir SK, Cherian SP, Collins N, Ng SC. Modulation of paclitaxel resistance by annexin IV in human cancer cell lines. Br J Cancer. 2000;83:83–88. doi: 10.1054/bjoc.2000.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogami T, Yokota N, Asai-Sato M, Yamada R, Koizume S, Sakuma Y, Yoshihara M, Nakamura Y, Takano Y, Hirahara F, Miyagi Y, Miyagi E. Annexin A4 is involved in proliferation, chemoresistance and migration and invasion in ovarian clear cell adenocarcinoma cells. PLoS One. 2013;8:e80359. doi: 10.1371/journal.pone.0080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin LL, Chen CN, Lin WC, Lee PH, Chang KJ, Lai YP, Wang JT, Juan HF. Annexin A4: a novel molecular marker for gastric cancer with Helicobacter pylori infection using proteomics approach. Proteomics Clin Appl. 2008;2:619–634. doi: 10.1002/prca.200780088. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto A, Serada S, Enomoto T, Kim A, Matsuzaki S, Takahashi T, Ueda Y, Yoshino K, Fujita M, Fujimoto M, Kimura T, Naka T. Annexin A4 induces platinum resistance in a chlorideand calcium-dependent manner. Oncotarget. 2014;5:7776–7787. doi: 10.18632/oncotarget.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng S, Wang J, Hou L, Li J, Chen G, Jing B, Zhang X, Yang Z. Annexin A1, A2, A4 and A5 play important roles in breast cancer, pancreatic cancer and laryngeal carcinoma, alone and/or synergistically. Oncol Lett. 2013;5:107–112. doi: 10.3892/ol.2012.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita T, Nagano K, Kanasaki S, Maeda Y, Furuya T, Inoue M, Nabeshi H, Yoshikawa T, Yoshioka Y, Itoh N, Abe Y, Kamada H, Tsutsumi Y, Tsunoda S. Annexin A4 is a possible biomarker for cisplatin susceptibility of malignant mesothelioma cells. Biochem Biophys Res Commun. 2012;421:140–144. doi: 10.1016/j.bbrc.2012.03.144. [DOI] [PubMed] [Google Scholar]
- 24.Kajihara H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, Yoshida S, Sado T, Oi H, Kobayashi H. Clear cell carcinoma of the ovary: potential pathogenic mechanisms (Review) Oncol Rep. 2010;23:1193. doi: 10.3892/or_00000750. [DOI] [PubMed] [Google Scholar]
- 25.Yao HS, Chang S, Li XX, Yi W, Jin KZ, Zhang XP, Hu ZQ. Annexin A4-nuclear factor-κB feedback circuit regulates cell malignant behavior and tumor growth in gallbladder cancer. Sci Rep. 2016;6:31056. doi: 10.1038/srep31056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon YJ, Kim DH, Jung H, Sang JC, Chi SW, Cho S, Sang CL, Park BC, Park SG, Bae KH. Annexin A4 interacts with the NF-κB p50 subunit and modulates NF-κB transcriptional activity in a Ca 2+ -dependent manner. Cell Mol Life Sci. 2010;67:2271–2281. doi: 10.1007/s00018-010-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C, Li H, Li J, Zhu Z, Yin S, Hao X, Yao M, Zheng S, Gu J. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis. 2008;29:2289–2297. doi: 10.1093/carcin/bgn223. [DOI] [PubMed] [Google Scholar]
- 28.Popescu M, Gurel Z, Ronni T, Song C, Hung KY, Payne KJ, Dovat S. Ikaros stability and pericentromeric localization are regulated by protein phosphatase 1. J Biol Chem. 2009;284:13869. doi: 10.1074/jbc.M900209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dovat S, Gurel Z, Payne KJ, Popescu M. Dephosphorylation of Ikaros by a specific phosphatase regulates its function in chromatin remodeling. Journal of Immunology [Google Scholar]
- 30.Yang L, Luo Y, Wei J. Integrative genomic analyses on Ikaros and its expression related to solid cancer prognosis. Oncol Rep. 2010;24:571–577. doi: 10.3892/or_00000894. [DOI] [PubMed] [Google Scholar]
- 31.Masse KL, Collins RJ, Bhamra S, Seville RA, Jones EA. Anxa4 genes are expressed in distinct organ systems in xenopus laevis and tropicalis but are functionally conserved. Organogenesis. 2007;3:83–92. doi: 10.4161/org.3.2.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang D, Golubkov VS, Han W, Correa RG, Zhou Y, Lee S, Strongin AY, Dong PD. Identification of annexin A4 as a hepatopancreas factor involved in liver cell survival. Dev Biol. 2014;395:96–110. doi: 10.1016/j.ydbio.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Deng L, Cai M, Zhuang H, Zhu L, Hao Y, Gao J, Liu J, Li X, Lin B. Annexin A4 fucosylation enhances its interaction with the NF-κB p50 and promotes tumor progression of ovarian clear cell carcinoma. Oncotarget. 2016 doi: 10.18632/oncotarget.10226. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaudio E, Paduano F, Spizzo R, Ngankeu A, Zanesi N, Gaspari M, Ortuso F, Lovat F, Rock J, Hill GA, Kaou M, Cuda G, Aqeilan RI, Alcaro S, Croce CM, Trapasso F. Fhit delocalizes annexin a4 from plasma membrane to cytosol and sensitizes lung cancer cells to paclitaxel. PLoS One. 2013;8:e78610. doi: 10.1371/journal.pone.0078610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwasa T, Takahashi R, Nagata K, Kobayashi Y. Suppression of MIP-2 or IL-8 production by annexins A1 and A4 during coculturing of macrophages with late apoptotic human peripheral blood neutrophils. Biochim Biophys Acta. 2012;1822:204–211. doi: 10.1016/j.bbadis.2011.10.013. [DOI] [PubMed] [Google Scholar]


