Abstract
Abnormal cellular energetics has emerged as a hallmark of cancer cells. Deregulating aerobic glycolysis can alter multiple metabolic and signaling pathways in cancer cells, and trigger unlimited growth and proliferation. Accumulating evidence suggests that elevated levels of protein modification with β-N-acetylglucosamine (O-GlcNAcylation) along with dysregulation of O-GlcNAc transferase (OGT) and/or O-GlcNAcase (OGA) levels may act as a nutrient sensor in cancer cells. However, the underlying mechanism of O-GlcNAcylation and the relationship between O-GlcNAcylation and glycolysis are largely unknown in pre-B acute lymphocytic leukemia (pre-B-ALL). In this study, CD19+ bone marrow mononuclear cells (BM-MNCs) from untreated pre-B-ALL patients displayed increased O-GlcNAcylation levels, upregulated OGT, and downregulated OGA. Patients with higher lactate dehydrogenase (LDH) levels exhibited higher O-GlcNAcylation levels with OGT upregulation and overactivation of the PI3K/Akt/c-Myc pathway. The extracellular acidification rate (ECAR) and the mRNA expression of key enzymes in glycolysis were determined to assess glycolysis activation. Our results revealed the existence of abnormal glycolysis in the CD19+ BM-MNCs of pre-B-ALL patients. The knockdown of OGT decreased the ECAR and downregulated glycolysis-related enzymes in Nalm-6 cells via the PI3K/Akt/c-Myc pathway. The suppression of OGT slowed the rate of proliferation and induced apoptosis in Nalm-6 cells. The glycolysis inhibitor 2-deoxy-D-glucose induced cytotoxicity of Nalm-6 cells, which was potentiated by OGT-siRNA. These findings suggested that O-GlcNAcylation could be a hallmark of pre-B-ALL, which has considerable therapeutic potential in clinical practice.
Keywords: O-GlcNAcylation, pre-B acute lymphocytic leukemia, O-GlcNAc transferase, glycolysis, PI3K/Akt/c-Myc pathway
Introduction
As an essential posttranslational modification (PTM) process for proteins, O-GlcNAcylation can alter the structures of proteins to improve the precision of their regulation [1]. Almost all subcellular compartments contain O-GlcNAcylation-related proteins; the process occurs in nuclear membranes and nuclei, particularly on nuclear pore complex proteins [2]. This modification is a dynamic and reversible process, which adds the monosaccharide β-N-acetylglucosamine (O-GlcNAc) to the serine and threonine residues of intracellular proteins (O-GlcNAcylation). In cells, 2%-5% of glucose enters via the hexosamine biosynthesis pathway (HBP). UDP-GlcNAc is an end product of the HBP [3] and a donor substrate in O-GlcNAcylation cycling. In contrast to other PTMs that are mediated by a series of enzymes to guarantee site specificity, O-GlcNAcylation is controlled by 2 key enzymes. O-GlcNAc transferase (OGT) appends UDP-GlcNAc to serine and threonine residues. The deglycosylating enzyme O-GlcNAcase (OGA) can cleave the modification [4]. Protein O-GlcNAcylation is involved in a series of complex biological processes, such as gene expression, transduction, protein degradation, immune protection, and cell proliferation [5].
Eighty years ago, Otto Warburg found that, in contrast to normal cells, tumor cells support their energy requirements through high levels of anaerobic glycolysis, even in the presence of sufficient oxygen [6]. The amount of ATP generated by glycolysis is considerably lower than that from oxidative phosphorylation, but macromolecules can be provided to satisfy the increased energy requirement [7]. In addition to solid tumors, leukemia cells undergo overactive anaerobic glycolysis to produce energy for survival [8]. Tumor cells consume and utilize considerably more glucose than normal cells [9]. Metabolic disorder has been considered an important hallmark of malignancy [10]. O-GlcNAcylation is sensitive to intracellular glucose levels and, thus, is used as a nutrient sensor and metabolic regulator [11]. Numerous proteins associated with tumorigenesis are also O-GlcNAcylated [12]. O-GlcNAcylation, OGT, and OGA levels are altered in the tumorigenesis and progression of various cancers, such as bladder [13], prostate [14], breast [15], human laryngeal [16], and colorectal cancer [17]. Therefore, we hypothesized that the O-GlcNAcylation level of proteins can be used as a tumor marker.
The PI3K/Akt pathway can regulate the uptake and utilization of glucose [18]. Aberrant PI3K/Akt pathway activity plays an important role in hematologic malignancies. This pathway may also serve as a pro-survival factor in leukemic stem cells and contribute to disease progression. Inhibitors of this pathway elicit therapeutic effects on leukemia [19]. The PI3K/Akt pathway also participates in cell survival and metabolic processes [20]. A well-known oncogene in leukemia, c-Myc, regulates tumor glycolytic activity [21]. It is also a key downstream molecule in the PI3K/Akt pathway [22]. However, its functions in the glycolytic progress of acute leukemia cells remain unclear.
Intracellular O-GlcNAcylation is associated with the pathogenesis of chronic lymphocytic leukemia [23], but the relationship between O-GlcNAcylation and glycolysis in human chronic or acute leukemia remains unknown. In this study, we found that O-GlcNAcylation was higher in CD19+ bone marrow mononuclear cells (BM-MNCs) from pre-B acute lymphocytic leukemia (pre-B-ALL) patients compared with cells from healthy donors, while OGT was upregulated and OGA was downregulated. O-GlcNAcylation correlated with lactate dehydrogenase (LDH) levels in pre-B-ALL patient cells. The suppression of OGT-mediated O-GlcNAcylation affected glycolysis via the PI3K/Akt/c-Myc pathway. The knockdown of OGT induced cytotoxicity to Nalm-6 cells and increased their sensitivity to the glycolysis inhibitor 2-deoxy-D-glucose (2-DG). Our results suggested that OGT-mediated O-GlcNAcylation could be a tumor marker and a novel therapeutic target for pre-B-ALL.
Materials and methods
Ethical statement
This study was carried out in conformity with ethical standards and was approved by Institutional Review Board of the Qilu Hospital Authority.
