Abstract
Pancreatic cancer is the fourth highest cause of cancer mortality in the world. It has very low survival rates owing to late diagnosis resulting from the absence of accurate diagnostic tools and effective therapies. Hence, there is a pressing need to develop new diagnostic and therapeutic tools. In the recent years, there has been new evidence implicating the importance of mucins in pancreatic carcinogenesis. Mucins belong to a group of heavily glycosylated proteins, and are often aberrantly expressed in a number of cancers such as pancreatic cancer. Therefore, this literature review will summarise the role of mucins and mucin expression in pancreatic neoplasms. Subsequently the paper will also discuss the most recent advances in the biological properties of mucins and their role in carcinogenesis and resistance to chemotherapy. Then it will conclude on the newest developments in diagnosis and therapy based on mucins for pancreatic cancer.
Keywords: Mucins, pancreatic cancer, carcinogenesis, resistance, prognostic biomarker, cancer therapy
Introduction
Pancreatic cancer is the fourth highest cause of cancer deaths in the United States and has contributed to over 40,000 deaths in 2016 [1], despite advances in cancer diagnosis and treatment methods. 95% percent of pancreatic cancer is classified as pancreatic ductal adenocarcinoma (PDAC), and more rarely it is acinous or endocrine in origin [2,3].
In 85% of cases, PDAC arises from pancreatic intraepithelial neoplasia (PanIN), categorised into PanIN 1A, PanIN 1B, PanIN 2 and PanIN 3. The remaining 15% arises from intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasms (MCN) [3-5].
Survival is poor, as the 5-year survival rate is less than 5% [6], whilst the median survival is less than 6 months. Surgery is the primary treatment, however only a minority are eligible for this treatment as 80% of patients have either metastatic or locally advanced tumours at diagnosis, leaving chemotherapy and radiotherapy with palliative intent as the only option [3,5,7-9]. Although the standard chemotherapy drug used is gemcitabine, less than 30% of patients respond to the treatment due to resistance [3,10]. Other alternative drugs include albumin-bound paclitaxel (nab paclitaxel), erlotinib or combination of leucovorin, 5-FU, irinotecan and oxaliplatin (FOLFIRINOX). However, these combination treatments have shown minimal survival advantage over gemcitabine monotherapy, and have serious adverse effects such as neutropenia and peripheral neuropathy [4,10,11]. Mucins, which are able to form a protective coat around cancer cells, are critical in the pathogenesis of pancreatic cancer, and are associated with resistance to cytotoxic drugs, invasiveness, metastases and cell proliferation. In recent years, there has been a number of studies on the irregular expression of mucins in malignancies such as pancreatic cancer and its potential as a drug target or prognostic biomarker [5]. This review aims to summarise the most recent evidence regarding the function of mucins in the development and advancement of pancreatic neoplasms.
Overview of mucins
Mucins are a family of heavy weight glycoproteins produced by the gastrointestinal, respiratory, reproductive, hepatic, pancreatic and renal epithelium [7,12]. They consist of a varying number of tandem repeat regions prolific in proline, serine and threonine [13] with abundant oligosaccharide chains attached by either N-glycosylation or O-glycosylation to serine or threonine residues [3,5,14] as represented in Figure 1. Due to the abundance of glycans that extend out from the peptide core, mucins can extend out to 200 nm into the lumen of ducts and glands [15].
Figure 1.
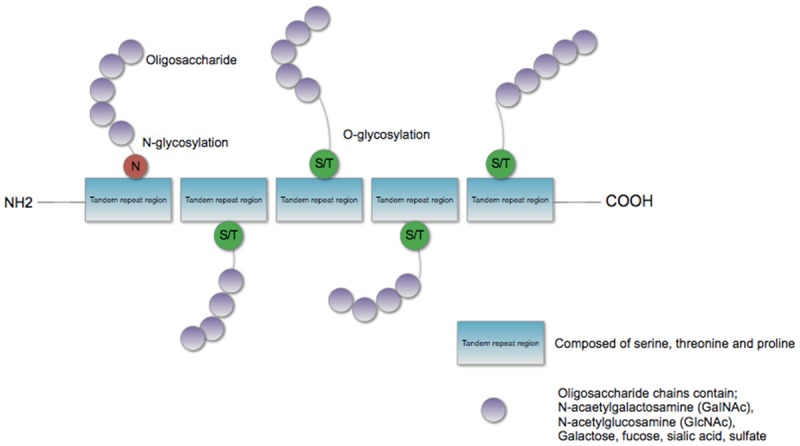
Basic structure of mucin.
There are 21 mucin members and they can be categorised into secreted and transmembrane mucins based upon their structural and functional roles. Secreted mucins form a viscoelastic gel on the surface of apical cells to protect the epithelium from inflammation, pH changes, toxins and pathogens [13,16]. The gel like property of secreted mucins is due to the fact that they are able to polymerize through the formation of glycosidic and disulphide bonds. The members include MUC2, MUC5AC, MUC5B, MUC6, MUC7, MUC8, MUC9 and MUC19 [17-19]. Membrane-bound or transmembrane mucins contain a hydrophobic region in the phospholipid bilayer and include MUC1, MUC3A/B, MUC4, MUC11, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20, MUC21 and MUC22. They contain a number of distinguishing components such as the N-glycans, O-glycans and the functional domains of epidermal growth factor, nidogen-like domain, sea urchin sperm protein-enterokinase-agrin, von Willebrand factor D domain, cysteine knots and the cytoplasmic tail, which are able to interact with receptors, signalling molecules and the extracellular matrix [3,5,9,15,18].
Carcinogenetic significance of mucins in pancreatic malignancies
Pancreatic cancer, along with a number of other malignancies including breast, ovarian, lung and gastrointestinal, are characterised by the aberrant expression, glycosylation and localisation of mucins as they develop from normal tissue to malignant cells as outlined in Figure 2 and Table 1 [17,18,20-22]. These alterations in mucin expression confer oncogenic properties, playing a critical role in carcinogenesis, metastasis and resistance to therapy [5]. Mucins can play a variety of roles in oncogenesis, such as limiting intracellular drug uptake, antagonising drug targets, evasion of the immune system and upregulating survival pathways [15,18,23-27]. This is supported by a number of clinical studies that link the aberrant expression of mucins to poor clinical prognosis [15,23-25,28]. Healthy pancreatic tissue may express low levels of mucins such as MUC1, however histological studies show that there is neo-expression and upregulation of mucins such as MUC4, MUC5AC & MUC16 in PanIN, IPMN and MCN [15,24,25,29]. Additionally major genetic mutations such as K-RAS are also implicated in alteration of mucin expression patterns [30].
