Abstract
The serine–threonine protein kinase encoded by the Tpl2 protooncogene transduces Toll-like and death receptor signals in a variety of cell types and plays an important role in innate immunity and inflammation. Differential translational initiation of the Tpl2 mRNA gives rise to 58-kDa (p58) and 52-kDa (p52) isoforms. In unstimulated cells, both isoforms are stabilized and inactivated by stoichiometric binding to NF-κB1/p105. After lipopolysaccharide or TNF-α stimulation, p58 is released from p105 preferentially relative to p52. The released p58 is active but unstable and undergoes rapid degradation via the proteasome. Recent studies revealed that Tpl2 undergoes phosphorylation at Thr-290 and that phosphorylation at this site is required for activation. Here, we present evidence showing that it is the p58 isoform that is preferentially phosphorylated at Thr-290 and that phosphorylation is more efficient when p58 is complexed to p52. Because p58 is preferentially released from p105 after stimulation, we examined whether Tpl2 phosphorylation at this site controls the dissociation of the two proteins in response to external signals and the subsequent events leading to the activation of Tpl2. The results showed that lipopolysaccharide-induced Tpl2 phosphorylation at Thr-290 in macrophages promotes the release of Tpl2 from p105, contributes to the enzymatic activation of the Tpl2 kinase, and is required for the degradation of Tpl2 via the proteasome.
Keywords: Tpl2/Cot, inflammation, endotoxin shock, mitogen-activated protein kinase, extracellular signal-regulated kinase
Tumor progression locus 2 (Tpl2) encodes a serine–threonine protein kinase that is activated by provirus integration in Moloney murine leukemia virus-induced T cell lymphomas and mouse mammary tumor virus-induced mammary adenocarcinomas (1–3). Provirus integration always occurs in the last intron and in the same transcriptional orientation as the Tpl2 gene. The mRNA transcribed from the mutant gene is truncated and encodes a protein with an altered C-terminal tail. In this protein, 43 C-terminal amino acids encoded by the last exon are replaced by 7 amino acids encoded by the intron (1). The C-terminally altered, tumor-specific Tpl2 exhibits enhanced kinase activity (1–3). mRNA transcribed from both the wild type (WT) and the mutant gene contains three ATGs at its 5′ end. Differential utilization of these ATGs in the WT mRNA gives rise to 467-, 464-, and 438-aa proteins. The 467- and 464-aa proteins, translated from the first two ATGs, are detected as a 58-kDa band (p58), whereas the 438-aa protein translated from the third ATG is detected as a 52-kDa (p52) band by Western blotting (4). The p58 and p52 isoforms encoded by endogenous Tpl2 in macrophages are expressed at equimolar levels. However, when Tpl2 is overexpressed in 293 cells, the 58-kDa isoform is predominant.
In the absence of outside signals, endogenous Tpl2 is inactive. When overexpressed in a variety of cell types, however, Tpl2 is constitutively active. Overexpressed Tpl2 activates all of the mitogen-activated protein kinase pathways (5–7), and the transcription factors NF-AT and NF-κB (8–11), transforms cells in culture (7), and promotes cellular proliferation (1, 12). Transgenic mice expressing the truncated, constitutively active form of Tpl2, but not the WT protein, from a T cell-specific promoter develop thymic lymphomas (3). Despite the profound effects of overexpressed Tpl2 on cell function, Tpl2-knockout mice appear healthy. These animals however, are resistant to lipopolysaccharide (LPS)-induced endotoxin shock, as well as to TNF-α-induced inflammatory bowel disease (13, 14). Based on these findings, we addressed the role of Tpl2 in Toll-like receptor and death-receptor signaling. Today, we know that Tpl2 plays an obligatory role in the transduction of extracellular signal-regulated kinase (ERK) activation signals induced by LPS and CD40L in macrophages and B cells and by TNF-α in macrophages (13, 15, 16). In addition, we know that Tpl2 is required for the transduction of TNF-α signals that activate ERK and other pathways in mouse embryo fibroblasts (S. Das, I. Lambertz, M. Kelliher, A. Eliopoulous, K. Du, and P.N.T., unpublished data).
The failure of ERK activation by LPS in Tpl2-/- macrophages gives rise to defects in the expression of cytokines, chemokines, and other molecules involved in the regulation of innate and adaptive immunity (13). The mechanism by which the Tpl2/ERK pathway regulates the expression of these molecules in macrophages is variable. Thus, the induction of TNF-α by LPS depends on Tpl2/ERK signals that target the AU-rich motif in the 3′ untranslated region of the TNF-α mRNA and regulate nucleocytoplasmic RNA transport (13). The induction of cyclooxygenase-2 depends on Tpl2/ERK signals that regulate the transcription and stability of the cyclooxygenase-2 mRNA (15), and the induction of IL-1β depends on Tpl2/ERK signals that regulate transcription (C. Dumitru and P.N.T., unpublished data).
Recent studies have shown that Tpl2 undergoes phosphorylation at Thr-290 and that a Tpl2 T290A mutant, which cannot be phosphorylated at this site, is inactive (17). Preliminary data from our laboratory (J.C., M. Melnick, G. Solidakis, and P.N.T., unpublished data) revealed that phosphorylation at this site is induced by LPS and TNF-α and plays an obligatory role in Tpl2 activation by external signals. The same studies revealed that phosphorylation at Thr-290 depends on the activity of IKKβ, an obligatory upstream regulator of Tpl2.