Isolation of human pre-B-ALL cells
BM samples were obtained from 24 pre-BALL patients at the time of initial diagnosis prior to chemotherapy and 10 healthy donors at Qilu Hospital of Shandong University from September 2015 to September 2016, with informed consent from the children’s legal guardians (clinical characteristics in Table 1). The experiments are in accord with the ethical standards established by the institutionThe specimens were collected in a sterile manner into preservative-free heparin. BM-MNCs and CD19+ BM-MNCs were isolated, respectively, with a lymphocyte separation medium (Haoyang, Tianjin, China) and through positive selection with CD19 MicroBeads (Miltenyi), according to the manufacturers’ instructions. BM-MNCs were then stored in a frozen stock solution containing 10% dimethyl sulfoxide and 90% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) for subsequent experiments. The LDH levels in patient plasma samples were collected from their laboratory tests upon hospital admission. Plasma LDH greater than 400 units/L were considered high (n = 15) and plasma LDH levels lower than 400 units/L were denoted as low (n = 9) [24].
Table 1.
Basic clinical characteristics of the patient
| Pre-B-ALL | Healthy donor | P | |
|---|---|---|---|
| Age (months), median (range) | 36 (14-69) | 35.5 (18-60) | 0.689 |
| Gender, male/female | 10/14 | 6/4 | 0.457 |
Cell culture and reagents
The human pre-B-ALL cell line Nalm-6 was provided by Prof. Daoxin Ma (Qilu Hospital, Jinan, Shandong, China). Nalm-6 cells were cultured in RPMI-1640 (Gibco) containing 10% FBS (Gibco) and 1% penicillin-streptomycin (Gibico) at 37°C in 5% CO2 and a normal level of oxygen. LY294002 and 2-DG were purchased from Sigma-Aldrich, and recombinant human insulin-like growth factor 1 (IGF-1) was purchased from PeproTech.
Western blot analysis
Protein extracts from isolated CD19+ BM-MNCs (prior to and following different in vitro treatments) were prepared in RIPA lysis buffer (Beyotime, Shanghai, China). Then, proteins (60 μg) were separated on 10% acrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). The following commercially available antibodies and dilutions were used for western blotting: anti-O-GlcNAc (RL2) (ab2739, Abcam; 1:1000), anti-OGT (ab96718, Abcam; 1:1000), anti-MGEA5 (OGA) (ab124807, Abcam; 1:1000), anti-PI3K (ab86714, Abcam; 1:1000), anti-AKT (ab81283, Abcam; 1:1000), anti-AKT (phosphoS473) (ab81283, Abcam; 1:1000), anti-c-Myc (ab32072, Abcam; 1:1000), anti-c-Myc (phosphoT58) (ab28842, Abcam; 1:1000), and anti-β-actin (60008-1-Ig, Proteintech; 1:2000). After incubation with primary antibodies overnight at 4°C, the PVDF membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse (SA00001-1, Proteintech, 1:10000) or -rabbit (SA00001-2, Proteintech, 1:10000) secondary antibody for 1 h at 25°C. Blots were developed using the ECL chemiluminescence detection kit (Millipore) and analyzed with Image Studio™ DiGits Version 4.0. All target protein signal intensities were normalized to β-actin signals.
Real-time polymerase chain reaction
Total RNA was extracted from cell lysates with TRIzol® reagent (Invitrogen Life Technologies), following the manufacturer’s protocol. We prepared cDNA using ReverTra Ace® qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan) according to the manufacturer’s instructions. The mRNA levels were quantified by quantitative real-time (qRT)-polymerase chain reaction (PCR) with 1×SYBR® Green PCR Master Mix (TOYOBO) in an Applied Biosystems® 7500 PCR system (Applied Biosystems). The relative expression of each target gene was normalized to β-actin using gene-specific primers (primer sequences in Table 2).
Table 2.
Primer sequences of genes in glycolysis
| Gene ID | Primer sequence (5’-3’) | Length |
|---|---|---|
| HK2 | Forward primer: TTCCCCTGCCACCAGACTAA | 20 |
| Reverse primer: TCAAAGTCCCCTCTCCTCTGG | 20 | |
| Glut1 | Forward primer: GTCTGGCATCAACGCTGTCT | 20 |
| Reverse primer: GTTGACGATACCGGAGCCAA | 20 | |
| HIF-α | Forward primer: ATTTTGGCAGCAACGACACA | 21 |
| Reverse primer: CGTTTCAGCGGTGGGTAATG | 20 | |
| LDHA | Forward primer: CCAGTGTGCCTGTATGGAGT | 20 |
| Reverse primer: CGTAAAGACCCTCTCAACCACC | 22 | |
| PFK1 | Forward primer: CAGGCTCCCTCCATCCTCA | 19 |
| Reverse primer: CTGCCTCCTAGCGACTCTTC | 20 |
siRNA transfection
Nalm-6 cells were transfected with siRNA targeting OGT (OGT-siRNA, GenePharma) or negative control (NC) siRNA (GenePharma) using Lipofectamine® 2000 (Invitrogen). The sequences of the siRNAs were: OGT-siRNA, sense, 5’-GCUUGCAAUUCAUCACUUUTT-3’, and anti-sense, 5’-AAAGUGAUGAAUUGCAAGCTT-3’; NC-siRNA, sense, 5’-UUCUCCGAACGUGUCACGUTT-3’, and anti-sense, 5’-ACGUGACACGUUCGGAGAATT-3’.
Extracellular flux analysis
We determined the bioenergetics of Nalm-6 cells using the XFe24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA). Cells (5×104) were cultured in specialized cell culture plates coated by Cell-Tak (BD Biosciences). The extracellular acidification rate (ECAR) was detected using the XF Glycolysis Stress Test kit (Seahorse Bioscience) strictly in accordance with the user’s guide.
Flow cytometry
The purity of sorted CD19+ BM-MNCs was detected by flow cytometry with APC-conjugated mouse anti-human CD19 antibody (555415, BD Biosciences) and APC-conjugated mouse IgG1, κ-isotype control (555751, BD Biosciences). To identify apoptotic cells after OGT-siRNA or 2-DG (0, 2.5, and 5 mmol/L) treatment, Nalm-6 cells were stained with a FITC Annexin V Apoptosis Detection Kit (BD Biosciences). Flow cytometry was performed with a Guava® easyCyte 6HT-2L (Millipore), and the results were analyzed with guavaSoft™ 3.1.1 software (Millipore).