Figure 2.
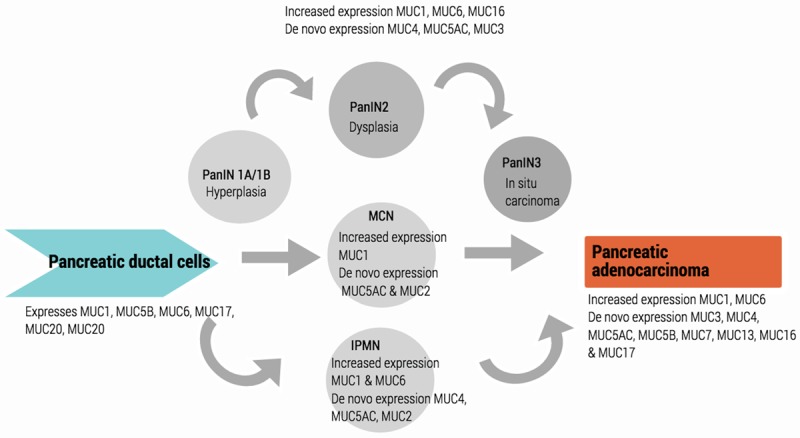
Relationship between pancreatic neoplasia and mucin expression.
Table 1.
| Mucin | Normal | PanIN | PDAC | Invasive IPMN | MCN | Studies | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| PanIN-I | PanIn-II | PanIN-III | Gastric | Intestinal | |||||
| MUC1 | L | L | M | H | H | H | H | M | [7,77,97,98] |
| MUC2 | N | N | N | N | ND | H | H | ND | [52,69,99,100] |
| MUC3 | C | L | L | L | M | C | C | ND | [38,60,69,101] |
| MUC4 | N | L | M | H | H | M | M | H | [14,36,38,41,42,56,73] |
| MUC5AC | N | H | H | H | H | H | H | H | [14,55,56,61,64,102] |
| MUC5B | C | ND | ND | ND | H | ND | M | ND | [16,69,99] |
| MUC6 | L | M | M | M | H | M | M | ND | [56,61,69,97,99] |
| MUC7 | N | ND | ND | ND | L | N | N | ND | [69,74,99] |
| MUC13 | C | ND | ND | ND | H | ND | ND | ND | [54,58,59,103] |
| MUC16 | N | L | M | M | H | ND | ND | ND | [16,17,48,51,104,105] |
| MUC17 | M | H | H | H | M | ND | ND | ND | [38,62] |
| MUC20 | L | ND | ND | ND | ND | ND | ND | ND | [106] |
| MUC21 | L | ND | ND | ND | ND | ND | ND | ND | [107] |
ND: no data is available.
N = negative expression, L = low or minimal expression, M = moderate expression, H = high or abundant expression, C = contradicting studies.
MUC1
MUC1 is a 300-600 kDA membrane bound mucin expressed by both pancreatic ductal and acinar cells. MUC1 plays a variety of physiological roles in the healthy pancreas involving both cell signalling and differentiation. However, MUC1 is atypically expressed in higher than 60% of PDAC and positively correlates with tumour size and dysplasia, which may suggest that it has a pivotal role in pancreatic cancer progression [5,7,31-33]. In addition, there are a number of clinical studies that link greater MUC1 expression with poorer prognosis [34]. In cancer cells, MUC1 binds to Epidermal Growth Factor Receptor (EGFR), ß-catenin and NF-KB to enhance cell proliferation through the MAPK, Akt or Wnt/ß-catenin pathways [7,33,35]. It also upregulates cdc-25c to increase cell division and increases the expression of glycolytic genes to enhance metabolism [14,33]. MUC1 also activates MMP13, Stat3 and PDGFR-ß, which induce epithelial mesenchymal transition (EMT) hence facilitating metastasis [7,18,31]. Additionally, MUC1 inhibits apoptosis through suppressing p53 [33] and activating anti-apoptotic pathways (Akt and Bcl-xL) [7]. Furthermore, it decreases caspase-3-activation, a mitochondrial apoptotic enzyme [18,36]. MUC1 also induces chemoresistance [4] through increasing expression of multidrug resistant genes [5,37].
MUC4
MUC4 is a large membrane bound glycoprotein that is over expressed in the majority of pancreatic cancers, detected as early as the PanIN-IA stages [14,38,39]. It is an independent marker of poor clinical prognosis [10] and imparts a number of oncogenic properties. MUC4 activates a number of proliferative pathways such as MAPK, PKC and RAS-REK, all of which induces cell growth and differentiation [5,40,41]. Cell proliferation is also aided by the large extracellular domain of MUC4, which allows it to evade recognition by the immune system [9,42]. Furthermore, MUC4 facilitates EMT through activating Akt, JNK/2 pathway and Src kinase and Wnt/ß catenin pathways [3,8,9,14,42,43]. These help promote extravasation and metastasis, playing a critical role in invasive cancer [10,44]. In addition, MUC4 allows cancer cells to evade apoptosis through sequestration of pro-apoptotic proteins and inhibition of anti-apoptotic proteins such as cytochrome C present in the mitochondria and caspase-9 [5,18,45,46]. Moreover, MUC4 imparts resistance to tumour cells against gemcitabine through upregulating anti-apoptotic pathways [47].
MUC16
Similar to MUC4, MUC16 is a transmembrane mucin that is also a predictor of poor prognosis and high recurrence rate [48,49]. However, it is expressed in both pancreatitis and pancreatic neoplasms [50], which can limit its role as a specific biomarker. MUC16 has been implicated in a number of oncogenic pathways, such as increasing nuclear translocation of JAK2 and upregulation of LMO2 to increase cell proliferation. Furthermore, it has been shown that inhibition of MUC16 decreases mTOR and c-MYC activity, both of which stimulates cell growth [17,48]. MUC16 also helps promote metastasis through activating matrix metalloproteinase-7 and the Akt and MAPK pathways. Furthermore, it is a ligand to E- and L-selectin, which helps promote adhesion and metastases [17,49]. Indeed inhibiting MUC16 decreases migration and survival of pancreatic tumour cells [16]. Additionally, MUC16 induces cancer cells to switch to anaerobic metabolism and also inhibits apoptosis, both of which increase survival [48,51].