In unstimulated cells, Tpl2 interacts stoichiometrically with NF-κB1/p105 (10). Tpl2 bound to p105 is stable but inactive. Treatment of macrophages with LPS activates Tpl2 by promoting the dissociation of the two proteins. Tpl2 released from p105 in response to LPS or TNF-α signals is active but unstable and undergoes rapid degradation via the proteasome (18, 19). Data presented in this work show that the release of Tpl2 from p105 and its subsequent activation and degradation in response to LPS depend on Tpl2 phosphorylation at Thr-290. The involvement of Thr-290 phosphorylation in the dissociation of Tpl2 from p105 was suggested by data showing that, of the p58 and p52 isoforms of Tpl2, the p58 isoform is preferentially released and degraded in response to LPS (18, 19). Although the degradation of p105 may contribute to the release of Tpl2, as suggested by recent reports (20, 21), our data indicate that the initiation of this process depends on Tpl2 phosphorylation at Thr-290.
Materials and Methods
Cell Culture. Bone marrow-derived macrophages were cultured as described in ref. 13. Cultures of bone marrow-derived macrophages were immortalized by infection with a pZIP-Neo/SV40 large T antigen retrovirus that was harvested from Y2 cells (kindly provided by Anthony DeFranco, University of California, San Francisco). The immortalized lines were maintained in macrophage medium (DMEM supplemented with 20% FBS, 30% L929 conditioned medium, nonessential amino acids, penicillin, and streptomycin). RAW264.7, Y2, 293, L929 (13), and Phoenix cells (kindly provided by Garry Nolan, Stanford University, Stanford, CA), used for the packaging of the retrovirus constructs, were maintained in DMEM supplemented with 10% FBS, nonessential amino acids, penicillin, and streptocycin. LPS (from Salmonella enteritidis) (1 μg/ml), used to stimulate immortalized macrophages and RAW264.7 cells, was purchased from Sigma. To inhibit the degradation of Tpl2 and p105 via the proteasome, cells were treated with 20 μM proteasome inhibitor MG132 (Calbiochem), starting 5 h before LPS stimulation. Treatment with MG132 continued after exposure to LPS.
Expression Constructs, Retrovirus Constructs, Site-Directed Mutagenesis, and Gene Transfer. Tpl2 tagged at the C terminus with a hemagglutinin (HA) epitope tag was cloned by PCR in pCMV5 between EcoRI and BamHI. Mutants were generated by using the QuikChange mutagenesis kit (Stratagene). For the coimmunoprecipitation experiments in Fig. 1, the p58 isoform of Tpl2 was N-terminally tagged with the Flag epitope. Retrovirus constructs were made, by using the pMSCV-based retrovirus vector pMigR1 (kindly provided by Warren Pear, University of Pennsylvania, Philadelphia). A myc-tagged pEF expression construct of p105 was kindly provided by Aristides Eliopoulos (University of Birmingham, Birmingham, U.K.). All constructs were confirmed by sequencing. Gene transfer was carried out by using standard procedures (for details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site).
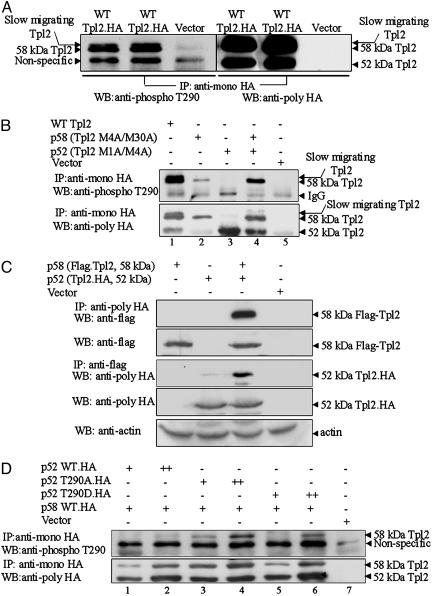
Fig. 1.