Cell counting kit-8 assay
We used the Cell Counting Kit-8 (CCK-8) assay (Dojindo) to measure the OGT-siRNA and 2-DG-induced inhibition of Nalm-6 proliferation. OGT-siRNA or 2-DG-treated cells (2×104) were cultured in a 96-well plate. After treatment, 10 μL of CCK-8 reagent was added per well. After 2 h of continuous culture in an incubator, the absorbance values of the plates at 450 nm were detected using a spectrophotometer (Bio-Rad Laboratories) and recorded for analysis. The times of treatment and the concentrations of reagents are shown in the relevant figures and/or the corresponding figure legends.
Statistical analysis
All data were expressed as mean ± SD. Unpaired parametric (2-tailed Student’s t-test) or nonparametric (Mann-Whitney U test) tests were used to compare between 2 independent groups. One-way ANOVA analysis was used when comparing differences among the means of 3 or more independent groups. All experiments were repeated independently 3 times. Prism 6 software (San Diego) and SPSS 18.0 (IBM) were used for statistical analyses, and differences where P < 0.05 were considered statistically significant.
Results
O-GlcNAcylation and glycolysis are increased in pre-B-ALL cells compared with normal B cells
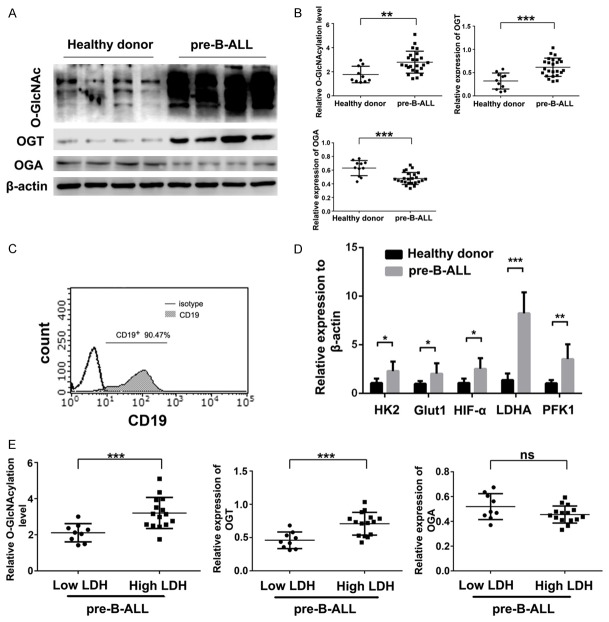
Abnormal O-GlcNAcylation levels and expression of the key enzymes OGT and OGA occur in numerous malignant diseases. However, no clear evidence has emerged in pre-B-ALL. Protein extracts from the CD19+ BM-MNCs of 24 pre-B-ALL patients and 10 healthy controls were immunoblotted with RL2 antibody, which recognizes O-GlcNAc moieties. In addition to the O-GlcNAcylation level, we also evaluated changes in the protein expression of OGT and OGA. We found that O-GlcNAcylation was markedly increased (P < 0.01) in CD19+ BM-MNCs from pre-B-ALL patients compared with those from healthy donors; in addition, the expression of OGT was upregulated (P < 0.001), while OGA was downregulated in pre-B-ALL cells (P < 0.001) (Figure 1A and 1B). The purity after CD19 MicroBead separation was up to 90.47% (Figure 1C).
Figure 1.
Pre-B-ALL cells exhibit elevated O-GlcNAcylation and overactive glycolysis. A, B. Western blot and statistical analyses of O-GlcNAcylation, and OGT and OGA expression from CD19+ BM-MNCs from 10 healthy donors and 24 pre-B-ALL patients. β-actin was used as a loading control. C. The separation purity after positive selection with CD19 MicroBeads compared to an isotype control. D. Levels of glycolysis-related enzyme mRNA quantified by qRT-PCR and normalized to β-actin. Differences were exhibited as fold changes compared with the healthy donor group. E. Statistical analysis of O-GlcNAcylation, OGT, and OGA isolated from CD19+ BM-MNCs from the low LDH group (n = 9) and the high LDH group (n = 15) by western blotting. *P < 0.05; **P < 0.01; ***P < 0.001. The results are expressed as mean ± SD.
O-GlcNAcylation is sensitive to metabolism, especially glycometabolism. Thus, we examined if pre-B-ALL CD19+ BM-MNCs had aberrant glycolysis. For this purpose, we measured several essential components of the glycolytic cycle, including hexokinase 2 (HK2), glucose transporter 1 (Glut1), hypoxia inducible factor 1 alpha (HIF1A), lactate dehydrogenase A (LDHA), and phosphofructokinase-1 (PFK1) via qRT-PCR. In comparison with the healthy cells, we found that pre-B-ALL cells had upregulated mRNA levels of all these factors, especially 5.951-fold and 3.366-fold increases in LDHA and PFK1, respectively (Figure 1D).
LDH is an important enzyme in glycolysis [25]. To further understand the relationship between O-GlcNAcylation and glycolysis, we compared the O-GlcNAcylation levels in groups of pre-B-ALL patients with different LDH levels. We found that patients with higher LDH levels exhibited considerably higher O-GlcNAcylation levels (P < 0.001) and OGT expression (P < 0.001), compared with the lower LDH patients; however, they did not have significantly different OGA levels (Figure 1E). These findings indicated a positive correlation between O-GlcNAcylation and plasma LDH levels in pre-B-ALL patients. These findings suggested that O-GlcNAcylation may be involved in the regulation of glycolysis.
The glycolytic pathway is regulated by the PI3K/Akt/c-Myc pathway in pre-B-ALL cells
To date, several reports have showed that c-Myc is a downstream signaling molecule in the PI3K/Akt pathway [26,27], but its function is unclear in pre-B-ALL cells. We employed Nalm-6 cells as a model for pre-B-ALL; we treated them with various concentrations of the PI3K/Akt pathway inhibitor LY294002 for 48 h, at which point phosphorylation of Akt (P < 0.001) and c-Myc (P < 0.001) were markedly decreased (Figure 2A and 2B). Therefore, the PI3K-Akt pathway may regulate the expression of c-Myc in pre-B-ALL.
Figure 2.
