Abstract
The purpose of this study was to explore the potential role of HOTAIR in thyroid cancer carcinogenesis. We found that HOTAIR was unregulated in human thyroid cancer and inversely correlated with miR-1. Functional assays indicated HOTAIR regulates miR-1 directly in thyroid cancer cells. We also revealed that HOTAIR promotes the processes of thyroid cancer cell malignancy through regulation of microRNA-1 (miR-1). Furthermore, we showed that HOTAIR could regulate a downstream target of miR-1, CCND2, in a miR-1-mediated manner. In addition, we also proved, using a tumor formation assay in nude mice, that silencing HOTAIR inhibited tumor formation in vivo. Therefore, our study demonstrated that HOTAIR promotes the development and progression of thyroid cancer through inhibition of microRNA-1 and activation of CCND2.
Keywords: HOTAIR, mircroRNA-1, thyroid cancer, migration and invasion, CCND2
Introduction
Thyroid cancer composes a large proportion (approximately 93%) of endocrine cancers and has substantially increased morbidity [1,2]. In 2015, there were 62,450 new thyroid cancer cases and approximately 1,950 thyroid cancer deaths [3]. Although the current treatment methods for thyroid cancer are improved, mainly surgical resection, radiotherapy and levothyroxine treatment [4], metastatic thyroid carcinoma has a poor prognosis [5,6]. Therefore, further studies on the molecular mechanisms and functions of thyroid cancer development are still urgently needed.
Long non-coding RNAs (lncRNAs), a new family of regulatory RNAs, regulate gene expression levels via transcription regulation, genomic imprinting and chromatin modification [7,8]. Studies have shown that lncRNAs are involved in the carcinogenesis of lung [9], liver [10] 55 vwV, and breast cancer [11,12]. In addition, several studies have shown that the lncRNA-HOTAIR is involved in various cancers [13], such as hepatocellular cancer [14], colorectal cancers [15], lung cancer [16], and gastric cancer [17]. However, it remains unclear whether HOTAIR plays a key role in thyroid cancer.
MicroRNAs (miRNAs) are highly conserved noncoding RNAs approximately 20 nucleotides in length that take part in post-transcriptional regulation of target genes and play important roles in tumor carcinogenesis [18,19]. A variety of studies have suggested that miR-1 is involved in lung cancer [20], bladder cancer [21], prostate cancer [22], colorectal cancer [23], and squamous cell cancer [24]. One study also demonstrated that miR-1 acts as a tumor suppressor though targeting CCND2 in thyroid cancer [25]. However, there is no evidence of a relationship between HOTAIR and miR-1 in thyroid cancer.
In our study, we indicated that HOTAIR promotes the processes of thyroid cancer through regulation of the miR-1-CCND2 axis. Therefore, our study has shown that HOTAIR/miR-1/CCND2 may serve as a potential target for thyroid cancer treatment.
Materials and methods
Patients and tissue samples
We collected 26 pairs of thyroid cancer tissues and adjacent noncancerous thyroid tissues from patients at The Second Hospital of Hebei Medical University. This study was approved by the Ethics Committee of the Second Hospital of Hebei Medical University. Informed consent was obtained from patients. All tissue samples were stored at -80°C until used.
Vector construction and transfections
The siRNA sequences targeting human HOTAIR (siHOTAIR1#: UAA CAA GAC CAG AGA GCU GUU; siHOTAIR2#: CCA CAU GAA CGC CCA GAG AUU; siHOTAIR3#: GAA CGG GAG UAC AGA GAG AUU) or negative control RNA (NC, CUA CAA CAG CCA CAA CGU CdT dT) were designed and purchased from GenePharma (GenePharma Co., Ltd., Shanghai, China). According to the manufacturer’s instructions, TPC-1 and FTC-133 cells were transfected with 50 nM si-HOTAIR1#, si-HOTAIR2#, si-HOTAIR3# and control using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). A total of 200 μl mature anti-NC and anti-miR-1 (GenePharma) were transfected into TPC-1 and FTC-133 cells, which have been transfected with si-HOTAIR3# and control for 72 hrs. Mature miR-1 (GenePharma) was transfected into TPC-1 and FTC-133 cells using Lipofectamine 2000 for 72 hrs. Constructed CCND2 and vector were transfected into TPC-1 and FTC-133 cells, which have been transfected with si-HOTAIR3# or miR-1 for 48 hrs., respectively.
Quantitative real-time reverse transcription PCR (qRT-PCR)
According to the manufacturer’s instructions, total RNA was extracted from thyroid cancer tissues and TPC-1 and FTC-133 cells. cDNAs were synthesized using the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher, Shanghai, China). The SYBR-Green PCR Master Mix Kit (Takara) was used to detect mRNA expression. The primer sequences for HOTAIR were 5’-TTT GGA CTG TAA AAT ATG GC-3’ (forward) and 5’- TTC TGA CAC TGA ACG GAC T-3’ (reverse). The primer sequences for miR-1 were 5’-GTA GGC ACC TGA AAT GGA A-3’ (forward) and 5’-TTG ATG GTG CCT ACA GTA CAT-3’ (reverse). The primer sequences for GAPDH were 5’-CCT CGT CTC ATA GAC AAG ATG GT-3’ (forward) and 5’-GGG TAG AGT CAT ACT GGA ACA TG-3’ (reverse) (internal control). The primer sequences for U6 were 5’- CTC GCT TCG GCA GCA CA-3’ (forward) and 5’- AAC GCT TCA CGA ATT TGC GT-3’ (reverse).