Other mucins
MUC2
MUC2 is occasionally present at low levels in healthy pancreatic tissue and IPMN, however it is absent in PDAC and MCN [5,14]. In IPMN intestinal type, expression of MUC2 is correlated with high grade dysplasia and invasiveness [52], whilst for the IPMN gastric type MUC2 is a marker of favourable prognosis [32].
MUC5AC/AB
MUC5AC is a secreted mucin that can be overexpressed in early neoplastic lesions such as PanIN and PDAC [53,54]. However, it is more commonly found in non-invasive IPMN and MCN [5]. As it is absent in the healthy pancreas, it is used as an indicator for distinguishing malignant from non-neoplastic cells [32]. Knockdown of MUC5AC in xenograft studies resulted in decreased growth and metastasis, suggesting it plays a role in tumour development [55]. However MUC5AC expression in PDAC and IPMN is associated with better clinical outcome [56,57].
MUC13
MUC13 is a transmembrane glycoprotein associated with proliferation, motility, invasion and tumour growth [18,54]. It can be expressed at low levels in the non-neoplastic pancreas, however is upregulated in pancreatic cancer as early as the PanIN stages [58]. MUC13 phosphorylates HER2 in PDAC, regulating growth, motility and differentiation of PDAC. Furthermore it activates PAK1, resulting in increased metastases [59]. MUC13 is also associated with expression of a number of other oncogenes including ERK, Akt and S100A4 and reduced expression of tumour suppressor genes such as p53 [54].
MUC3, MUC 6, MUC15, MUC17, MUC20
Cysteine domains present in MUC3 can suppress the intrinsic apoptotic pathway and increase invasion [60]. Expression of MUC3 also correlates with the level of dysplasia in cancer cells [5,38]. MUC6 can be present in the non-neoplastic tissue and is not associated with either clinical prognosis or outcome [5,61]. There are limited studies on MUC15, 17 and 20, however some studies have indicated that they are increased in pancreatic cancer cell lines as compared to the healthy pancreas [62,63].
Mucins as diagnostic biomarker in pancreatic malignancies
The unavailability of reliable biomarkers for the timely diagnosis of pancreatic cancer is a major contributor to the high mortality rates. Current available biomarkers have limited sensitivity and specificity [5,64,65]. Cancer Antigen 19-9 (CA-19-9) is the most widely used biomarker [66], whilst others include, pyruvate kinase isoenzyme type 2, MIC-1, CEA and CA242. However the expression of these antigens in benign conditions such as chronic pancreatitis, along with variable sensitivity and specificity limits their utility as a diagnostic tool [64,67]. As a result, mucins have garnered attention as promising biomarkers to be utilised in the clinical setting, as certain mucins have a high specificity for pancreatic cancer cells [14,16].
EUS-DNA and circulating tumour markers
Endoscopic ultrasonography-guided, fine-needle aspiration (EUS-FNA) is a relatively harmless method for staging and diagnosing pancreatic malignancies. In previous studies using EUS-FNA, MUC1 (CA15-3), MUC2, MUC7, MUC4, MUC16 (CA125) and MUC5AC has been shown to be overexpressed compared to benign conditions or the healthy pancreas [18,68-71]. In EUS, the use of MUC4 and MUC16 were 100% specific in diagnosing malignant pancreatic cancer from benign conditions with 63% and 67% sensitivity [71]. Circulating tumour markers have also been explored as a potential approach. A recent study evaluating a panel of serum tumour markers (such as CA19-9, CEA, CA242, CA72-4, CA50 and CA125) has shown that MUC16 had the highest association with metastatic disease and was able to best predict recurrent disease after surgical resection [67]. To improve the diagnostic sensitivity of the CA19-9 test, it has been used in combination with mucins such as MUC1 and MUC5AC [64,72]. Mucins have been used to predict prognosis, and elevated serum CA125 levels were strongly associated with a shorter survival time [65]. Similarly tumours that expressed a high level of MUC4 were more likely to metastasize [36,73,74], whereas patients with a low MUC4 expression receiving adjuvant gemcitabine treatment had a longer survival time [13]. In summary, MUC1, MUC4 and MUC16 may be able to diagnose pancreatic cancer, forecast prognosis and monitor treatment with the intention of improving patient survival.
Mucins as a therapeutic target
Since mucins play an important role in oncogenesis, immune system evasion, metastasis and chemotherapeutic resistance [75], they are a target for the development of new therapies as well as to enhance the potency of cytotoxic drugs [7,37,76,77].
Agents that can affect the structural features of mucin and production of glycol-oncoproteins
Several studies have shown that the dense mucin mesh, due to heavy O-glycosylation, decreases the efficacy of cytotoxic drugs. It is postulated that the mucin forms a physical barrier thus inhibiting the intracellular drug uptake and thereby affecting cytotoxicity of the drug [78]. Hence, there has been investigation into dissolving mucin using mucolytic agents bromelain and N-acetylcysteine (NAC) [79-81]. Bromelain is a cysteine proteinase from Ananas comosus that is composed of thiol endopeptidases as well as other enzymes such as phosphatases, glucosidases and cellulases and is able to break the glycosidic bonds within mucin. NAC, a mucolytic agent used in respiratory conditions is able to denature the disulphide bonds in mucins through reduction reactions [19]. The use of bromelain and NAC in suitable combination, also resulted in decreased expression of mucin in vitro and in vivo. The advantage of this treatment is that they have very low toxicity profiles and also contributed to increased chemosensitivity, laying the basis for its use as an adjunct to cytotoxic drugs [82].
Agents involving the immune system
Agents involving the immune system range from the use of peptide vaccinations, adoptive immunotherapy or the use of mucin specific antibodies [14]. Peptide vaccinations enhance the T helper cell or cytotoxic T cell response against tumour cells expressing mucins [5,33]. A vaccination comprising of a MUC1 peptide with five TRR, with either a BCG (Bacillus Calmette-Gurein) or Freund’s adjuvant, has been explored through a Phase 1 trial on patients with metastatic or localised pancreatic cancer. Patients had minimal side effects and results showed that they had an increase in mucin specific cytotoxic T cells [83-85]. In adoptive immunotherapy, patients are injected with dendritic cells expressing the mucin peptides or cytotoxic T cells sensitized to mucin [86]. Single treatment with either approach showed negligible improvement in survival rates, but in metastatic disease, the one-year survival was greater than 20% for the combination approach [87]. PankoMab, an anti-tumour MUC1 antibody, has also been investigated in pancreatic cancer. The advantage of these antibodies is that it differentiates between malignant MUC1 and non-malignant MUC1 antigens whilst generating a strong antibody dependent killing response [88]. Anti-mucin antibodies, conjugated to radioactive iodine, technetium or yttrium, have also been evaluated in clinical trials. However, patients only develop a partial response to this treatment and were exposed to unacceptable levels of radioactivity resulting in neutropenia and thrombocytopenia [89-91]. Although many of these studies and trials show promising results, they are limited in that they are mainly targeting MUC1, besides only involving a small cohort of patients.