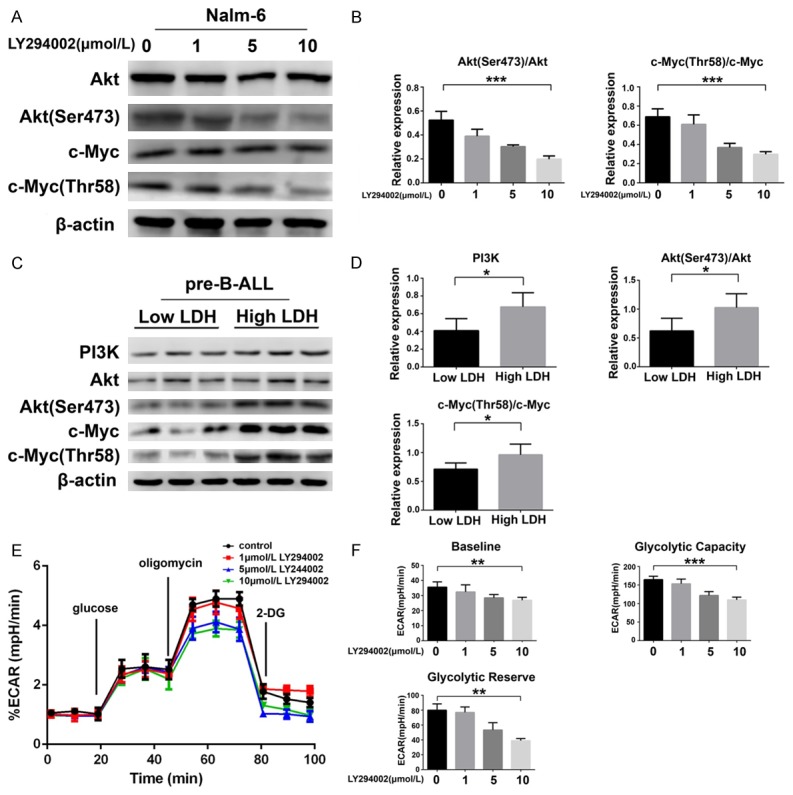
Glycolysis is regulated by the PI3K/Akt/c-Myc pathway in pre-B-ALL cells. A, B. Western blot and statistical analyses of the phosphorylation of Akt (Ser473) and c-Myc (Thr58) in Nalm-6 cells treated with LY294002 (0, 1, 5, and 10 μmol/L). C, D. Western blotting of proteins in the PI3K/Akt/c-Myc signaling pathway, and statistical analysis of PI3K expression, and the phosphorylation of Akt (Ser473) and c-Myc (Thr58) in CD19+ BM-MNCs from low LDH and high LDH pre-B-ALL patients. E, F. ECAR in Nalm-6 cells treated with LY294002 (0, 1, 5, and 10 μmol/L) for 48 h. The relative ECAR (% ECAR) is shown as a percentage of the baseline measurement (set as 100%). Statistical analyses of the baseline ECAR, glycolytic capacity, and glycolytic reserve are also shown. *P < 0.05; **P < 0.01; ***P < 0.001. The results are expressed as mean ± SD.
To investigate the relationship between the PI3K/AKT/c-Myc pathway and glycolysis, we investigated if the PI3K/Akt/c-Myc pathway is more active in the high LDH pre-B-ALL CD19+ BM-MNCs than in cells from the low LDH group. As expected, we found that the protein expression of PI3K (P < 0.05) and the phosphorylation of Akt (P < 0.05) and c-Myc (P < 0.05) were markedly increased in the high LDH pre-B-ALL group (Figure 2C and 2D). To further substantiate that glycolysis can be regulated by the PI3K/Akt/c-Myc pathway, we cultivated Nalm-6 cells with LY294002 and measured the production of lactic acid via the ECAR to evaluate the impact on glycolysis. As expected, treatment with LY294002 resulted in an evident decrease in the baseline ECAR (P < 0.01), glycolytic capacity (P < 0.001), and glycolytic reserve (P < 0.01) (Figure 2E and 2F). Taken together, these results indicate that the PI3K/Akt/c-Myc pathway is essential for glycolysis in pre-B-ALL cells.
Suppression of OGT-mediated O-GlcNAcylation affects glycolysis via the PI3K/Akt/c-Myc pathway
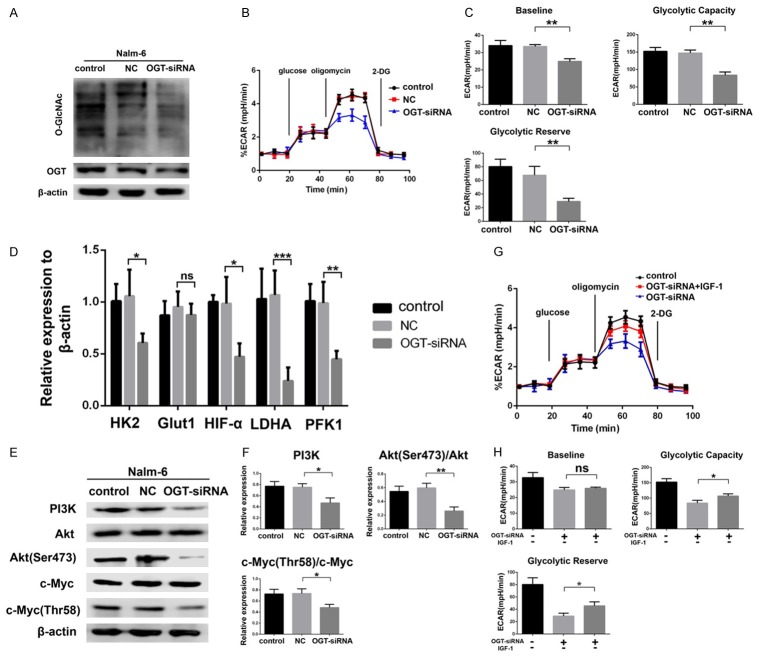
To investigate if the change in O-GlcNAcylation is related to the inhibition of glycolysis in pre-B-ALL cells, we downregulated O-GlcNAcylation with OGT-siRNA and harvested the cells 48 h after transfection. To confirm the interference efficiency, we measured the protein expression of OGT by western blot, which indicated high efficiency by the OGT-siRNA (Figure 3A).
Figure 3.