Phosphorylation at Thr-290 targets preferentially the p58 isoform of Tpl2, which forms heterodimers with the p52 isoform. (A) An anti-HA mAb was used to immunoprecipitate Tpl2.HA from lysates of 293 cells transiently transfected with a Tpl2.HA construct or with the empty vector. A Western blot of the immunoprecipitates was probed with a rabbit polyclonal Ab raised against a phosphorylated Tpl2 peptide encompassing Thr-290 (CKDLRGT(PO4)EIYMSPE) (Left). The blot then was stripped and reprobed with a rabbit anti-HA polyclonal Ab (Right). The faster-migrating band detected with the phosphospecific Ab is a nonspecific band and not the p52 isoform of Tpl2 (see Results for details). (B) HA-tagged constructs of WT Tpl2 and the ATG mutants of Tpl2 (p58 or Tpl2 M4A/M30A and p52 or Tpl2 M1A/M4A) were transiently transfected, singly or in combination, in 293 cells. Control cells were transfected with the empty vector. WT and Tpl2 mutants immunoprecipitated with the anti-HA mAb were probed with the phospho-Thr-290 Tpl2 Ab (Upper) or with an anti-HA polyclonal Ab (Lower). The ratio of the intensity of the p58 bands detected with the anti-HA Ab in lanes 4 and 2 (≈2.2) was lower than that of the p58 bands detected with the anti-phospho-Thr-290 Ab (≈8). (C) We transfected 293 cells with the indicated expression constructs. p58 was immunoprecipitated with an anti-Flag Ab, and p52 was immunoprecipitated with an anti-HA Ab from cell lysates harvested 48 h later. Western blots of the immunoprecipitates and of straight cell lysates were probed with the indicated Abs. (D) The p58 isoform of Tpl2.HA was transfected into 293 cells in combination with increasing concentrations of WT p52.HA, p52T290A.HA, or p52T290D.HA. Tpl2 immunoprecipitates were probed with the phospho-Thr-290 Ab or with an anti-HA Ab, which detects both Tpl2 isoforms. Both the WT p52 and the p52 mutants enhanced phosphorylation of the p58 isoform (Upper). The ratio of the intensity of the p58 bands detected with the anti-HA Ab in lanes 4 and 3 (≈1.5) was lower than that of the p58 bands detected in the same lanes with the phospho-Thr-290 Ab (≈4).
Antibodies (Abs), Immunoprecipitation, and Immunoblotting. Abs against phospho-ERK1/2 (Thr-202/Tyr-204), phosphomitogen-activated protein kinase kinase1/2 (MEK1/2) (Ser-217/Ser-221), total ERK1/2, and total MEK1/2 were purchased from Cell Signaling Technology (Beverly, MA). Anti-p105 Ab was from Delta Biolabs (Campbell, CA), and anti-Tpl2 (M20) Ab was from Santa Cruz Biotechnology. Finally anti-HA monoclonal Abs (mAbs) and polyclonal Abs were from Covance (Princeton). The phospho-Thr-290 Ab was raised by injecting rabbits with the peptide CKDLRGT (PO4) EIYMSPE (J.C., M. Melnick, G. Solidakis, and P.N.T., unpublished work). Antirabbit and anti-mouse IgG-horseradish peroxidase were from Amersham Pharmacia, and protein G-Sepharose was from Invitrogen. Immunoprecipitation and immunoblotting were carried out by following standard procedures (see Supporting Materials and Methods for details).
Results
Phosphorylation at Thr-290 Targets Preferentially the p58 Isoform of Tpl2, Which Forms a Complex with the p52 Isoform. An Ab specifically recognizing Tpl2 phosphorylated at Thr-290 was raised in rabbits (see Materials and Methods). This Ab was used to probe Western blots of Tpl2 immunoprecipitated with a mouse anti-HA mAb from lysates of 293 cells transiently transfected with an expression construct of HA-tagged WT Tpl2 or with the empty vector. The phosphospecific Ab detected two bands that appeared to correspond to the 52- and 58-kDa Tpl2 isoforms. Surprisingly however, the p52-like band was detected in Tpl2.HA immunoprecipitates from all cell lysates, including those transfected with the empty vector. To determine whether the p52-like band was the 52-kDa Tpl2 isoform, the blot probed with the phosphospecific Ab was stripped and reprobed with a polyclonal anti-HA (Tpl2) Ab. The results showed that the faster migrating band detected with the phosphospecific Ab is a nonspecific band that migrates more slowly than p52. These results also showed that only the p58 isoform of Tpl2 is detectable with the phosphospecific Ab and suggested that only the p58 isoform undergoes phosphorylation at Thr-290.
Whereas translational initiation from the first two ATGs at the 5′ end of the Tpl2 mRNA gives rise to the p58 isoform of Tpl2, translational initiation from the third ATG gives rise to the p52 isoform (4). To confirm that only the p58 isoform is phosphorylated at Thr-290, we generated ATG mutants of Tpl2 that express one isoform at a time. Tpl2 was immunoprecipitated with an anti-HA mAb from lysates of 293 cells transiently transfected with expression constructs of the mutant or WT Tpl2. Probing a Western blot of the immunoprecipitates with the phospho-Thr-290 Tpl2 Ab confirmed that the p52 isoform is phosphorylated very weakly, if at all, at this site (Fig. 1B). Because p58, of the two Tpl2 isoforms, also is preferentially released and degraded in response to LPS, these findings suggested that phosphorylation at Thr-290 may promote the release of Tpl2 from p105.