Protein extraction and Western blot
Transfected cells were lysed by lysis buffer supplemented with a protease inhibitor cocktail (Sigma-Aldrich). Equal concentrations of proteins were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). Western blotting analysis was performed as previously described [26]. The primary antibody used was the anti-CCND2 antibody (1:200, Abcam, Cambridge, UK); the anti-GAPDH antibody (1:4000, Beverly, MA, USA) was used as internal control.
Luciferase reporter assay
Cells (5×104 cells/well) were cultured in a 24-well plate and co-transfected with wild type (HOTAIR-WT) or mutant (HOTAIR-Mut) HOTAIR and miR-1 using Lipofectamine 2000 (Invitrogen) for 48 hrs. The pRL-CMV Renilla was used as an internal control. According to the manufacturer’s instructions, the luciferase activities were measured by the Dual-Luciferase Reporter Assay System (Promega, Madison, WI).
RNA immunoprecipitation assay
For the RNA immunoprecipitation (RIP) assay, the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) was used. qRT-PCR was used to detect the co-precipitated RNAs.
Colony-forming unit assay
Treated cells (2×103 cells) were seeded in 100-mm dishes in complete medium for 10 days at 37°C and 5% CO2. The cells were fixed and stained with 0.1% crystal violet and colony-forming units were counted.
Proliferation assay
Treated cells (5×103 cells /well) were seeded in a 96-well plate and incubated at 37°C. At 0, 12, 24, and 48 hrs., 20 μl of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, 5 mg/mL) were added to each well. The original culture was removed, and 100 μl of a dimethyl sulfoxide (DMSO) solution were added to dissolve the crystal after 4 hrs. Absorbance was measured at 570 nm by a micro-plate reader (Bio Tek Instruments).
Wound healing assay
As previously described [27], treated cells were seeded in a 6-well plate. Small wounds were created and cell fragments were gently washed away. Images were taken under a microscope at 0 and 3 days.
Migration and invasion assays
For the migration assay, treated TPC-1 and FTC-133 cells (1×105 cells/well in 200 μl of serum-free medium) were seeded on the upper side of a polycarbonate membrane (8-mm pore), and 600 μl of complete medium were added to the lower chamber. After 24 hrs., the cells at on the upper side of the chamber were removed, and the cells on the bottom side of the chamber were fixed and stained with a crystal violet solution (Sigma-Aldrich). The migrated cells were obtained and counted. For the invasion assay, the diluted Matrigel (BD Biosciences) was pre-applied to the upper side of the polycarbonate membrane for 1 h at 37°C.
Flow cytometric analysis of the cell cycle and apoptosis
Cells were analyzed by flow cytometry as previously described [28]. Briefly, cells were harvested, fixed, and stained. Images of the cell cycle and apoptosis were obtained by the FACS Calibur system (BD Biosciences).
Tumor formation in nude mice
This study was approved by the Institutional Committee of the Second Hospital of Hebei Medical University. Treated TPC-1 and FTC-133 cells (1×107 cells in 200 μl serum-free medium) were subcutaneously injected into 5-week-old BALB/c male nude mice. At specific times, the mice were killed, and the weight and volume of the tumors were determined.
Statistical analysis
All experiments were performed independently three times. Data were expressed as the mean ± S.D. Graph Prism 5.0 software (GraphPad Prism, San Diego, CA) was used to analyze the results by Student’s t-test or one-way analysis of variance (ANOVA). P < 0.05 was regarded as statistically significant.
Results
LncRNA HOTAIR is highly expressed in human thyroid cancer
The expression of HOTAIR was detected in thyroid cancer tissues and adjacent noncancerous tissues from 26 patients. The results proved that HOTAIR expression was higher in thyroid cancer tissues than adjacent noncancerous tissues (P < 0.001) (Figure 1A). To further study the role of HOTAIR in thyroid cancer cells, qRT-PCR was used to detect the expression of HOTAIR in a human thyroid follicular epithelial cell line (Nthy-ori 3-1) and thyroid cancer cell lines (TPC-1, FTC-133, B-CPAP, and SW579). We found that HOTAIR expression was higher in the thyroid cancer cell lines than the Nthy-ori 3-1 cells (P < 0.05) (Figure 1B).
Figure 1.