OGT-mediated O-GlcNAcylation could regulate glycolysis via the PI3K/Akt/c-Myc pathway. (A) Cell lysates from Nalm-6 cells transfected with double-distilled H2O (control), negative control siRNA (NC), or OGT-siRNA by Lipofectamine® 2000 for 48 h were collected for western blot and statistical analysis of O-GlcNAcylation and OGT expression. (B) ECAR was measured in Nalm-6 cells transfected with control, NC, or OGT-siRNA. The relative ECAR (% ECAR) is shown as a percentage of the baseline measurement (set as 100%). Statistical analyses of baseline ECAR, glycolytic capacity, and glycolytic reserve shown in (C). (D) Levels of glycolysis-related enzyme mRNA quantified by qRT-PCR and normalized to β-actin in Nalm-6 cells transfected with control, NC, or OGT-siRNA for 48 h. (E, F) Western blot of proteins in the PI3K/Akt/c-Myc signaling pathway and statistical analyses of the phosphorylation of PI3K, Akt (Ser473), and c-Myc (Thr58) in Nalm-6 cells transfected with control, NC, or OGT-siRNA for 48 h. (G, H) The ECAR was measured in the OGT-siRNA group and the group that received co-treatment with OGT-siRNA and IGF-1 (an activator of PI3K). *P < 0.05; **P < 0.01. The results are expressed as mean ± SD.
Given that we had successfully constructed a low O-GlcNAcylation cell model, we next examined if the suppression of OGT affected glycolysis. Nalm-6 cells transfected with OGT-siRNA exhibited a decrease in their baseline ECAR (P < 0.01), glycolytic capacity (P < 0.01), and glycolytic reserve (P < 0.01) (Figure 3B and 3C). To confirm that the knockdown of OGT regulated glycolytic metabolism, we measured specific glycolysis-related molecules using qRT-PCR, which revealed that the mRNA levels of HK2, HIF-α, LDHA, and PFK1 were markedly deceased compared to the levels in the nontransfected cells (Figure 3D). PI3K, Akt, and c-Myc are O-GlcNAcylated; thus, it is likely that OGT regulates the PI3K/Akt/c-Myc pathway in pre-B-ALL cells. As expected, the knockdown of OGT decreased the expression of PI3K, as well as the phosphorylation of Akt and c-Myc (Figure 3E and 3F).
To further demonstrate the function of the PI3K/Akt/c-Myc pathway in O-GlcNAcylation-induced alterations to glycolysis, we measured the ECAR in Nalm-6 cells treated with IGF-1 (an activator of PI3K) after OGT-siRNA knockdown. IGF-1 rescued the reduction in the ECAR caused by OGT knockdown (Figure 3G and 3H). Overall, our data suggested that the suppression of OGT-mediated O-GlcNAcylation affects glycolysis via the PI3K/Akt/c-Myc pathway.
Downregulation of OGT induces cell apoptosis and potentiates the cytotoxicity of 2-DG in Nalm-6 cells
Given that OGT is a critical regulator of glycolysis, we examined if changes in glycolysis influenced O-GlcNAcylation and OGT. 2-DG is a known glycolysis inhibitor that targets HK2 [28]. HK2 is reported to be antineoplastic via targeting metabolic pathways in cancer cells [29,30], but its function in pre-B-ALL cells is unclear. After the treatment of Nalm-6 cells with different concentrations of 2-DG for 72 h, western blotting revealed that O-GlcNAcylation and OGT expression were reduced (Figure 4A and 4B). This finding indicated that OGT-mediated O-GlcNAcylation is suppressed when glycolysis is inhibited.
Figure 4.
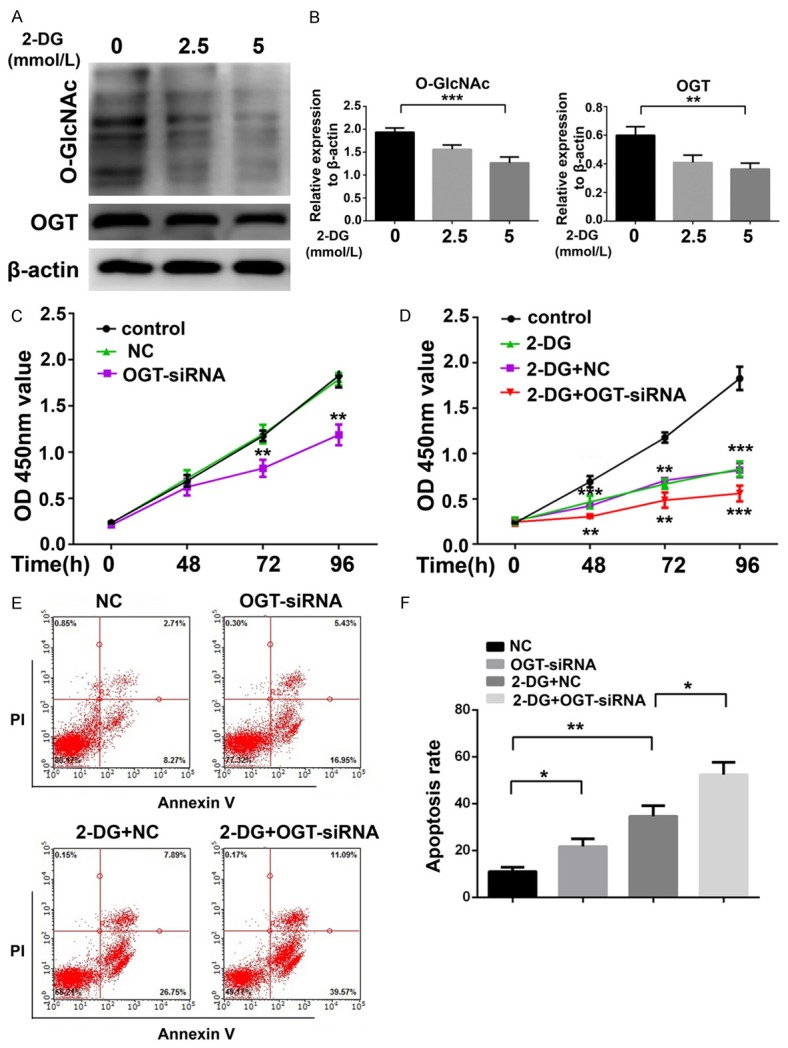
Suppression of OGT induces cell apoptosis and potentiates the cytotoxicity of 2-DG in Nalm-6 cells. A, B. Western blot and statistical analyses of O-GlcNAc and OGT in Nalm-6 cells treated with 2-DG (0, 2.5, and 5 mmol/L) for 48 h. C. Proliferation of Nalm-6 cells transfected with OGT-siRNA and NC-siRNA measured by CCK-8 assay. D. Proliferation of Nalm-6 cells transfected with OGT-siRNA or co-treated with OGT-siRNA and 2-DG (2.5 mmol/L) measured by CCK-8 assay. E, F. Cell apoptosis and statistical analyses of Nalm-6 cells transfected with OGT-siRNA, or co-treatment with OGT-siRNA and 2-DG (2.5 mmol/L) for 72 h. *P < 0.05; **P < 0.01; ***P < 0.001. The results are expressed as mean ± SD.