The data in Fig. 1B also show that the expression of p58 was up-regulated when p58 and p52 were coexpressed. However, the ratio of the p58 bands detected with the anti-HA Ab in cells expressing both isoforms, or only p58 (≈2.2), was lower than the ratio of the p58 bands detected with the anti-phospho-Thr-290 Ab in the same cells (≈8) (Fig. 1B), suggesting that phosphorylation of p58 was inefficient when p58 was expressed alone and became more efficient when it was coexpressed with p52. To address the molecular mechanism of this phenomenon, we first examined whether p58 and p52 interact when coexpressed in transiently transfected 293 cells. The Flag-tagged p58 and HA-tagged p52 isoforms of Tpl2 were transiently transfected into 293 cells alone or in combination, as indicated. The interaction of the two isoforms was demonstrated by immunoprecipitation either with anti-HA Ab or anti-Flag Ab, followed by Western blotting with anti-flag or anti-HA Abs, respectively. Expression of the transfected constructs was monitored by Western blotting with anti-HA or anti-Flag Abs, combined with anti-actin Ab to monitor the loading (Fig. 1C). The results of this experiment confirmed the interaction of the two isoforms. Subsequently, we carried out transient transfections of 293 cells, by using constant amounts of WT p58 and increasing amounts of WT, T290A, and T290D p52. The results (Fig. 1D) revealed that the expression of p58 increases in parallel with the expression of p52, suggesting that the p52/p58 interaction may stabilize p58. Because the half-life of p52 is longer than that of p58 (30 vs. 10 min) (4), the stability of p58 indeed may be enhanced by binding to p52. The apparent increase in p58 phosphorylation may be due to the higher levels of p58 in cells expressing both p58 and p52. However, because the ratio of the intensity of the p58 bands detected with the anti-HA Ab in lanes 4 and 3 (≈1.5) was lower than that of the p58 bands detected in the same lanes with the phospho-Thr-290 Ab (≈4), we conclude that the p58/p52 complex is likely to be a better substrate for the kinase phosphorylating Tpl2 at Thr-290.
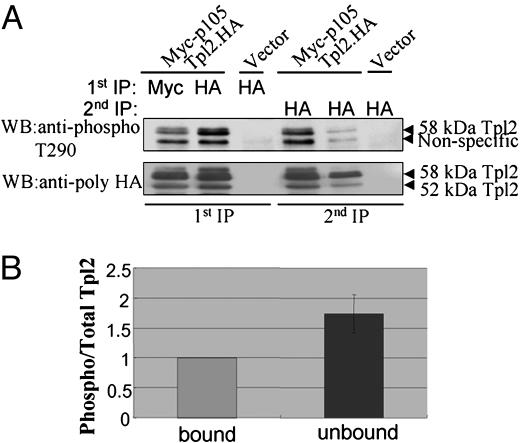
Phosphorylation at Thr-290 Decreases the Affinity of Tpl2 to p105. Tpl2 is stoichiometrically bound to p105 in unstimulated cells. p105-bound Tpl2 is stable but inactive (18, 19, 22). LPS stimulation of macrophages promotes the release of p58, but not p52, from p105 (18). Because only the p58 isoform is phosphorylated at Thr-290, the question was raised of whether the release of Tpl2 from p105 is induced by phosphorylation at this site. To address this question, we first examined whether the p105-bound and unbound fractions of Tpl2 differed with regard to the stoichiometry of phosphorylation at Thr-290. To this end, 293 cells were transiently transfected with Myc-p105 and Tpl2.HA or with the empty vector. Myc-p105 and Tpl2.HA were immunoprecipitated from transfected cell lysates with the mouse anti-myc or anti-HA mAbs, respectively. The immunoprecipitates were probed with rabbit polyclonal anti-HA and anti-phospho-Thr-290 Tpl2 Abs. Tpl2.HA detected with the anti-HA Ab in the Myc-p105 immunoprecipitates represents the p105-bound fraction of Tpl2.HA (Fig. 2A, lane 1). Tpl2.HA remaining in the supernatant of the first immunoprecipitation contains the unbound fraction of Tpl2 as well as a subfraction of p105-bound Tpl2 that failed to be immunoprecipitated with the anti-myc Ab (Fig. 2 A, lane 4). Tpl2.HA detected in the supernatants of the anti-HA (Tpl2) immunoprecipitates, represents the fraction of Tpl2 that was not immunoprecipitated quantitatively during the first immunoprecipitation. The latter appears to be a small fraction of the total protein (Fig. 2 A, lane 5). To compare the stoichiometry of Thr-290 phosphorylation in the p105-bound and unbound fractions of Tpl2.HA, we quantitated the intensity of the phosphorylated and total Tpl2.HA bands in the p105 bound fraction in lane 1 and the putative mixture of unbound and bound Tpl2 in lane 4. The ratio of phosphorylated to total Tpl2.HA in the bound fraction in lane 1 was given the arbitrary value of 1. The ratio of phosphorylated to total Tpl2.HA in the putative mixture of unbound and bound fractions in lane 4 was measured relative to the bound fraction. This experiment was repeated five times. The combined data in Fig. 2B show that the phosphorylated protein is preferentially unbound and suggest that phosphorylation at Thr-290 decreases the affinity of Tpl2 to p105. The same question was addressed in a parallel, simpler experiment that is presented in Fig. 7, which is published as supporting information on the PNAS web site. The results, which were fully reproducible in three consecutive experiments, showed again that the stoichiometry of phosphorylation is lower in the p105-bound fraction.
Fig. 2.