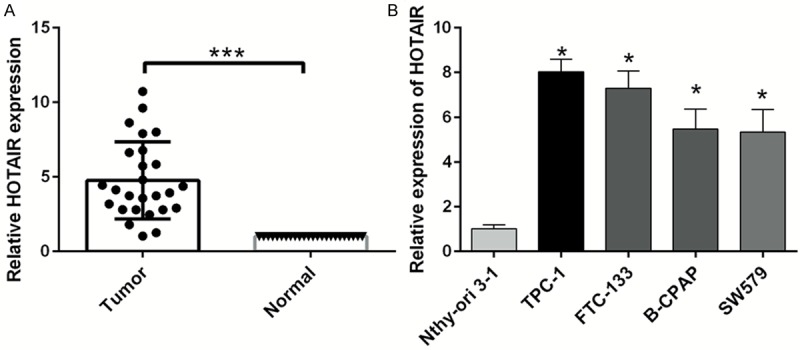
HOTAIR is highly expressed in human thyroid cancer. A. The expression level of HOTAIR was detected by qRT-PCR in 26 thyroid cancer tissues and paired adjacent normal tissues. B. The expression level of HOTAIR was measured by qRT-PCR in the human thyroid follicular epithelial cell line (Nthy-ori 3-1) and thyroid cancer cell lines (TPC-1, FTC-133, B-CPAP, and SW579) (*P < 0.05, ***P < 0.001).
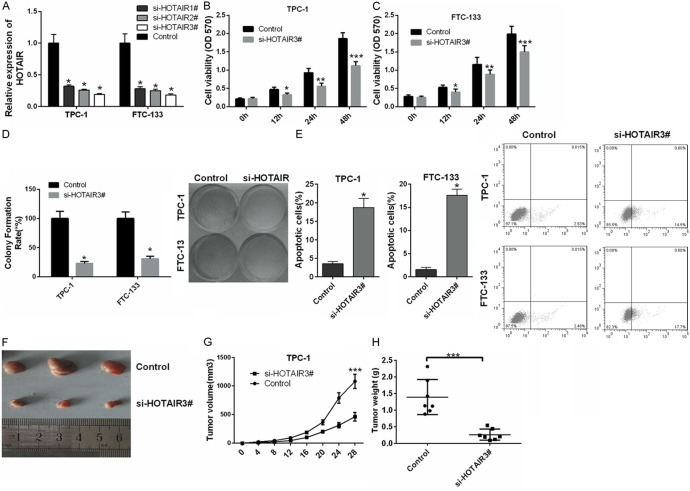
Silencing HOTAIR expression inhibits thyroid cancer cell growth in vivo and in vitro
To understand the functions of HOTAIR in thyroid cancer, TPC-1 and FTC-133 cells were transfected with siRNAs of HOTAIR, including si-HOTAIR1#, si-HOTAIR2#, si-HOTAIR3# and control. qRT-PCR was used to detect the effects of si-HOTAIR1#, si-HOTAIR2#, and si-HOTAIR3# in TPC-1 and FTC-133 cells. Our results showed that the expression of HOTAIR was markedly decreased in TPC-1 and FTC-133 cells transfected with si-HOTAIRs compared with control. si-HOTAIR3# had the highest silencing efficiency (Figure 2A); thus, si-HOTAIR3# was used for further studies. MTT assays indicated that knockdown of HOTAIR significantly inhibited the proliferation of TPC-1 (Figure 2B) and FTC-133 cells (Figure 2C) (P < 0.05, P < 0.01, P < 0.001). Colony-forming unit assay results also proved that silencing HOTAIR significantly suppressed thyroid cancer cell proliferation (P < 0.05) (Figure 2D). Furthermore, silencing HOTAIR promoted apoptosis of TPC-1 and FTC-133 cells (P < 0.05) (Figure 2E). We further explored the effect of HOTAIR on thyroid cancer tumorigenesis in vivo. TPC-1 cells stably transfected with si-HOTAIR3# or control were subcutaneously injected into nude mice, and the tumor was excised at 28 days (Figure 2F). Tumor volume was smaller in the si-HOTAIR3#-TPC-1 group than in the control group (P < 0.001) (Figure 2G). The tumor weight followed the same pattern and was smaller in the si-HOTAIR3#-TPC-1 group than in the control group (P < 0.001) (Figure 2H).
Figure 2.
Silencing HOTAIR inhibits thyroid cancer cell growth. A. The expression level of HOTAIR was measured by qRT-PCR in TPC-1 and FTC-133 cells transfected with siRNAs of HOTAIR (si-HOTAIR1#, si-HOTAIR2#, si-HOTAIR3#) and control. B, C. Silencing HOTAIR by si-HOTAIR3# significantly inhibited proliferation of TPC-1 and FTC-133 cells at 12 hrs., 24 hrs., and 48 hrs. Cell proliferation was detected by MTT assay. D. Cell proliferation was detected by colony-forming unit assay in TPC-1 and FTC-133 cells transfected with si-HOTAIR3# and control. E. Apoptosis was measured by Annexin V-APC/7-AAD staining in TPC-1 and FTC-133 cells transfected with si-HOTAIR3# and control. F. Tumors were collected from athymic mice injected with TPC-1 cells transfected with si-HOTAIR3# or control. G. The tumor volume was analyzed at specific times. H. The tumor weight was measured at specific times (n = 7). (*P < 0.05, **P < 0.01, ***P < 0.001).