To elucidate the function of OGT in 2-DG-induced cytotoxicity to Nalm-6 cells, we performed CCK-8 assays and flow cytometric analyses of annexin V/propidium iodide staining to assess cell proliferation and apoptosis. The CCK-8 assay indicated that treatment with OGT-siRNA alone induced the suppression of Nalm-6 proliferation at 72 and 96 h (P < 0.01 for OGT-siRNA versus NC, Figure 4C). However, decreased Nalm-6 cell proliferation was observed after treatment with 2.5 mM 2-DG for 48 h (P < 0.001 for 2-DG versus control, Figure 4D). Significantly higher cytotoxicity was detected in Nalm-6 cells treated with both OGT-siRNA and 2-DG than in cells treated with 2-DG alone at 48 h (P < 0.01 for 2-DG+OGT-SiRNA versus 2-DG). By contrast, a negligible effect was detected when these cells were treated with OGT-siRNA alone at 48 h (Figure 4C and 4D). On the basis of the CCK-8 assay results, we next used flow cytometric analysis to assess cell apoptosis. We found that the knockdown of OGT for 72 h induced an approximately 2-fold increase in cell apoptosis over the NC group (21.72 ± 1.903% versus 11.07 ± 1.069%, Figure 4E and 4F). Consistent with the CCK-8 assay results, treatment with 2-DG caused cell apoptosis (34.66 ± 2.566% versus 11.07 ± 1.069%), which was potentiated by OGT-siRNA (52.40 ± 3.037% versus 34.66 ± 2.566%, Figure 4E and 4F) in Nalm-6 cells.
Discussion
Pre-B-ALL is a hematopoietic malignancy defined as the malignant proliferation of early B lymphoid precursors that turns into clonal expansion [31]. Although pre-B-ALL is sensitive to chemotherapy, 20% to 30% of pre-B-ALL patients cannot achieve complete remission and eventually relapse because of drug resistance and immune escape [32]. Moreover, although the pathogenesis of pre-B-ALL at the genetic level is known, the role of O-GlcNAcylation of proteins in pre-B-ALL is not fully understood.
The results of this study demonstrated for the first time that the level of O-GlcNAcylation in CD19+ pre-B-ALL BM-MNCs was significantly higher than in healthy cells, and was accompanied by the upregulation of OGT and the downregulation of OGA. Furthermore, higher O-GlcNAcylation levels and overactivation of the PI3K/Akt/c-Myc pathway were detected in pre-B-ALL cells from patients with higher LDH levels. Knockdown of OGT downregulated the key enzymes in glycolysis and induced a significant decrease in the ECAR; however, IGF-1, an activator of PI3K, rescued the inhibition. In addition, knockdown of OGT slowed proliferation and caused apoptosis in Nalm-6 cells. Combination treatment with 2-DG and OGT-siRNA led to more serious cytotoxicity to Nalm-6 cells. These results demonstrated the ability of O-GlcNAcylation to aggravate pre-B-ALL through regulation of glycolysis via the PI3K/Akt/c-Myc pathway.
Increasing evidence suggests that O-GlcNAcylation may be strongly related to the pathogenesis and progression of certain cancers [33], such as prostate [34], breast [35], colorectal [36], liver [37], and pancreatic cancers [38]. At present, aberrant O-GlcNAcylation and its 2 key enzymes are regarded as biomarkers of certain cancers. An earlier report suggested that the gene expression of OGA decreases by about 56% when screening from 40 candidate genes in breast cancer [39]. More recently, O-GlcNAcylation levels were found to be associated with poor prognosis in prostate cancer patients, and siRNA-mediated OGT knockdown enhanced prostate cancer cell proliferation and invasion [40]. In our study, we found that O-GlcNAcylation is significantly enhanced in CD19+ pre-B-ALL BM-MNC protein extracts, along with increased OGT and decreased OGA expression. Furthermore, the knockdown of OGT expression via siRNA resulted in not only decreased cell proliferation, but also increased cell apoptosis in Nalm-6 cells. Therefore, increased O-GlcNAcylation and aberrant expression of the O-GlcNAc cycling enzymes could be regarded as a biomarker for the oncogenesis and tumor progression of pre-B-ALL. Moreover, OGT targeting may be a novel treatment for pre-B-ALL.
Despite increasing evidence for the critical role of O-GlcNAcylation in various cancers, the molecular mechanisms of the PTM in leukemia are unclear. Here, we detected a positive correlation between O-GlcNAcylation levels and plasma LDH from clinical cases. LDH, which includes the subunits LDHA and LDHB, is critical in aerobic glycolysis [41]. Recently, LDHA was established to play an essential role in the maintenance and progression of tumors [42]. Furthermore, cancer cells rely on aerobic glycolysis to generate energy to maintain cell growth [18]. Therefore, we investigated if O-GlcNAcylation correlated with glycolysis in pre-B-ALL. We first found that glycolysis is overactive in pre-B-ALL cells. Then, we utilized siRNA to downregulate the expression of OGT and found decreased mRNA expression of glycolytic-related enzymes). Among the glycolytic enzymes examined, LDHA and PFK1 were markedly reduced, as was previously reported with regard to PFK1 modification by O-GlcNAc at Ser-529 [43]. These data provide evidence that the molecular mechanism of O-GlcNAcylation-induced cytotoxicity may be the inhibition of glycolysis.