Relative affinity of Thr-290-phosphorylated and -nonphosphorylated Tpl2 to p105. (A) We transiently transfected 293 cells with Myc-p105 and Tpl2.HA or with the empty vector in the indicated combinations. Myc-p105 and Tpl2.HA were immunoprecipitated from cell lysates, and the immunoprecipitates were probed with anti-phospho-Thr-290 or anti-HA Abs as indicated. Tpl2.HA was immunoprecipitated with an anti-HA Ab from the supernatants of the first immunoprecipitation, and the immunoprecipitates were again probed with the anti-phospho-Thr-290 and the anti-HA Abs (see Results for details and interpretation). (B) The stoichiometry of phosphorylation is higher in the unbound relative to the p105-bound Tpl2, suggesting that phosphorylation at Thr-290 decreases the affinity of Tpl2 to p105. The ratio of phosphorylated/total Tpl2 in the bound and unbound fractions of Tpl2, shown in the figure, was calculated from quantitative measurements in five independent experiments. Unbound Tpl2 may be a mixture that also contains a fraction of bound Tpl2 that failed to be coimmunoprecipitated with Myc-p105 (see Results for details).
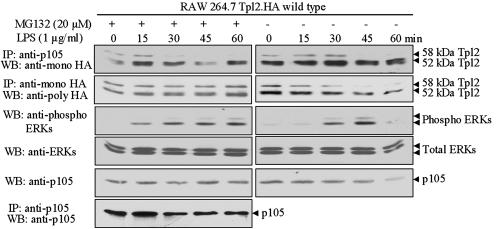
Tpl2 Is Released from p105 in LPS-Treated Macrophages Even If p105 Degradation Is Blocked by Proteasome Inhibitors. The preceding data, although compelling, do not address whether phosphorylation at Thr-290 plays a physiological role in the release of Tpl2 from p105. To address this question, RAW264.7 cells expressing stably near-physiological levels of WT Tpl2.HA were stimulated with LPS in the presence or absence of the proteasome inhibitor MG132. Tpl2.HA was immunoprecipitated from lysates of these cells, and the immunoprecipitates were probed with a rabbit anti-HA polyclonal Ab. Western blots of the same lysates were probed with the p105 Ab. The results confirmed that MG132 blocks the degradation of both Tpl2.HA and p105 after LPS stimulation (Fig. 3). Next, we immunoprecipitated p105 from the same lysates and we probed a Western blot of the immunoprecipitates with a mouse anti-HA mAb. To confirm that p105 was efficiently immunoprecipitated, the same blot also was probed with the p105 Ab. The results showed that the fraction of Tpl2.HA coimmunoprecipitating with p105 decreased after stimulation in both the MG132-treated and untreated cells (Fig. 3). We conclude that the release of Tpl2.HA from p105 in response to LPS is independent of the proteasomal degradation of either Tpl2.HA or p105. Instead, it is due to changes in the affinity of binding between the two proteins, which, according to the preceding experiment, may be the result of Tpl2 phosphorylation at Thr-290.
Fig. 3.
The release of Tpl2 from p105 in response to LPS is independent of the proteasomal degradation of either Tpl2 or p105. RAW264.7 cells engineered to stably express near-physiological levels of WT Tpl2.HA were stimulated with LPS, in the presence or absence of the proteasome inhibitor MG132 (20 μM). p105 and Tpl2.HA immunoprecipitated from lysates of cells harvested at the indicated time points were probed with the anti-HA Ab. Western blots of the same lysates were probed with Abs against p105 and against phosphorylated and total ERK. The efficiency of p105 immunoprecipitation was monitored by probing the p105 immunoprecipitates from the MG132-treated cell lysates with the anti-p105 Ab.
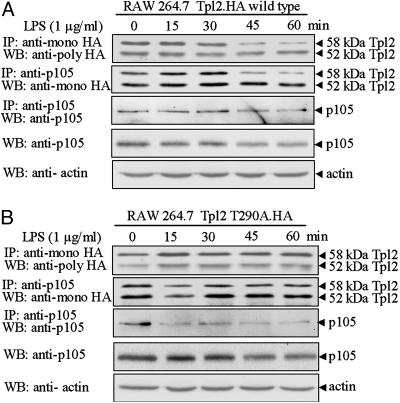
Phosphorylation at Thr-290 Is Required for the Release of Tpl2 from p105 in LPS-Stimulated Macrophages. The data in Figs. 2 and 3 suggest but do not prove that phosphorylation at Thr-290 may be responsible for the release of Tpl2 from p105. To address this hypothesis, we examined the dissociation of Tpl2 T290A from p105 in LPS-stimulated RAW264.7 cells. Cells expressing stably near-physiological levels of WT Tpl2.HA or Tpl2 T290A.HA were generated by retroviral infection. All cell lines were stimulated with LPS. Total Tpl2.HA and p105-bound Tpl2.HA were immunoprecipitated with anti-HA and anti-p105 Abs, respectively. Probing these immunoprecipitates with the anti-HA Ab revealed that, whereas the p58 isoform of WT Tpl2 is released (Fig. 4A, second blot from the top) and degraded (Fig. 4A, first and second blots from the top) in response to LPS, the Tpl2 T290A mutant remains bound (Fig. 4B, second blot from the top) and stable (Fig. 4B, first and second blots from the top). Taken together, these data show that the release of Tpl2 from p105 is indeed regulated by phosphorylation at Thr-290.
Fig. 4.