Silencing HOTAIR inhibits migration and invasion of thyroid cancer cells
Silencing HOTAIR significantly inhibited the invasion of TPC-1 (Figure 3A) and FTC-133 cells (Figure 3B) (P < 0.05). Representative invasion images are shown in Figure 3C. The results of the migration assay also indicated that migration was decreased in TPC-1 and FTC-133 cells transfected with si-HOTAIR3# compared with control cells (P < 0.05) (Figure 3D-F). Wound healing assay results also revealed that silencing HOTAIR significantly inhibited the migration of TPC-1 and FTC-133 cells (Figure 3G, 3H).
Figure 3.
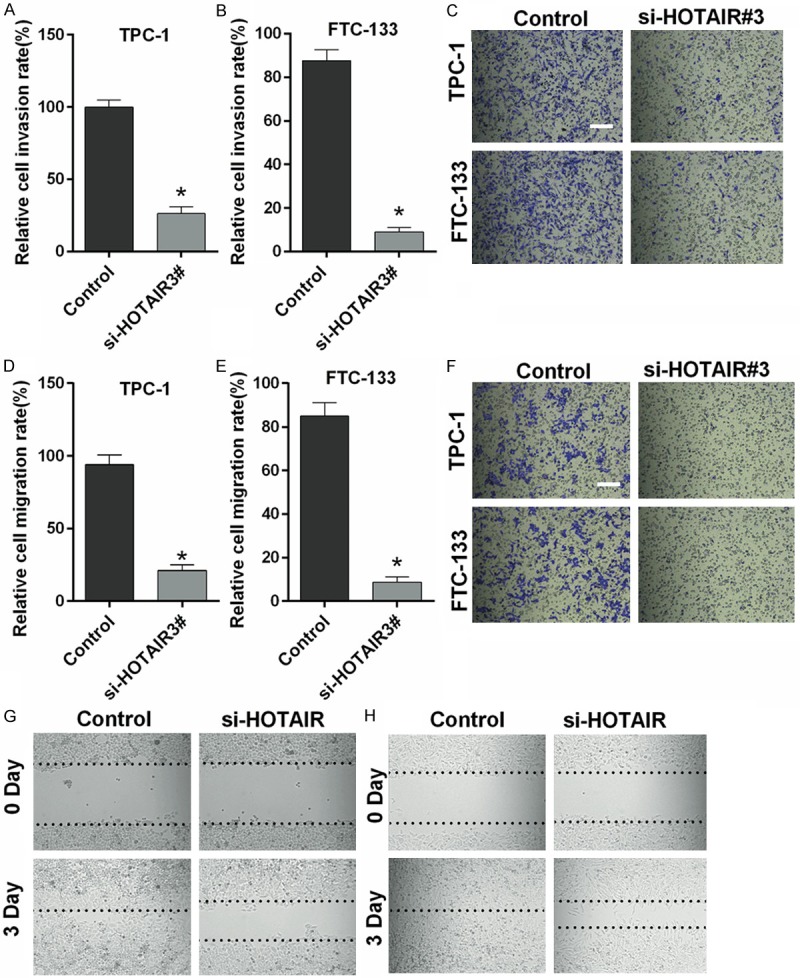
Silencing HOTAIR inhibits migration and invasion of thyroid cancer cells. A-C. Silencing HOTAIR significantly inhibited the invasion of TPC-1 and FTC-133 cells in a Transwell assay. D-F. Silencing HOTAIR inhibited the migration of TPC-1 and FTC-133 cells in Transwell assay. G, H. Silencing HOTAIR inhibited the migration of TPC-1 and FTC-133 cells in wound healing assay. (Magnification 200×, Scale bars = 10 μm, *P < 0.05).
HOTAIR negatively regulates miR-1 in thyroid cancer cells
To further explore the molecular mechanisms of HOTAIR in thyroid cancer, TPC-1 and FTC-133 cells were transfected with a HOTAIR overexpression vector and a control vector. Gene microarray results showed that miR-1, miR-148a, miR-141, and miR-27b expression were decreased in TPC-1 and FTC-133 cells transfected with HOTAIR compared with control, while miR-21 and miR-27a were increased (Figure 4A). As shown in Figure 4B, the putative binding site of miR-1 and HOTAIR was predicted by starBase v2.0. qRT-PCR results indicated that the expression of miR-1 was significantly lower in TPC-1 and FTC-133 cells transfected with pGL3-HOTAIR than control (P < 0.05) (Figure 4C), while miR-1 was significantly higher in TPC-1 and FTC-133 cells transfected with si-HOTAIR3# than control (P < 0.05) (Figure 4D). The luciferase reporter assay indicates the interaction between miR-1 and HOTAIR disappeared when the binding sites were mutated (P < 0.05) (Figure 4E). In addition, the interaction between HOTAIR and miR-1 was analyzed by RIP assay in thyroid cancer cells. Endogenous miR-1 was highly enriched by HOTAIR RIP assay compared with control, indicating that HOTAIR could act as a miR-1 sponge in thyroid cancer cells (Figure 4F).
Figure 4.