The PI3K/Akt pathway is aberrantly active in a large number of malignancies, including leukemia. Consistent with previous research [26,27], our study confirmed that c-Myc is a downstream molecule in the PI3K/Akt pathway and that constitutively activated PI3K/Akt/c-Myc existed in the cells of pre-B-ALL patients with high LDH. Akt and c-Myc undergo both phosphorylation and O-GlcNAcylation [44]. However, the relationship between OGT and PI3K/Akt/c-Myc in glycolysis remains unclear. In this study, we silenced the expression of OGT via OGT-siRNA and found that PI3K and the phosphorylation of Akt and c-Myc were all decreased in Nalm-6 cells. The findings indicate that OGT may participate in regulating the activation of the PI3K pathway in pre-B-ALL. To evaluate the change in glycolysis, we directly measured lactic acid production via an XF instrument to determine ECAR values. Glycolytic capacity is the maximum potential ECAR level when mitochondrial ATP production is inhibited by oligomycin. A decreased ECAR caused by blocking glycolysis with 2-DG is defined as the glycolytic reserve. When Nalm-6 cells were treated with a PI3K inhibitor or OGT-siRNA, their baseline ECAR level, glycolytic capacity, and reserve deceased to varying degrees. In addition, co-treatment of Nalm-6 cells with OGT-siRNA and IGF-1 (an activator of PI3K) increased their ECAR compared with that of the OGT knockdown group. These results reveal that the PI3K/Akt/c-Myc pathway plays a key role in the changes in glycolysis caused by the knockdown of OGT.
Numerous cancer cells satisfy more of their energy requirements by glycolysis than by mitochondrial oxidative phosphorylation [45]. Although glycolysis generates less ATP, the rate of production can be 100 times faster to meet energy demands [46]. Various glycolytic enzymes, such as GLUT1 [47], hexokinase [48], and LDHA [49], have been targeted for therapy in tumors. Here, we demonstrated that the HK2 inhibitor 2-DG could induce cytotoxicity to Nalm-6 cells. Nalm-6 cells treated with 2-DG displayed significant upregulation of OGT protein expression and higher O-GlcNAcylation levels. Moreover, we found that OGT knockdown could potentiate the cytotoxicity caused by 2-DG. Our findings suggest that a combination therapy of glycolytic inhibitors with an O-GlcNAcylation inhibitor may be a powerful and effective treatment for human leukemia.
Therefore, our study is the first to demonstrate, using clinical cases, that O-GlcNAcylation and its key enzymes could be regarded as pre-B-ALL biomarkers. This study is also the first to examine the key role of OGT in glycolysis mediated by the PI3K/Akt/c-Myc signaling pathway. OGT targeting could be applied therapeutically in the future. A combination of glycolytic inhibitors with O-GlcNAcylation inhibitors may be an effective therapy for clinical use in pre-B-ALL.
Acknowledgements
We thank Prof. Daoxin Ma (Qilu Hospital, Jinan, Shandong, China) for providing the Nalm-6 cells. We thank all of the patients and healthy donors for agreeing to participate in our study. This work was supported by Shenzhen Science and Technology Research and Development Fund (JCYJ20140418115449178), and Shandong Province Natural Science Fund (2014GSF118131).
Disclosure of conflict of interest
None.
References
- 1.Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. 2010;1800:80–95. doi: 10.1016/j.bbagen.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986;261:8049–8057. [PubMed] [Google Scholar]
- 3.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucoseinduced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 4.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 5.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 7.Cheong H, Lu C, Lindsten T, Thompson CB. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol. 2012;30:671–678. doi: 10.1038/nbt.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villalba M, Lopez-Royuela N, Krzywinska E, Rathore MG, Hipskind RA, Haouas H, Allende-Vega N. Chemical metabolic inhibitors for the treatment of blood-borne cancers. Anticancer Agents Med Chem. 2014;14:223–232. doi: 10.2174/18715206113136660374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Ruan HB, Singh JP, Li MD, Wu J, Yang X. Cracking the O-GlcNAc code in metabolism. Trends Endocrinol Metab. 2013;24:301–309. doi: 10.1016/j.tem.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch TP, Reginato MJ. O-GlcNAc transferase: a sweet new cancer target. Cell Cycle. 2011;10:1712–1713. doi: 10.4161/cc.10.11.15561. [DOI] [PubMed] [Google Scholar]
- 13.Rozanski W, Krzeslak A, Forma E, Brys M, Blewniewski M, Wozniak P, Lipinski M. Prediction of bladder cancer based on urinary content of MGEA5 and OGT mRNA level. Clin Lab. 2012;58:579–583. [PubMed] [Google Scholar]
- 14.Itkonen HM, Gorad SS, Duveau DY, Martin SE, Barkovskaya A, Bathen TF, Moestue SA, Mills IG. Inhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolism. Oncotarget. 2016;7:12464–12476. doi: 10.18632/oncotarget.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sodi VL, Khaku S, Krutilina R, Schwab LP, Vocadlo DJ, Seagroves TN, Reginato MJ. mTOR/MYC axis regulates O-GlcNAc transferase expression and O-GlcNAcylation in breast cancer. Mol Cancer Res. 2015;13:923–933. doi: 10.1158/1541-7786.MCR-14-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starska K, Forma E, Brzezinska-Blaszczyk E, Lewy-Trenda I, Brys M, Jozwiak P, Krzeslak A. Gene and protein expression of O-GlcNAccycling enzymes in human laryngeal cancer. Clin Exp Med. 2015;15:455–468. doi: 10.1007/s10238-014-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phueaouan T, Chaiyawat P, Netsirisawan P, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J, Champattanachai V. Aberrant O-GlcNAc-modified proteins expressed in primary colorectal cancer. Oncol Rep. 2013;30:2929–2936. doi: 10.3892/or.2013.2794. [DOI] [PubMed] [Google Scholar]