Gene based
Currently there are no clinically available forms of gene therapy. However, there are a number of laboratory based and clinical studies that have down-regulated mucin through direct targeting and epigenetic regulation at the transcriptional level [28,54,92,93]. Down regulation of MUC13 through transfecting human pancreatic cancer cells with microRNA-145 has resulted in decreased tumour growth and invasion [54]. Similarly, tumour expression of mucins has been targeted with short interfering RNA that resulted in reduced tumour invasion and metastasis in in vitro and in vivo models [94,95]. Apart from the silencing of mucin genes, another approach involves taking advantage of the mucin gene promoter. This technique involves inserting suicide genes, for example the herpes simplex thymidine kinase, into the promoter region, therefore up-regulating the enzyme. This enzyme helps convert prodrugs into more potent cytotoxic agents, that has been tested in a number of neoplastic pancreatic cell lines successfully, resulting in increased cell death [96]. Although these studies indicate the potential efficacy of gene therapies, they do not address the challenge of delivering the gene in vivo and thus warrants future work on the practicalities of the treatment.
Conclusion and directions for future research
Pancreatic cancer is notorious for its low survival rates. Effective treatment remains a challenge due to lack of effective therapy and resistance. Over the past forty years, the significance of mucins in the carcinogenesis, progression and metastasis of pancreatic neoplasms has been established. Whilst the role of MUC1, MUC4 and MUC16 in pancreatic cancer is well elucidated, there is a lack of data on the significance of other mucins in this disease. However, gaining a deeper understanding of mucins will be critical to developing improved diagnostic tools and novel therapies. Additionally, whilst there has been a number of studies on MUC1 therapy, there is an urgent need to target other mucins such as MUC4 and MUC16, perhaps through combination therapy with the mucolytic agents, bromelain and N-acetylcysteine. Although therapy in pancreatic cancer has not improved, with growing knowledge on the role of mucins in pancreatic cancer, the development of a therapy aimed at improving the survival and outcomes of pancreatic cancer in the near future is inevitable.
Review criteria
Relevant literature on mucins and pancreatic cancer was identified by searching PubMed for articles published until March 2017. Search terms used in combination with mucin included “pancreatic cancer”, “carcinogenesis”, “expression”, “therapy”, “diagnosis” and “biomarker”. Full text articles published in peer reviewed journals were used.
Disclosure of conflict of interest
None.
Abbreviations
- PDAC
pancreatic ductal adenocarcinoma
- PanIN
Pancreatic intraepithelial neoplasia
- IPMN
Intraductal papillary mucinous neoplasms
- MUC
mucin
- EMT
epithelial mesenchymal transition
References
- 1.Pancreatic Cancer Treatment (PDQ(R)): Health Professional Version BTI-PDQ Cancer Information Summaries [Google Scholar]
- 2.Gu YL, Lan C, Pei H, Yang SN, Liu YF, Xiao LL. Applicative value of serum CA19-9, CEA, CA125 and CA242 in diagnosis and prognosis for patients with pancreatic cancer treated by concurrent chemoradiotherapy. Asian Pac J Cancer Prev. 2015;16:6569–6573. doi: 10.7314/apjcp.2015.16.15.6569. [DOI] [PubMed] [Google Scholar]
- 3.Vasseur R, Skrypek N, Duchêne B, Renaud F, Martínez-Maqueda D, Vincent A, Porchet N, Van Seuningen I, Jonckheere N. The mucin MUC4 is a transcriptional and post-transcriptional target of K-ras oncogene in pancreatic cancer. Implication of MAPK/AP-1, NF-kappaB and RalB signaling pathways. Biochim Biophys Acta. 2015;1849:1375–84. doi: 10.1016/j.bbagrm.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Lo ST, Pantazopouos P, Medarova Z, Moore A. Presentation of underglycosylated mucin 1 in pancreatic adenocarcinoma (PDAC) at early stages. Am J Cancer Res. 2016;6:1986–1995. [PMC free article] [PubMed] [Google Scholar]
- 5.Jonckheere N, Skrypek N, Van Seuningen I. Mucins and pancreatic cancer. Cancers. 2010;2:1794–1812. doi: 10.3390/cancers2041794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendy M. An Australasian perspective on the curative treatment of patients with pancreatic cancer, supportive care, and future directions for management. Ecancermedicalscience. 2016;10:700. doi: 10.3332/ecancer.2016.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tréhoux S, Duchêne B, Jonckheere N, Van Seuningen I. The MUC1 oncomucin regulates pancreatic cancer cell biological properties and chemoresistance. Implication of p42-44 MAPK, Akt, Bcl-2 and MMP13 pathways. Biochem Biophys Res Commun. 2015;456:757–762. doi: 10.1016/j.bbrc.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Zhi X, Tao J, Xie K, Zhu Y, Li Z, Tang J, Wang W, Xu H, Zhang J, Xu Z. MUC4-induced nuclear translocation of β-catenin: a novel mechanism for growth, metastasis and angiogenesis in pancreatic cancer. Cancer Lett. 2014;346:104–113. doi: 10.1016/j.canlet.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Pai P, Rachagani S, Dhawan P, Batra SK. Mucins and Wnt/β-catenin signaling in gastrointestinal cancers: an unholy nexus. Carcinogenesis. 2016;37:223–232. doi: 10.1093/carcin/bgw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansari D, Urey C, Hilmersson KS, Bauden MP, Ek F, Olsson R, Andersson R. Apicidin sensitizes pancreatic cancer cells to gemcitabine by epigenetically regulating MUC4 expression. Anticancer Res. 2014;34:5269–5276. [PubMed] [Google Scholar]
- 11.Thota R, Pauff JM, Berlin JD. Treatment of metastatic pancreatic adenocarcinoma: a review. Treatment of metastatic pancreatic adenocarcinoma: a review. Oncology (Williston Park) 2014;28:70–4. [PubMed] [Google Scholar]
- 12.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urey C, Andersson B, Ansari D, Sasor A, Said-Hilmersson K, Nilsson J, Andersson R. Low MUC4 expression is associated with survival benefit in patients with resectable pancreatic cancer receiving adjuvant gemcitabine. Scand J Gastroenterol. 2017;52:595–600. doi: 10.1080/00365521.2017.1290134. [DOI] [PubMed] [Google Scholar]