The release of Tpl2 from p105 is regulated by phosphorylation at Thr-290. RAW264.7 cells engineered to express WT Tpl2.HA (A) or Tpl2 T290A.HA (B) were stimulated with LPS. Probing p105-bound and total Tpl2.HA with the anti-HA Ab revealed that whereas the p58 isoform of WT Tpl2.HA was released and degraded in response to LPS, the p58 isoform of Tpl2 T290A remained bound and stable. The efficiency of p105 immunoprecipitation in both panels was monitored by probing the p105 immunoprecipitates with the anti-p105 Ab.
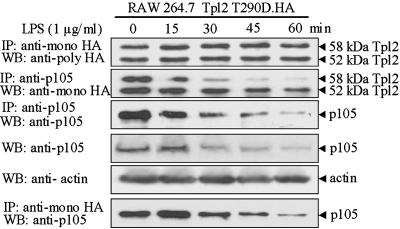
Phosphorylation at Thr-290 Is Required for the Degradation of Tpl2 That Is Released from p105 by LPS in Macrophages. Next, we examined the dissociation and degradation of Tpl2 T290D.HA and p105 in response to LPS in RAW264.7 cells expressing stably Tpl2 T290D.HA. The experimental protocol was identical to the one in the preceding experiment addressing the behavior of Tpl2 T290A. The results showed that Tpl2 T290D.HA was released from p105 in response to LPS but was not degraded (Fig. 5). The finding that Tpl2 T290D.HA failed to undergo degradation after its dissociation from p105 suggests that phosphorylation at Thr-290 is required for targeting the unbound, active protein to the proteasome. The finding that T290D is bound to p105 in unstimulated cells, but, unlike T290A, is rapidly released in response to LPS stimulation, suggests that phosphorylation at Thr-290 is necessary but not sufficient for dissociation.
Fig. 5.
The degradation of Tpl2 after its release from p105 in LPS-stimulated macrophages depends on phosphorylation at Thr-290. RAW264.7 cells engineered to stably express Tpl2 T290D.HA were stimulated with LPS. Probing p105-bound and total Tpl2.HA with the anti-HA Ab revealed that while Tpl2 T290D.HA was released from the p105 complex, it remained stable, suggesting that phosphorylation at Thr-290 targets Tpl2 for degradation. The efficiency of p105 immunoprecipitation in both panels was monitored by probing the p105 immunoprecipitates with the anti-p105 Ab.
Discussion
In resting cells, Tpl2 is stoichiometrically bound to NF-κB1/p105 (10). p105-bound Tpl2 is stable but inactive (10, 18, 22). LPS stimulation activates Tpl2, in part, by promoting its dissociation from p105. In this work, we addressed the molecular mechanism responsible for the dissociation of the two proteins and the activation of Tpl2 in response to LPS. Recent studies published while this manuscript was being prepared, showed that Tpl2 undergoes phosphorylation at Thr-290 and that, whereas a T290D mutant is active, a T290A mutant is not (17). Moreover, preliminary studies from our laboratory (J.C., M. Melnick, G. Solidakis, and P.N.T., unpublished data) have shown that Tpl2 phosphorylation at Thr-290 is induced by LPS via an IKKβ-dependent mechanism and that phosphorylation at this site is required for the physiological activation of Tpl2 and for Tpl2 signaling in macrophages. Here, we show that, of the p58 and p52 isoforms of Tpl2, p58 is preferentially phosphorylated at this site. Given that p58 is also preferentially released from p105 in response to LPS (18), we hypothesized that phosphorylation at Thr-290 promotes the dissociation of the two proteins. The data we present confirms that Thr-290 phosphorylation promotes the release, activation, and degradation of Tpl2 in response to LPS (Fig. 6).
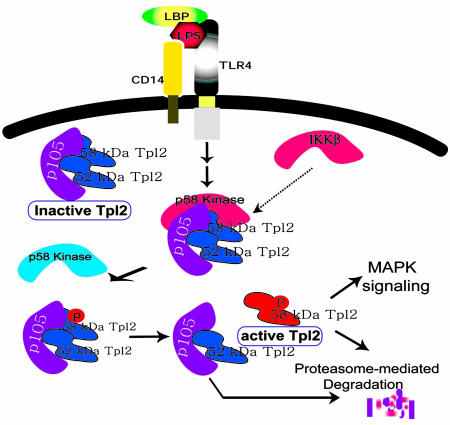
Fig. 6.
A model of the role of Thr-290 phosphorylation in Tpl2 activation by LPS. The p52 and p58 isoforms of Tpl2 form a stable complex with p105 in resting cells. Binding to p105 stabilizes Tpl2 and maintains it in an inactive state. Stimulation of CD14/TLR4 with LPS initiates a signaling cascade that leads to the phosphorylation of the p58 isoform at Thr-290. Phosphorylation promotes the release of p58 from the p105 complex and is required for the enzymatic activation of the Tpl2 kinase. The parallel degradation of p105 may contribute to the release of Tpl2 by preventing the reassociation of the two proteins. Unbound, active Tpl2 transduces downstream signals that activate ERK and is rapidly degraded via the proteasome. Targeting to the proteasome also depends on phosphorylation at Thr-290. Thus, phosphorylation at this site couples Tpl2 activation with degradation. MAPK, mitogen-activated protein kinase.