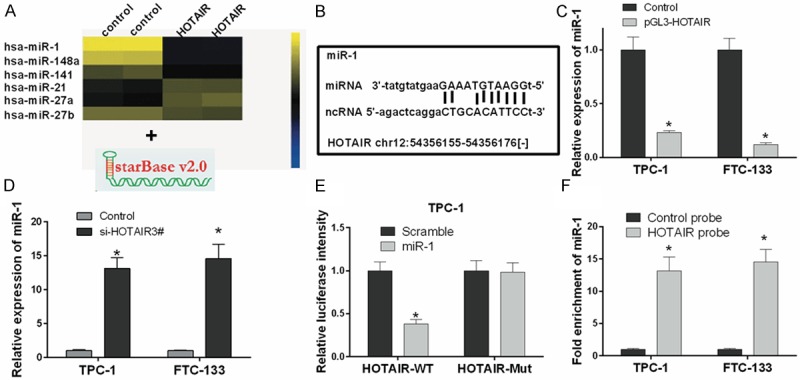
HOTAIR negatively regulates miR-1 in thyroid cancer cells. A. Cluster heat map of significantly differentially expressed miRNAs in TPC-1 cells transfected with a HOTAIR overexpression or control vector on a scale from low (blue) to high (yellow). Individual cell subsets are depicted as columns. B. The putative binding site of miR-1 and HOTAIR is shown. C, D. The expression level of miR-1 was measured by qRT-PCR in TPC-1 and FTC-133 cells transfected with pGL3-HOTAIR, si-HOTAIR3# or controls. E. Luciferase activity was measured in TPC-1 cells co-transfected with wild type (HOTAIR-WT) or mutant (HOTAIR-Mut) HOTAIR and miR-1 using the luciferase reporter assay. F. miR-1 was significantly enriched by RNA immunoprecipitation (RIP) assay in the HOTAIR group compared with control (*P < 0.05).
HOTAIR promotes thyroid cancer cell growth and invasion through inhibition of miRNA-1
To understand the roles of HOTAIR and miR-1 in thyroid cancer, TPC-1 and FTC-133 cells were transfected with si-HOTAIR3# and anti-miR-1, si-HOTAIR3# and anti-NC, or control and anti-NC. Compared with controls, the expression of miR-1 was significantly up-regulated by si-HOTAIR3# and was significantly down-regulated by anti-miR-1 (P < 0.05) (Figure 5A). MTT assay results indicated that HOTAIR promoted the cell growth of TPC-1 and FTC-133, and this growth could be reversed by anti-miRNA-1 (P < 0.05) (Figure 5B, 5C). Colony-forming unit assay data further revealed that HOTAIR promoted thyroid cancer cell growth through inhibition of miRNA-1 (P < 0.05) (Figure 5D, 5E). Transwell assay results also indicated that HOTAIR promoted thyroid cancer cell invasion through inhibition of miRNA-1 (P < 0.05) (Figure 5F).
Figure 5.
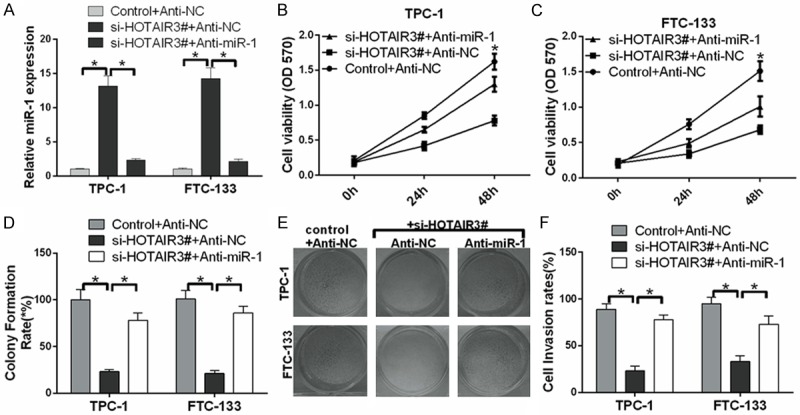
HOTAIR promotes thyroid cancer cell growth and invasion through inhibition of miRNA-1. A. The expression level of miR-1 was measured by qRT-PCR in TPC-1 and FTC-133 cells transfected with si-HOTAIR3# and anti-miR-1, si-HOTAIR3# and anti-NC, and control and anti-NC. B and C. Proliferation was detected by MTT assay in treated TPC-1 and FTC-133 cells. D, E. Proliferation was detected by colony-forming unit assay in treated TPC-1 and FTC-133 cells. F. The effect of HOTAIR on thyroid cancer cell invasion through miRNA-1 by Transwell assays (*P < 0.05).