- 14.Moschovis D, Bamias G, Delladetsima I. Mucins in neoplasms of pancreas, ampulla of Vater and biliary system. World J Gastrointest Oncol. 2016;8:725–734. doi: 10.4251/wjgo.v8.i10.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607–620. doi: 10.1038/nrgastro.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Lee J, Yun JH, Jeong DG, Kim JH. DUSP28 links regulation of Mucin 5B and Mucin 16 to migration and survival of AsPC-1 human pancreatic cancer cells. Tumour Biol. 2016;37:12193–12202. doi: 10.1007/s13277-016-5079-x. [DOI] [PubMed] [Google Scholar]
- 17.Muniyan S, Haridas D, Chugh S, Rachagani S, Lakshmanan I, Gupta S, Seshacharyulu P, Smith LM, Ponnusamy MP, Batra SK. MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism. Genes Cancer. 2016;7:110–24. doi: 10.18632/genesandcancer.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonckheere N, Skrypek N, Van Seuningen I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim Biophys Acta. 2014;1846:142–151. doi: 10.1016/j.bbcan.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Pillai K, Akhter J, Chua TC, Morris DL. A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei. Int J Cancer. 2014;134:478–86. doi: 10.1002/ijc.28380. [DOI] [PubMed] [Google Scholar]
- 20.Pan S, Brentnall TA, Chen R. Glycoproteins and glycoproteomics in pancreatic cancer. World J Gastroenterol. 2016;22:9288–9299. doi: 10.3748/wjg.v22.i42.9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue T, Goldstein IJ, Hollingsworth MA, Kaul K, Brand RE, Haab BB. The prevalence and nature of glycan alterations on specific proteins in pancreatic cancer patients revealed using antibody-lectin sandwich arrays. Mol Cell Proteonomics. 2009;8:1697–707. doi: 10.1074/mcp.M900135-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalra AV, Campbell RB. Mucin impedes cytotoxic effect of 5-FU against growth of human pancreatic cancer cells: overcoming cellular barriers for therapeutic gain. Br J Cancer. 2007;97:910–8. doi: 10.1038/sj.bjc.6603972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633–1638. doi: 10.1038/sj.bjc.6602163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, Yonezawa S. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–254. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 25.Torres MP, Chakraborty S, Souchek J, Batra SK. Mucin-based targeted pancreatic cancer therapy. Curr Pharm Des. 2012;18:2472–2481. doi: 10.2174/13816128112092472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonckheere N, Skrypek N, Van Seuningen I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim Biophys Acta. 2014;1846:142–51. doi: 10.1016/j.bbcan.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Besmer DM, Curry JM, Roy LD, Tinder TL, Sahraei M, Schettini J, Hwang SI, Lee YY, Gendler SJ, Mukherjee P. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res. 2011;71:4432–42. doi: 10.1158/0008-5472.CAN-10-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao CV, Janakiram NB, Madka V, Kumar G, Scott EJ, Pathuri G, Bryant T, Kutche H, Zhang Y, Biddick L, Gali H, Zhao YD, Lightfoot S, Mohammed A. Small-molecule inhibition of GCNT3 disrupts mucin biosynthesis and malignant cellular behaviors in pancreatic cancer. Cancer Res. 2016;76:1965–1974. doi: 10.1158/0008-5472.CAN-15-2820. [DOI] [PubMed] [Google Scholar]
- 29.Moniaux N, Chaturvedi P, Varshney GC, Meza JL, Rodriguez-Sierra JF, Aubert JP, Batra SK. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer. 2007;97:345–357. doi: 10.1038/sj.bjc.6603868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonckheere N, Vasseur R, Van Seuningen I. The cornerstone K-RAS mutation in pancreatic adenocarcinoma: from cell signaling network, target genes, biological processes to therapeutic targeting. Crit Rev Oncol Hematol. 2017;111:7–19. doi: 10.1016/j.critrevonc.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Tréhoux S, Lahdaoui F, Delpu Y, Renaud F, Leteurtre E, Torrisani J, Jonckheere N, Van Seuningen I. Micro-RNAs miR-29a and miR-330-5p function as tumor suppressors by targeting the MUC1 mucin in pancreatic cancer cells. Biochim Biophys Acta. 2015;1853:2392–2403. doi: 10.1016/j.bbamcr.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 32.Yonezawa S, Higashi M, Yamada N, Yokoyama S, Goto M. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:108–24. doi: 10.1007/s00534-009-0174-7. [DOI] [PubMed] [Google Scholar]
- 33.Besmer DM, Curry JM, Roy LD, Tinder TL, Sahraei M, Schettini J, Hwang SI, Lee YY, Gendler SJ, Mukherjee P. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res. 2011;71:4432–42. doi: 10.1158/0008-5472.CAN-10-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai K, Akhter J, Chua TC, Morris DL. Anticancer property of bromelain with therapeutic potential in malignant peritoneal mesothelioma. Cancer Invest. 2013;31:241–250. doi: 10.3109/07357907.2013.784777. [DOI] [PubMed] [Google Scholar]
- 35.Nath S, Roy LD, Grover P, Rao S, Mukherjee P. Mucin 1 regulates Cox-2 gene in pancreatic cancer. Pancreas. 2015;44:909–17. doi: 10.1097/MPA.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama S, Higashi M, Kitamoto S, Oeldorf M, Knippschild U, Kornmann M, Maemura K, Kurahara H, Wiest E, Hamada T, Kitazono I, Goto Y, Tasaki T, Hiraki T, Hatanaka K, Mataki Y, Taguchi H, Hashimoto S, Batra SK, Tanimoto A, Yonezawa S, Hollingsworth MA. Aberrant methylation of MUC1 and MUC4 promoters are potential prognostic biomarkers for pancreatic ductal adenocarcinomas. Oncotarget. 2016;7:42553–42565. doi: 10.18632/oncotarget.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nath S, Daneshvar K, Roy LD, Grover P, Kidiyoor A, Mosley L, Sahraei M, Mukherjee P. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis. 2013;2:e51. doi: 10.1038/oncsis.