The role of Thr-290 phosphorylation in the release of Tpl2 from p105 was confirmed by several complementary observations. First, the stoichiometry of phosphorylation of p105-bound Tpl2 in transiently transfected 293 cells was significantly lower than that of unbound Tpl2 in the same cells. This finding suggested that Thr-290-phosphorylated Tpl2 exhibits lower affinity toward p105 than nonphosphorylated Tpl2 and that phosphorylation may promote its release. To determine whether phosphorylation at Thr-290 contributes to the physiological regulation of Tpl2 in LPS-stimulated macrophages, we first examined whether LPS induced the release of Tpl2 from p105 in macrophages pretreated with the proteasome inhibitor MG132, which stabilizes both Tpl2 and p105. In addition, we examined whether LPS treatment of macrophages stably expressing Tpl2 T290A promotes the release of the mutant protein from p105. The results showed that WT Tpl2 was released in response to LPS even after stabilization of p105 and Tpl2 by MG132. More importantly, Tpl2 T290A failed to be released by LPS, whereas Tpl2 T290D was released. These data together confirm that phosphorylation at Thr-290 promotes the release of Tpl2 from p105.
p105 undergoes degradation after stimulation with LPS (23). Recent studies were interpreted to suggest that p105 degradation may be responsible for the release of Tpl2 from p105 (20, 21). The data presented in this report, however, clearly show that even though the degradation of p105 could be a contributing factor in the release of Tpl2 in response to LPS, phosphorylation of Tpl2 at Thr-290 is an obligatory event that may initiate the process.
Tpl2 expressed at physiological levels is quantitatively bound to p105 and is inactive (10, 18, 22). When overexpressed in transiently transfected cells, a fraction of WT Tpl2 undergoes phosphorylation at Thr-290. This fraction is preferentially unbound and catalytically active. Interestingly, when overexpressed in 293 cells, the T290A mutant is inactive, whereas the T290D mutant is active. Because the level of endogenous p105 is not sufficient to quantitatively bind all Tpl2 transiently expressed in 293 cells, a fraction of both the T290A and T290D mutants should remain unbound. The fact that Tpl2 T290A, despite being partially unbound, is inactive, whereas Tpl2 T290D is active, suggests that phosphorylation at Thr-290, in addition to promoting the release of Tpl2 from p105, also may be required for the enzymatic activation of the unbound protein.
Once released from p105, Tpl2 becomes enzymatically active and undergoes rapid degradation via the proteasome (18). Proteins destined to undergo proteasome-mediated degradation usually are marked by phosphorylation. This observation raises the question of whether phosphorylation at Thr-290 promotes not only the release and the enzymatic activation of Tpl2, but also its degradation via the proteasome. To address this question we compared the release, activation, and degradation of Tpl2 T290A and Tpl2 T290D in LPS-stimulated RAW264.7 macrophages engineered to constitutively express the two mutants. Data presented in Fig. 5 clearly show that, unlike Tpl2 T290A, Tpl2 T290D is rapidly released from p105 in response to LPS. However, unlike the WT protein, Tpl2 T290D is not degraded, suggesting that phosphorylation at this site is required for Tpl2 degradation. The rate of p105 degradation in cells expressing Tpl2 T290D appears to be faster than that in cells expressing Tpl2 T290A, whereas the rate of p105 degradation in cells expressing WT Tpl2 appears to be intermediate. This result was surprising because, although Tpl2 is stoichiometrically bound to p105, only a fraction of p105 is bound to Tpl2. This finding provides support to the hypothesis that p105 is not only a regulator but also a target of Tpl2 (10). Thus, whereas p105 regulates Tpl2 stability and catalytic activity, Tpl2 may regulate p105 degradation in response to external signals. However, further studies will be needed to confirm this hypothesis.
Translation of the Tpl2 mRNA can be in initiated from one of three potential ATGs at its 5′ end. The first and second ATG are three codons apart. Initiation at either of the two gives rise to the p58 isoform of Tpl2. Translational initiation at the third ATG, which is located 90 nucleotides from the first ATG, gives rise to the p52 isoform (4). Based on this information, we generated mutants that express the two isoforms one at a time. By using these mutants, we confirmed that the p58 isoform is preferentially phosphorylated at Thr-290. To our surprise however, the p58 isoform was phosphorylated very poorly when expressed in the absence of the p52 isoform. Data presented in Fig. 1 show that p52 and p58 form heterodimeric complexes and that p52 up-regulates the expression and phosphorylation of p58. The apparent increase in p58 phosphorylation may be due to the higher levels of p58 in cells expressing both isoforms. In addition however, the p58/p52 complex appears to be a better phosphorylation target than p58 for the kinase that phosphorylates Tpl2 at Thr-290. These findings provide a teleological justification for the concerted translation of Tpl2 from the three alternative ATGs.
In summary, the data presented in this work show that LPS signals promote the phosphorylation of Tpl2 at Thr-290 and that phosphorylation at this site promotes the release from p105, the enzymatic activation, and the degradation of the Tpl2 kinase in response to LPS.