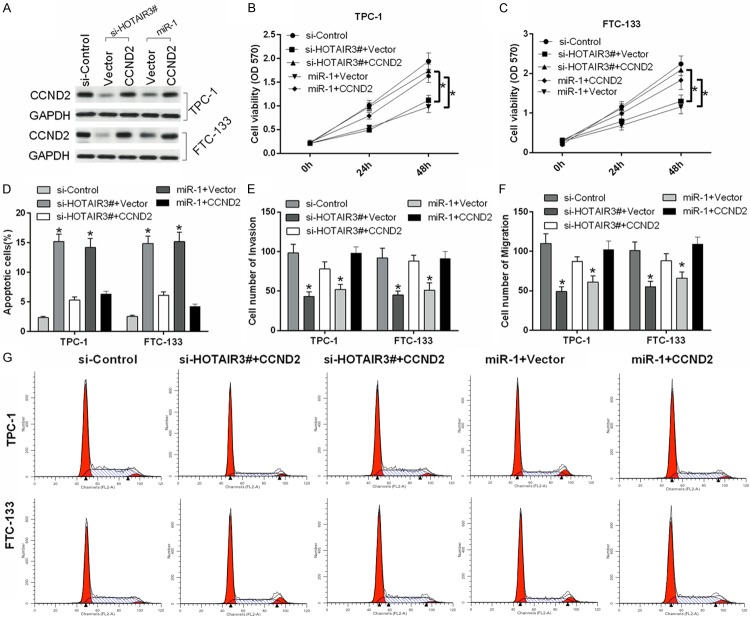
HOTAIR promotes the processes of thyroid cancer cell tumorigenesis through the miRNA-1/CCND2 axis
Previous research has shown that miR-1 serves as a tumor suppressor by targeting CCND2 in thyroid cancer [25]. In our study, we demonstrated the relationships among HOTAIR, miR-1, and CCND2 and their effects on the development and progression of thyroid cancer. First, TPC-1 and FTC-133 cells were transfected with si-control, si-HOTAIR3# and vector, si-HOTAIR3# and CCND2, miR-1 and vector, and miR-1 and CCND2. The protein level of CCND2 was downregulated by si-HOTAIR3# and miR-1 in TPC-1 and FTC-133 cells and restored by CCND2 overexpression (Figure 6A). Furthermore, our study showed that HOTAIR promoted the growth of TPC-1 (Figure 6B) and FTC-133 cells (Figure 6C); inhibited apoptosis of TPC-1 and FTC-133 cells through inhibition of miRNA-1 and activation of CCND2 (Figure 6D); accelerated invasion of TPC-1 and FTC-133 cells through inhibition of miRNA-1 and activation of CCND2 (Figure 6E); and accelerated migration of TPC-1 and FTC-133 cells (Figure 6F) through inhibition of miRNA-1 and activation of CCND2. Finally, cell cycle arrest induced by HOTAIR silencing in TPC-1 and FTC-133 cells was detected by flow cytometry. The growth-inhibiting effect of HOTAIR was prevented through suppressing arrest at the G1/S-phase transition. Therefore, HOTAIR inhibited cell cycle arrest in TPC-1 and FTC-133 cells through inhibition of miRNA-1 and activation of CCND2 (Figure 6G). A model of the functions and regulatory mechanisms of the HOTAIR/miR-1-CCND2 axis in thyroid cancer development was established (Figure 7).
Figure 6.
HOTAIR promotes thyroid cancer processes through inhibition of miRNA-1 and activation of CCND2. A. Western blot revealed the protein expression level of CCND2 in transfected TPC-1 and FTC-133 cells. GAPDH was used as a protein-loading control. B, C. Proliferation was measured by MTT assay in treated TPC-1 and FTC-133 cells. D. Apoptosis was detected by Annexin V-APC/7-AAD staining in treated TPC-1 and FTC-133 cells. E, F. Migration and invasion were measured by Transwell assays in treated TPC-1 and FTC-133 cells. G. Cell cycle distributions were detected by flow cytometry in treated TPC-1 and FTC-133 cells (*P < 0.05).
Figure 7.
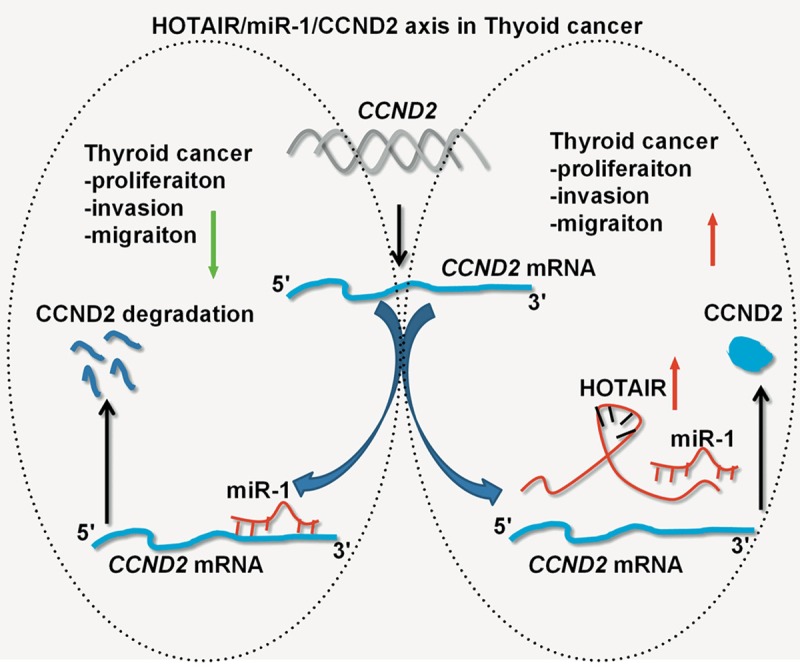
A diagram showing the functions and regulatory mechanisms of the HOTAIR/miR-1-CCND2 axis in thyroid cancer. In the absence of HOTAIR, miR-1 suppresses thyroid cancer cell growth, cell cycle, migration and invasion, and promotes apoptosis by targeting CCND2. Meanwhile, HOTAIR acts as a sponge to inhibit miR-1 expression, which then activates CCND2 and promotes thyroid cancer cell growth, migration and invasion.