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park HU, Kim JW, Kim GE, Bae HI, Crawley SC, Yang SC, Gum JR Jr, Batra SK, Rousseau K, Swallow DM, Sleisenger MH, Kim YS. Aberrant expression of MUC3 and MUC4 membraneassociated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003;26:e48–54. doi: 10.1097/00006676-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Cin Pathol. 2002;117:791–6. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Zhang JJ, Peng YP, Liu X, Xie KL, Tang J, Jiang KR, Gao WT, Tian L, Zhang K, Xu ZK, Miao Y. NIDO, AMOP and vWD domains of MUC4 play synergic role in MUC4 mediated signaling. Oncotarget. 2017;8:10385–10399. doi: 10.18632/oncotarget.14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lakshmanan I, Seshacharyulu P, Haridas D, Rachagani S, Gupta S, Joshi S, Guda C, Yan Y, Jain M, Ganti AK, Ponnusamy MP, Batra SK. Novel HER3/MUC4 oncogenic signaling aggravates the tumorigenic phenotypes of pancreatic cancer cells. Oncotarget. 2015;6:21085–99. doi: 10.18632/oncotarget.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia P, Choi AH, Deng Z, Yang Y, Zhao J, Wang Y, Hardwidge PR, Zhu G. Cell membrane-anchored MUC4 promotes tumorigenicity in epithelial carcinomas. Oncotarget. 2017;8:14147–14157. doi: 10.18632/oncotarget.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarhan YE, Kato T, Jang M, Haga Y, Ueda K, Nakamura Y, Park JH. Morphological changes, cadherin switching, and growth suppression in pancreatic cancer by GALNT6 knockdown. Neoplasia. 2016;18:265–272. doi: 10.1016/j.neo.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komatsu M, Tatum L, Altman NH, Carothers Carraway CA, Carraway KL. Potentiation of metastasis by cell surface sialomucin complex (rat MUC4), a multifunctional anti-adhesive glycoprotein. Int J Cancer. 2000;87:480–6. doi: 10.1002/1097-0215(20000815)87:4<480::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Xie K, Zhi X, Tang J, Zhu Y, Zhang J, Li Z, Tao J, Xu Z. Upregulation of the splice variant MUC4/Y in the pancreatic cancer cell line MIA PaCa-2 potentiates proliferation and suppresses apoptosis: new insight into the presence of the transcript variant of MUC4. Oncol Rep. 2014;31:2187–94. doi: 10.3892/or.2014.3113. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y, Zhang JJ, Liang WB, Zhu R, Wang B, Miao Y, Xu ZK. Pancreatic cancer counterattack: MUC4 mediates Fas-independent apoptosis of antigen-specific cytotoxic T lymphocyte. Oncol Rep. 2014;31:1768–76. doi: 10.3892/or.2014.3016. [DOI] [PubMed] [Google Scholar]
- 47.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101:1155–61. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukla SK, Gunda V, Abrego J, Haridas D, Mishra A, Souchek J, Chaika NV, Yu F, Sasson AR, Lazenby AJ, Batra SK, Singh PK. MUC16-mediated activation of mTOR and c-Myc reprograms pancreatic cancer metabolism. Oncotarget. 2015;6:19118–31. doi: 10.18632/oncotarget.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu A, Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Kitahata Y, Nakamura Y, Noda T, Yokoyama S, Yamaue H. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012;103:739–746. doi: 10.1111/j.1349-7006.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takaori K, Hruban RH, Maitra A, Tanigawa N. Pancreatic intraepithelial neoplasia. Pancreas. 2004;28:257–62. doi: 10.1097/00006676-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Das S, Rachagani S, Torres-Gonzalez MP, Lakshmanan I, Majhi PD, Smith LM, Wagner KU, Batra SK. Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget. 2015;6:5772–87. doi: 10.18632/oncotarget.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masuda A, Arisaka Y, Hara S, Matsumoto I, Takenaka M, Sakai A, Shiomi H, Matsuki N, Sugimoto M, Fujita T, Hayakumo T, Ku Y, Ogino S, Azuma T, Kutsumi H. MUC2 expression and prevalence of high-grade dysplasia and invasive carcinoma in mixed-type intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2013;13:583–588. doi: 10.1016/j.pan.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Le N, Sund M, Vinci A GEMS Collaborating Group of Pancreas 2000. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig Liver Dis. 2016;48:223–30. doi: 10.1016/j.dld.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Khan S, Ebeling MC, Zaman MS, Sikander M, Yallapu MM, Chauhan N, Yacoubian AM, Behrman SW, Zafar N, Kumar D, Thompson PA, Jaggi M, Chauhan SC. MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget. 2014;5:7599–609. doi: 10.18632/oncotarget.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoshi H, Sawada T, Uchida M, Saito H, Iijima H, Toda-Agetsuma M, Wada T, Yamazoe S, Tanaka H, Kimura K, Kakehashi A, Wei M, Hirakawa K, Wanibuchi H. Tumor-associated MUC5AC stimulates in vivo tumorigenicity of human pancreatic cancer. Int J Oncol. 2011;38:619–27. doi: 10.3892/ijo.2011.911. [DOI] [PubMed] [Google Scholar]
- 56.Higashi M, Yokoyama S, Yamamoto T, Goto Y, Kitazono I, Hiraki T, Taguchi H, Hashimoto S, Fukukura Y, Koriyama C, Mataki Y, Maemura K, Shinchi H, Jain M, Batra SK, Yonezawa S. Mucin expression in endoscopic ultrasoundguided fine-needle aspiration specimens is a useful prognostic factor in pancreatic ductal adenocarcinoma. Pancreas. 2015;44:728–34. doi: 10.1097/MPA.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yonezawa S, Nakamura A, Horinouchi M, Sato E. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328–41. doi: 10.1007/s005340200037. [DOI] [PubMed] [Google Scholar]
- 58.Nishii Y, Yamaguchi M, Kimura Y, Hasegawa T, Aburatani H, Uchida H, Hirata K, Sakuma Y. A newly developed anti-Mucin 13 monoclonal antibody targets pancreatic ductal adenocarcinoma cells. J Oncol. 2015;46:1781–1787. doi: 10.3892/ijo.2015.2880. [DOI] [PubMed] [Google Scholar]
- 59.Khan S, Sikander M, Ebeling MC, Ganju A, Kumari S, Yallapu MM, Hafeez BB, Ise T, Nagata S, Zafar N, Behrman SW, Wan JY, Ghimire HM, Sahay P, Pradhan P, Chauhan SC, Jaggi M. MUC13 interaction with receptor tyrosine kinase HER2 drives pancreatic ductal adenocarcinoma progression. Oncogene. 2017;36:491–500. doi: 10.1038/onc.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho SB, Dvorak LA, Moor RE, Jacobson AC, Frey MR, Corredor J, Polk DB, Shekels LL. Cysteinerich domains of muc3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenterology. 2006;131:1501–17. doi: 10.1053/j.gastro.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Jinfeng M, Kimura W, Hirai I, Sakurai F, Moriya T, Mizutani M. Expression of MUC5AC and MUC6 in invasive ductal carcinoma of the pancreas and relationship with prognosis. Int J Gastrointest Cancer. 2003;34:9–18. doi: 10.1385/IJGC:34:1:09. [DOI] [PubMed] [Google Scholar]