Supplementary Material
Acknowledgments
We thank K. Du, L. Feig, N. Rosenberg, A. Roy, and C. D. Dumitru for helpful discussions. We also thank A. DeFranco, A. Eliopoulos, G. Nolan, and W. Pear for reagents and the Center for Gastroenterology Research on Absorptive and Secretory Processes (Tufts–New England Medical Center) for providing core services to this project. Finally, we thank R. Pfau and G. P. Solidakis for technical assistance. This work was supported in part by National Institutes of Health Grant CA R01 38047. J.C. is a Ph.D. student in the Genetics program of the Sackler School of Biomedical Sciences, Tufts University School of Medicine.
Author contributions: P.N.T. and J.C. designed research; J.C. performed research; J.C. contributed new reagents/analytic tools; P.N.T. and J.C. analyzed data; and J.C. and P.N.T. wrote the paper.
Abbreviations: ERK, extracellular signal-regulated kinase; HA, hemagglutinin; LPS, lipopolysaccharide.
References
- 1.Patriotis, C., Makris, A., Bear, S. E. & Tsichlis, P. N. (1993) Proc. Natl. Acad. Sci. USA 90, 2251-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erny, K. M., Peli, J., Lambert, J. F., Muller, V. & Diggelmann, H. (1996) Oncogene 13, 2015-2020. [PubMed] [Google Scholar]
- 3.Ceci, J. D., Patriotis, C. P., Tsatsanis, C., Makris, A. M., Kovatch, R., Swing, D. A., Jenkins, N. A., Tsichlis, P. N. & Copeland, N. G. (1997) Genes Dev. 11, 688-700. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, M., Hamada, F., Sugimoto, T., Sumida, S., Akiyama, T. & Toyoshima, K. (1993) J. Biol. Chem. 268, 22723-22732. [PubMed] [Google Scholar]
- 5.Patriotis, C., Makris, A., Chernoff, J. & Tsichlis, P. N. (1994) Proc. Natl. Acad. Sci. USA 91, 9755-9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmeron, A., Ahmad, T. B., Carlile, G. W., Pappin, D., Narsimhan, R. P. & Ley, S. C. (1996) EMBO J. 15, 817-826. [PMC free article] [PubMed] [Google Scholar]
- 7.Chiariello, M., Marinissen, M. J. & Gutkind, J. S. (2000) Mol. Cell. Biol. 20, 1747-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsatsanis, C., Patriotis, C. & Tsichlis, P. N. (1998) Oncogene 17, 2609-2618. [DOI] [PubMed] [Google Scholar]
- 9.Tsatsanis, C., Patriotis, C., Bear, S. E. & Tsichlis, P. N. (1998) Proc. Natl. Acad. Sci. USA 95, 3827-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belich, M. P., Salmeron, A., Johnston, L. H. & Ley, S. C. (1999) Nature 397, 363-368. [DOI] [PubMed] [Google Scholar]
- 11.Lin, X., Cunningham, E. T., Jr., Mu, Y., Geleziunas, R. & Greene, W. C. (1999) Immunity 10, 271-280. [DOI] [PubMed] [Google Scholar]
- 12.Velasco-Sampayo, A. & Alemany, S. (2001) J. Immunol. 166, 6084-6090. [DOI] [PubMed] [Google Scholar]
- 13.Dumitru, C. D., Ceci, J. D., Tsatsanis, C., Kontoyiannis, D., Stamatakis, K., Lin, J. H., Patriotis, C., Jenkins, N. A., Copeland, N. G., Kollias, G. & Tsichlis, P. N. (2000) Cell 103, 1071-1083. [DOI] [PubMed] [Google Scholar]
- 14.Kontoyiannis, D., Boulougouris, G., Manoloukos, M., Armaka, M., Apostolaki, M., Pizarro, T., Kotlyarov, A., Forster, I., Flavell, R., Gaestel, M., et al. (2002) J. Exp. Med. 196, 1563-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliopoulos, A. G., Dumitru, C. D., Wang, C. C., Cho, J. & Tsichlis, P. N. (2002) EMBO J. 21, 4831-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliopoulos, A. G., Wang, C. C., Dumitru, C. D. & Tsichlis, P. N. (2003) EMBO J. 22, 3855-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luciano, B. S., Hsu, S., Channavajhala, P. L., Lin, L. L. & Cuozzo, J. W. (2004) J. Biol. Chem. 279, 52117-52123. [DOI] [PubMed] [Google Scholar]
- 18.Waterfield, M. R., Zhang, M., Norman, L. P. & Sun, S. C. (2003) Mol. Cell 11, 685-694. [DOI] [PubMed] [Google Scholar]
- 19.Gandara, M. L., Lopez, P., Hernando, R., Castano, J. G. & Alemany, S. (2003) Mol. Cell. Biol. 23, 7377-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterfield, M., Jin, W., Reiley, W., Zhang, M. & Sun, S. C. (2004) Mol. Cell. Biol. 24, 6040-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beinke, S., Robinson, M. J., Hugunin, M. & Ley, S. C. (2004) Mol. Cell. Biol. 24, 9658-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beinke, S., Deka, J., Lang, V., Belich, M. P., Walker, P. A., Howell, S., Smerdon, S. J., Gamblin, S. J. & Ley, S. C. (2003) Mol. Cell. Biol. 23, 4739-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donald, R., Ballard, D. W. & Hawiger, J. (1995) J. Biol. Chem. 270, 9-12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.