Discussion
Numerous studies have shown that HOTAIR participates in cancer development. For instance, HOTAIR was highly expressed in primary breast cancer [29]. Knockdown of HOTAIR suppressed invasion and reversed epithelial-mesenchymal transition in gastric cancer [30]. HOTAIR expression was associated with poor prognosis and accelerated metastasis in non-small cell lung cancer [31]. In our study, the expression of HOTAIR was increased in thyroid cancer tissues compared with adjacent noncancerous tissues. Silencing HOTAIR inhibited proliferation and tumor formation and accelerated apoptosis of thyroid cancer cells. In addition, we indicated that silencing HOTAIR inhibited migration and invasion of thyroid cancer cells. These findings will provide significant contributions to the treatment of thyroid cancer.
Recently, numerous studies have shown that miR-1 plays important roles in the development of cancers. For example, miR-1 suppressed tumorigenicity of lung cancer cells [20], exerted a tumor-suppressive function by targeting TAGLN2 in bladder cancer [21], served as a tumor suppressor though targeting purine nucleoside phosphorylase (PNP) in prostate cancer [22], inhibited EMT in human colon cancer [32], and regulated cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2 [33]. Our results also demonstrated that miR-1 inhibited cell growth, cell cycle, migration and invasion and promoted cell apoptosis in thyroid cancer.
In recent years, ceRNA has been regarded as a new mechanism of post-transcriptional regulation through miRNA competition [34]. CeRNA targets and miRNA can combine to form a complex ceRNA network [35]. Some evidence has shown that ceRNA crosstalk has great impact on essential cellular processes, and its imbalance will affect various diseases [36]. In this study, the lncRNA-HOTAIR negatively regulated miR-1 by competitively binding to the miR-1 site directly and participated in regulating thyroid cancer cell carcinogenesis. However, the ceRNA network is so complex that we still need to improve our understanding of the HOTAIR-miR-1 regulation network in thyroid cancer.
CCND2 (Cyclin D2) is a member of the conserved cyclin family, which controls periodicity through the cell cycle. CCND2 was required for cell cycle G1/S transition [37]. Other research also revealed that CCND2 was highly expressed in ovarian and testicular tumors [38]. Our results showed that CCND2 was a functional effector of the HOTAIR/miR-1 axis in promoting thyroid cancer cell growth, cycle, migration and invasion.
In summary, the overexpression of HOTAIR promotes thyroid cancer cell growth, cycle, migration and invasion and suppresses apoptosis through inhibition of miRNA-1 and activation of CCND2. Therefore, our study implies the HOTAIR/miR-1-CCND2 axis may provide a potential target for thyroid cancer treatment.
Acknowledgements
We thank Prof. Wang Xiaolu of the Second Hospital of Hebei Medical University for his kindly support of this study.
Disclosure of conflict of interest
None.
References
- 1.Erler P, Keutgen XM, Crowley MJ, Zetoune T, Kundel A, Kleiman D, Beninato T, Scognamiglio T, Elemento O, Zarnegar R. Dicer expression and microRNA dysregulation associate with aggressive features in thyroid cancer. Surgery. 2014;156:1342–1350. doi: 10.1016/j.surg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Curado M, Edwards B, Shin H, Storm H, Ferlay J, Heanue M, Boyle P. Cancer incidence in five continents. Lyon 9: International Agency for Research on Cancer; 2007. [Google Scholar]
- 3.Vriens MR, Weng J, Suh I, Huynh N, Guerrero MA, Shen WT, Duh QY, Clark OH, Kebebew E. MicroRNA expression profiling is a potential diagnostic tool for thyroid cancer. Cancer. 2012;118:3426–3432. doi: 10.1002/cncr.26587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol. 2011;5:51–56. doi: 10.1007/s12105-010-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Q, Ji M, Guan H, Shi B, Hou P. Shikonin inhibits thyroid cancer cell growth and invasiveness through targeting major signaling pathways. J Clin Endocrinol Metab. 2013;98:E1909–E1917. doi: 10.1210/jc.2013-2583. [DOI] [PubMed] [Google Scholar]
- 6.Vasko VV, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007;19:11–17. doi: 10.1097/CCO.0b013e328011ab86. [DOI] [PubMed] [Google Scholar]
- 7.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Morales DR, Thomas K, Presser A, Bernstein BE, van Oudenaarden A. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enfield KS, Pikor LA, Martinez VD, Lam WL. Mechanistic roles of noncoding RNAs in lung cancer biology and their clinical implications. Genet Res Int. 2012;2012:737416. doi: 10.1155/2012/737416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang F, Zhang L, Huo XS, Yuan JH, Xu D, Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689. doi: 10.1002/hep.24563. [DOI] [PubMed] [Google Scholar]
- 11.Piao Hl, Ma L. Non-coding RNAs as regulators of mammary development and breast cancer. J Mammary Gland Biol Neoplasia. 2012;17:33–42. doi: 10.1007/s10911-012-9245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, An Y, Liang Y, Xie X. Role of HOTAIR long noncoding RNA in metastatic progression of lung cancer. Eur Rev Med Pharmacol Sci. 2014;18:1930–1936. [PubMed] [Google Scholar]