- 62.Moniaux N, Junker WM, Singh AP, Jones AM, Batra SK. Characterization of human mucin MUC17. Complete coding sequence and organization. J Biol Chem. 2006;281:23676–85. doi: 10.1074/jbc.M600302200. [DOI] [PubMed] [Google Scholar]
- 63.Komatsu H, Tanji E, Sakata N, Aoki T, Motoi F, Naitoh T, Katayose Y, Egawa S, Unno M, Furukawa T. A GNAS mutation found in pancreatic intraductal papillary mucinous neoplasms induces drastic alterations of gene expression profiles with upregulation of mucin genes. PLoS One. 2014;9:e87875. doi: 10.1371/journal.pone.0087875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaur S, Smith LM, Patel A, Menning M, Watley DC, Malik SS, Krishn SR, Mallya K, Aithal A, Sasson AR, Johansson SL, Jain M, Singh S, Guha S, Are C, Raimondo M, Hollingsworth MA, Brand RE, Batra SK. A combination of MUC5AC and CA19-9 improves the diagnosis of pancreatic cancer: a multicenter study. Am J Gastroenterol. 2017;112:172–183. doi: 10.1038/ajg.2016.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gu YL, Lan C, Pei H, Yang SN, Liu YF, Xiao LL. Applicative value of serum CA19-9, CEA, CA125 and CA242 in diagnosis and prognosis for patients with pancreatic cancer treated by concurrent chemoradiotherapy. Asian Pac J Cancer Prev. 2015;16:6569–73. doi: 10.7314/apjcp.2015.16.15.6569. [DOI] [PubMed] [Google Scholar]
- 66.Ringel J, Lohr M. The MUC gene family: their role in diagnosis and early detection of pancreatic cancer. Mol Cancer. 2003;2:9. doi: 10.1186/1476-4598-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu L, Xu HX, Wang WQ, Wu CT, Xiang JF, Liu C, Long J, Xu J, Fu de L, Ni QX, Houchen CW, Postier RG, Li M, Yu XJ. Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis-associated burden. Oncotarget. 2016;7:5943–56. doi: 10.18632/oncotarget.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Gao J, Li Z, Jin Z, Gong Y, Man X. Diagnostic value of mucins (MUC1, MUC2 and MUC5AC) expression profile in endoscopic ultrasound-guided fine-needle aspiration specimens of the pancreas. Int J Cancer. 2007;121:2716–22. doi: 10.1002/ijc.22997. [DOI] [PubMed] [Google Scholar]
- 69.Carrara S, Cangi MG, Arcidiacono PG, Perri F, Petrone MC, Mezzi G, Boemo C, Talarico A, Cin ED, Grassini G, Doglioni C, Testoni PA. Mucin expression pattern in pancreatic diseases: findings from EUS-guided fine-needle aspiration biopsies. Am J Gastroenterol. 2011;106:1359–63. doi: 10.1038/ajg.2011.22. [DOI] [PubMed] [Google Scholar]
- 70.Jhala N, Jhala D, Vickers SM, Eltoum I, Batra SK, Manne U, Eloubeidi M, Jones JJ, Grizzle WE. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–9. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 71.Horn A, Chakraborty S, Dey P, Haridas D, Souchek J, Batra SK, Lele SM. Immunocytochemistry for MUC4 and MUC16 is a useful adjunct in the diagnosis of pancreatic adenocarcinoma on fine-needle aspiration cytology. Arch Pathol Lab Med. 2013;137:546–51. doi: 10.5858/arpa.2011-0229-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yue T, Maupin KA, Fallon B, Li L, Partyka K, Anderson MA, Brenner DE, Kaul K, Zeh H, Moser AJ, Simeone DM, Feng Z, Brand RE, Haab BB. Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA19-9 antigen on specific protein carriers. PLoS One. 2011;6:e29180. doi: 10.1371/journal.pone.0029180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang X, Wang X, Lu SM, Chen C, Wang J, Zheng YY, Ren BH, Xu L. Clinicopathological and prognostic significance of MUC4 expression in cancers: evidence from meta-analysis. Int J Clin Exp Med. 2015;8:10274–83. [PMC free article] [PubMed] [Google Scholar]
- 74.Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, Büchler MW, Aubert JP, Batra SK. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–40. [PubMed] [Google Scholar]
- 75.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skrypek N, Duchêne B, Hebbar M, Leteurtre E, van Seuningen I, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the concentrative nucleoside transporter family. Oncogene. 2011;32:1714–23. doi: 10.1038/onc.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tréhoux S, Duchêne B, Jonckheere N, Van Seuningen I. The MUC1 oncomucin regulates pancreatic cancer cell biological properties and chemoresistance. Implication of p42-44 MAPK, Akt, Bcl-2 and MMP13 pathways. Biochem Biophys Res Commun. 2015;456:757–762. doi: 10.1016/j.bbrc.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 78.Kalra AV, Campbell RB. Mucin overexpression limits the effectiveness of 5-FU by reducing intracellular drug uptake and antineoplastic drug effects in pancreatic tumours. Eur J Cancer. 2009;45:164–73. doi: 10.1016/j.ejca.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 79.Pillai K, Ehteda A, Akhter J, Chua TC, Morris DL. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anticancer Drugs. 2014;25:150–60. doi: 10.1097/CAD.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 80.Pillai K, Akhter J, Chua TC, Morris DL. Anticancer property of bromelain with therapeutic potential in malignant peritoneal mesothelioma. Cancer Invest. 2013;31:241–250. doi: 10.3109/07357907.2013.784777. [DOI] [PubMed] [Google Scholar]
- 81.Amini A, Masoumi-Moghaddam S, Ehteda A, Liauw W, Morris DL. Potentiation of chemotherapeutics by bromelain and N-acetylcysteine: sequential and combination therapy of gastrointestinal cancer cells. Am J Cancer Res. 2016;6:350–369. [PMC free article] [PubMed] [Google Scholar]