- 18.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertacchini J, Heidari N, Mediani L, Capitani S, Shahjahani M, Ahmadzadeh A, Saki N. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci. 2015;72:2337–2347. doi: 10.1007/s00018-015-1867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayley JP, Devilee P. The Warburg effect in 2012. Curr Opin Oncol. 2012;24:62–67. doi: 10.1097/CCO.0b013e32834deb9e. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HF, Wu C, Alshareef A, Gupta N, Zhao Q, Xu XE, Jiao JW, Li EM, Xu LY, Lai R. The PI3K/AKT/c-MYC axis promotes the acquisition of cancer stem-like features in esophageal squamous cell carcinoma. Stem Cells. 2016;34:2040–2051. doi: 10.1002/stem.2395. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, Zuccolo J, Deans JP, Hart GW, Spaner DE. Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia. 2010;24:1588–1598. doi: 10.1038/leu.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu W, Wang X, Yang R. Evaluation of D-dimer and lactate dehydrogenase plasma levels in patients with relapsed acute leukemia. Oncol Lett. 2016;12:591–596. doi: 10.3892/ol.2016.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kachel P, Trojanowicz B, Sekulla C, Prenzel H, Dralle H, Hoang-Vu C. Phosphorylation of pyruvate kinase M2 and lactate dehydrogenase A by fibroblast growth factor receptor 1 in benign and malignant thyroid tissue. BMC Cancer. 2015;15:140. doi: 10.1186/s12885-015-1135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold HK, Zhang X, Daniel CJ, Tibbitts D, Escamilla-Powers J, Farrell A, Tokarz S, Morgan C, Sears RC. The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J. 2009;28:500–512. doi: 10.1038/emboj.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai WB, Aiba I, Long Y, Lin HK, Feun L, Savaraj N, Kuo MT. Activation of Ras/PI3K/ERK pathway induces c-Myc stabilization to upregulate argininosuccinate synthetase, leading to arginine deiminase resistance in melanoma cells. Cancer Res. 2012;72:2622–2633. doi: 10.1158/0008-5472.CAN-11-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forte N, Medrihan L, Cappetti B, Baldelli P, Benfenati F. 2-Deoxy-d-glucose enhances tonic inhibition through the neurosteroid-mediated activation of extrasynaptic GABAA receptors. Epilepsia. 2016;57:1987–2000. doi: 10.1111/epi.13578. [DOI] [PubMed] [Google Scholar]
- 29.Davidescu M, Macchioni L, Scaramozzino G, Cristina Marchetti M, Migliorati G, Vitale R, Corcelli A, Roberti R, Castigli E, Corazzi L. The energy blockers bromopyruvate and lonidamine lead GL15 glioblastoma cells to death by different p53-dependent routes. Sci Rep. 2015;5:14343. doi: 10.1038/srep14343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Huang F, Wang J, Luo H, Wang Z. 2-DG-regulated RIP and c-FLIP effect on liver cancer cell apoptosis induced by TRAIL. Med Sci Monit. 2015;21:3442–3448. doi: 10.12659/MSM.895034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moorman AV. The clinical relevance of chromosomal and genomic abnormalities in B-cell precursor acute lymphoblastic leukaemia. Blood Rev. 2012;26:123–135. doi: 10.1016/j.blre.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, Ebell W, Escherich G, Schrappe M, Klingebiel T, Fengler R, Henze G, von Stackelberg A. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J. Clin. Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 33.Slawson C, Hart GW. O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer. 2011;11:678–684. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of OLinked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012;287:11070–11081. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 2010;70:6344–6351. doi: 10.1158/0008-5472.CAN-09-1887. [DOI] [PubMed] [Google Scholar]
- 36.Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I. O-linked beta-N-acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-beta-D-glucosaminidase silencing on cell phenotype and transcriptome. J Biol Chem. 2012;287:28755–28769. doi: 10.1074/jbc.M112.345546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L, Xing C, Zhang F, Zheng S. O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol. 2012;29:985–993. doi: 10.1007/s12032-011-9912-1. [DOI] [PubMed] [Google Scholar]
- 38.Ma Z, Vocadlo DJ, Vosseller K. Hyper-OGlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem. 2013;288:15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahl E, Sadr-Nabavi A, Klopocki E, Betz B, Grube S, Kreutzfeld R, Himmelfarb M, An HX, Gelling S, Klaman I, Hinzmann B, Kristiansen G, Grutzmann R, Kuner R, Petschke B, Rhiem K, Wiechen K, Sers C, Wiestler O, Schneider A, Hofler H, Nahrig J, Dietel M, Schafer R, Rosenthal A, Schmutzler R, Durst M, Meindl A, Niederacher D. Systematic identification and molecular characterization of genes differentially expressed in breast and ovarian cancer. J Pathol. 2005;205:21–28. doi: 10.1002/path.1687. [DOI] [PubMed] [Google Scholar]
- 40.Kamigaito T, Okaneya T, Kawakubo M, Shimojo H, Nishizawa O, Nakayama J. Overexpression of O-GlcNAc by prostate cancer cells is significantly associated with poor prognosis of patients. Prostate Cancer Prostatic Dis. 2014;17:18–22. doi: 10.1038/pcan.2013.56. [DOI] [PubMed] [Google Scholar]
- 41.Zhai X, Yang Y, Wan J, Zhu R, Wu Y. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol Rep. 2013;30:2983–2991. doi: 10.3892/or.2013.2735. [DOI] [PubMed] [Google Scholar]
- 42.Girgis H, Masui O, White NM, Scorilas A, Rotondo F, Seivwright A, Gabril M, Filter ER, Girgis AH, Bjarnason GA, Jewett MA, Evans A, Al-Haddad S, Siu KM, Yousef GM. Lactate dehydrogenase A is a potential prognostic marker in clear cell renal cell carcinoma. Mol Cancer. 2014;13:101. doi: 10.1186/1476-4598-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jozwiak P, Forma E, Brys M, Krzeslak A. O-GlcNAcylation and metabolic reprograming in cancer. Front Endocrinol (Lausanne) 2014;5:145. doi: 10.3389/fendo.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Pan C, Guo L, Wu M, Guo J, Peng S, Wu Q, Zuo Q. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. J Hematol Oncol. 2016;9:76. doi: 10.1186/s13045-016-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, Gao G, Zhang A, Xia X, Brasher H, Widger W, Ellis LM, Weihua Z. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun L, Liu X, Fu H, Zhou W, Zhong D. 2-Deoxyglucose suppresses ERK phosphorylation in LKB1 and ras wild-type non-small cell lung cancer cells. PLoS One. 2016;11:e0168793. doi: 10.1371/journal.pone.0168793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizwan A, Serganova I, Khanin R, Karabeber H, Ni X, Thakur S, Zakian KL, Blasberg R, Koutcher JA. Relationships between LDH-A, lactate, and metastases in 4T1 breast tumors. Clin Cancer Res. 2013;19:5158–5169. doi: 10.1158/1078-0432.CCR-12-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]