- 13.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Zhou L, Wu LM, Lai MC, Xie HY, Zhang F, Zheng SS. Overexpression of long noncoding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 15.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 16.Loewen G, Jayawickramarajah J, Zhuo Y, Shan B. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol. 2014;7:90. doi: 10.1186/s13045-014-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan W, Liu L, Wei J, Ge Y, Zhang J, Chen H, Zhou L, Yuan Q, Zhou C, Yang M. A functional lncRNA HOTAIR genetic variant contributes to gastric cancer susceptibility. Mol Carcinog. 2016;55:90–96. doi: 10.1002/mc.22261. [DOI] [PubMed] [Google Scholar]
- 18.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNA Cancer Regulation. Springer; 2013. MicroRNAs in human cancer; pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasinski AL, Slack FJ. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasser MW, Datta J, Nuovo G, Kutay H, Motiwala T, Majumder S, Wang B, Suster S, Jacob ST, Ghoshal K. Down-regulation of micro-RNA-1 (miR-1) in lung cancer suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. J Biol Chem. 2008;283:33394–33405. doi: 10.1074/jbc.M804788200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Yoshino H, Chiyomaru T, Enokida H, Kawakami K, Tatarano S, Nishiyama K, Nohata N, Seki N, Nakagawa M. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br J Cancer. 2011;104:808–818. doi: 10.1038/bjc.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima S, Chiyomaru T, Kawakami K, Yoshino H, Enokida H, Nohata N, Fuse M, Ichikawa T, Naya Y, Nakagawa M. Tumour suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br J Cancer. 2012;106:405–413. doi: 10.1038/bjc.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid JF, Sokolova V, Zoni E, Lampis A, Pizzamiglio S, Bertan C, Zanutto S, Perrone F, Camerini T, Gallino G. miRNA profiling in colorectal cancer highlights miR-1 involvement in METdependent proliferation. Mol Cancer Res. 2012;10:504–515. doi: 10.1158/1541-7786.MCR-11-0342. [DOI] [PubMed] [Google Scholar]
- 24.Nohata N, Sone Y, Hanazawa T, Fuse M, Kikkawa N, Yoshino H, Chiyomaru T, Kawakami K, Enokida H, Nakagawa M. miR-1 as a tumor suppressive microRNA targeting TAGLN2 in head and neck squamous cell carcinoma. Oncotarget. 2011;2:29–42. doi: 10.18632/oncotarget.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leone V, D’Angelo D, Rubio I, de Freitas PM, Federico A, Colamaio M, Pallante P, Medeiros-Neto G, Fusco A. MiR-1 is a tumor suppressor in thyroid carcinogenesis targeting CCND2, CXCR4, and SDF-1α. J Clin Endocrinol Metab. 2011;96:E1388–E1398. doi: 10.1210/jc.2011-0345. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L, Chen Y, Chan Cy, Wang X, Lin L, He Ml, Lin MC, Yew DT, Sung JJ, Li JC. Down-regulation of stathmin is required for TGF-β inducible early gene 1 induced growth inhibition of pancreatic cancer cells. Cancer Lett. 2009;274:101–108. doi: 10.1016/j.canlet.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Madhyastha H, Radha K, Nakajima Y, Omura S, Maruyama M. uPA dependent and independent mechanisms of wound healing by Cphycocyanin. J Cell Mol Med. 2008;12:2691–2703. doi: 10.1111/j.1582-4934.2008.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V, Borbone E, Petrocca F, Alder H, Croce CM. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer. 2007;14:791–798. doi: 10.1677/ERC-07-0129. [DOI] [PubMed] [Google Scholar]
- 29.Bhan A, Hussain I, Ansari KI, Kasiri S, Bashyal A, Mandal SS. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425:3707–3722. doi: 10.1016/j.jmb.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, Yu PF, Cheng XD. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587–597. doi: 10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Xh, Liu Zl, Sun M, Liu J, Wang ZX, De W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13:1. doi: 10.1186/1471-2407-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migliore C, Martin V, Leoni VP, Restivo A, Atzori L, Petrelli A, Isella C, Zorcolo L, Sarotto I, Casula G. MiR-1 downregulation cooperates with MACC1 in promoting MET overexpression in human colon cancer. Clin Cancer Res. 2012;18:737–747. doi: 10.1158/1078-0432.CCR-11-1699. [DOI] [PubMed] [Google Scholar]
- 33.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 34.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3:1113–1121. doi: 10.1158/2159-8290.CD-13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter T, Pines J. Cyclins and cancer II: cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 38.Büschges R, Weber RG, Actor B, Lichter P, Collins VP, Reifenberger G. Amplification and expression of cyclin D genes (CCND1 CCND2 and CCND3) in human malignant gliomas. Brain Pathol. 1999;9:435–442. doi: 10.1111/j.1750-3639.1999.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]