- 82.Afshin A, Masoumi-Moghaddam S, Ehteda A, Liauw W, Morris DL. Depletion of mucin in mucin-producing human gastrointestinal carcinoma: results from in vitro and in vivo studies with bromelain and N-acetylcysteine. Oncotarget. 2015;6:33329–33344. doi: 10.18632/oncotarget.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamamoto K, Ueno T, Kawaoka T, Hazama S, Fukui M, Suehiro Y, Hamanaka Y, Ikematsu Y, Imai K, Oka M, Hinoda Y. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005;25:3575–9. [PubMed] [Google Scholar]
- 84.Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, Warnick E, Whiteside T, Osborne J, Kim H, Day R, Troetschel M, Finn OJ. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–64. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT. A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res. 1996;63:298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- 86.Kawaoka T, Oka M, Takashima M, Ueno T, Yamamoto K, Yahara N, Yoshino S, Hazama S. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep. 2008;20:155–63. [PubMed] [Google Scholar]
- 87.Kondo H, Hazama S, Kawaoka T, Yoshino S, Yoshida S, Tokuno K, Takashima M, Ueno T, Hinoda Y, Oka M. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28:379–87. [PubMed] [Google Scholar]
- 88.Danielczyk A, Stahn R, Faulstich D, Löffler A, Märten A, Karsten U, Goletz S. PankoMab: a potent new generation anti-tumour MUC1 antibody. Cancer Immunol Immunother. 2006;55:1337–47. doi: 10.1007/s00262-006-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu D, Chang CH, Gold DV, Goldenberg DM. Identification of PAM4 (clivatuzumab)-reactive epitope on MUC5AC: a promising biomarker and therapeutic target for pancreatic cancer. Oncotarget. 2015;6:4274–4285. doi: 10.18632/oncotarget.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han S, Jin G, Wang L, Li M, He C, Guo X, Zhu Q. The role of PAM4 in the management of pancreatic cancer: diagnosis, radioimmunodetection, and radioimmunotherapy. J Immunol Res. 2014;2014:268479. doi: 10.1155/2014/268479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cardillo TM, Ying Z, Gold DV. Therapeutic advantage of (90)yttrium-versus (131)iodine-labeled PAM4 antibody in experimental pancreatic cancer. Clin Cancer Res. 2001;7:3186–92. [PubMed] [Google Scholar]
- 92.Jonckheere N, Lahdaoui F, Van Seuningen I. Targeting MUC4 in pancreatic cancer: miRNAs. Oncoscience. 2015;2:799–800. doi: 10.18632/oncoscience.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295:69–84. doi: 10.1016/j.canlet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, Wu C, Chen T, Zhang J, Liu G, Pu Y, Zhu J, Shen C, Zhang Y, Zeng N, Zhang X. Effects of RNAi-mediated MUC4 gene silencing on the proliferation and migration of human pancreatic carcinoma BxPC-3 cells. Oncol Rep. 2016;36:3449–3455. doi: 10.3892/or.2016.5152. [DOI] [PubMed] [Google Scholar]
- 95.Tsutsumida H, Swanson BJ, Singh PK, Caffrey TC, Kitajima S, Goto M, Yonezawa S, Hollingsworth MA. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12:2976–87. doi: 10.1158/1078-0432.CCR-05-1197. [DOI] [PubMed] [Google Scholar]
- 96.Ring CJ, Blouin P, Martin LA, Hurst HC, Lemoine NR. Use of transcriptional regulatory elements of the MUC1 and ERBB2 genes to drive tumour-selective expression of a prodrug activating enzyme. Gene Ther. 1997;4:1045–52. doi: 10.1038/sj.gt.3300510. [DOI] [PubMed] [Google Scholar]
- 97.Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, Yonezawa S. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–54. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 98.Yonezawa S, Taira M, Osako M, Kubo M, Tanaka S, Sakoda K, Takao S, Aiko T, Yamamoto M, Irimura T, Kim YS, Sato E. MUC-1 mucin expression in invasive areas of intraductal papillary mucinous tumors of the pancreas. Pathol Int. 1998;48:319–22. doi: 10.1111/j.1440-1827.1998.tb03913.x. [DOI] [PubMed] [Google Scholar]
- 99.Terris B, Dubois S, Buisine MP, Sauvanet A, Ruszniewski P, Aubert JP, Porchet N, Couvelard A, Degott C, Fléjou JF. Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632–7. doi: 10.1002/path.1146. [DOI] [PubMed] [Google Scholar]
- 100.Yonezawa S, Sueyoshi K, Nomoto M, Kitamura H, Nagata K, Arimura Y, Tanaka S, Hollingsworth MA, Siddiki B, Kim YS, Sato E. MUC2 gene expression is found in noninvasive tumors but not in invasive tumors of the pancreas and liver: its close relationship with prognosis of the patients. Hum Pathol. 1997;28:344–52. doi: 10.1016/s0046-8177(97)90134-9. [DOI] [PubMed] [Google Scholar]
- 101.Park HU, Kim JW, Kim GE, Bae HI, Crawley SC, Yang SC, Gum JR Jr, Batra SK, Rousseau K, Swallow DM, Sleisenger MH, Kim YS. Aberrant expression of MUC3 and MUC4 membraneassociated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003;26:e48–54. doi: 10.1097/00006676-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 102.Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, Nagata K, Tanaka S, Sakoda K, Aikou T, Sato E. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45–54. doi: 10.1046/j.1440-1827.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- 103.Chauhan SC, Ebeling MC, Maher DM, Koch MD, Watanabe A, Aburatani H, Lio Y, Jaggi M. MUC13 mucin augments pancreatic tumorigenesis. Mol Cancer Ther. 2012;11:24–33. doi: 10.1158/1535-7163.MCT-11-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haridas D, Chakraborty S, Ponnusamy MP, Lakshmanan I, Rachagani S, Cruz E, Kumar S, Das S, Lele SM, Anderson JM, Wittel UA, Hollingsworth MA, Batra SK. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS One. 2011;6:e26839. doi: 10.1371/journal.pone.0026839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shimizu A, Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Kitahata Y, Nakamura Y, Noda T, Yokoyama S, Yamaue H. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012;103:739–46. doi: 10.1111/j.1349-7006.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, Waga I, Nanayama T, Yamamoto Y, Munger W, Sun HW, Falk RJ, Jennette JC, Alcorta DA, Li H, Yamamoto T, Saito Y, Nakamura M. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem. 2004;279:1968–79. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 107.Itoh Y, Kamata-Sakurai M, Denda-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M, Irimura T. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]


