Abstract
Assembly of Ig genes in B lineage cells involves two distinct DNA rearrangements. In early B cell development, site-specific double strand breaks (DSBs) at germ-line V, D, and J gene segments are joined via nonhomologous end-joining (NHEJ) to form variable region exons. Activated mature B cells can change expressed Ig heavy chain constant region exons by class switch recombination (CSR), which also involves DSB intermediates. Absence of any known NHEJ factor severely impairs joining of cleaved V, D, and J segments. In NHEJ, DNA-dependent protein kinase (DNA-PK), which is comprised of the Ku70/80 end-binding heterodimer and the catalytic subunit (DNA-PKcs), activates Artemis to generate a nuclease that processes DSBs before ligation. Because inactivation of DNA-PKcs components also severely affects CSR, we tested whether DNA-PK also functions in CSR via activation of Artemis. To obviate the requirement for V(D)J recombination, we generated DNA-PKcs- and Artemis-deficient B cells that harbored preassembled Ig heavy chain and κ-light chain “knock-in” (HL) alleles. We found that Artemis-deficient HL B cells undergo robust CSR, indicating that DNA-PKcs functions in CSR via an Artemis-independent mechanism. To further elucidate potential Artemis-independent functions of DNA-PKcs, we asked whether the embryonic lethality associated with double-deficiency for DNA-PKcs and the related ataxia-telangiectasia-mutated (ATM) kinase was also observed in mice doubly deficient for ATM and Artemis. We found that ATM/Artemis double-deficient mice were viable and born in normal Mendelian numbers. Therefore, we conclude that DNA-PKcs has Artemis-independent functions in CSR and normal development.
Keywords: ATM, DNA-PKcs
Precursor B and T cells assemble antigen receptor genes from component, germ-line V, D, and J segments via V(D)J recombination (1). V(D)J recombination is initiated by the lymphoid-specific RAG1/2 endonuclease (RAG), which introduces DNA double strand breaks (DSBs) between V(D)J coding sequences and flanking recombination signal (RS) sequences. RAG cleavage generates two distinct intermediates: hairpin coding ends and blunt RS ends, which are then fused via the nonhomologous end-joining (NHEJ) pathway of DSB repair (2). There are six known mammalian NHEJ factors (2). XRCC4 and DNA-ligase IV function as a ligation complex, whereas Ku70 and Ku80 form the Ku DNA end-binding heterodimer, which associates with DNA-dependent protein kinase catalytic subunit (DNA-PKcs) to form the DNA-dependent protein kinase (DNA-PK). DNA-PK, in turn, associates with Artemis and activates its endonuclease activity (3). XRCC4, ligase IV, Ku70, and Ku80 are evolutionarily conserved and are required to repair all DSBs joined by NHEJ, including coding and RS ends. In contrast, DNA-PKcs and Artemis are relatively dispensable for RS joining but required for coding joining. In this context, the DNA-PKcs-activated Artemis endonuclease activity is required for processing V(D)J coding hairpins and also appears to function similarly in end-processing in general DNA DSB repair (4).
Ig heavy chain (IgH) class switch recombination (CSR) allows mature IgM+ B cells to change the class (isotype) and effector function of their IgH through replacement of the Cμ constant region with one of several downstream IgH constant region (CH) exons (5, 6). CSR employs a recombination/deletion event that links two participating switch (S) regions, which are repetitive sequences that lie 5′ of individual sets of CH exons. Like V(D)J recombination, CSR appears to proceed through DSB intermediates (reviewed in ref. 5). CSR requires activation-induced cytidine deaminase (AID) (7, 8), which deaminates cytidines to uridines within S regions in a transcription-dependent fashion, which can lead to DSBs (reviewed in ref. 5). AID-induced S-region DSBs include staggered-end intermediates (9, 10), which might require end-processing activities for resolution (11).
NHEJ has been implicated in the joining phase of CSR based on lack of CSR in Ku-deficient mice in which the mature IgM+ B cell population was reconstituted with knock-in IgH and Ig light chain alleles with preassembled variable region exons [IgH and κ-light chain knock-in (HL)-reconstituted] (12–14). However, HL-reconstituted DNA-PKcs-null (DP-T/HL) B cells are defective for CSR to all IgH isotypes except IgG1, despite a lack of growth defects (15), whereas HL-reconstituted severe combined immunodeficiency (DNA-PKcs kinase-inactive) B cells also show CSR defects, albeit more modest ones (16, 17). Based on these findings, one might speculate that the DNA-PK holoenzyme plays a similar role in CSR as it does in V(D)J recombination. In this scenario, some, but not all, CSR DSBs might require the DNA-PKcs/Artemis processing activities, leading to less severe CSR defects in DNA-PKcs-deficient mice than in Ku-deficient mice. On the other hand, DNA-PKcs may have Artemis-independent functions in NHEJ, CSR, and other processes. In this regard, DNA-PKcs-deficiency has a modest impact on the fidelity of RS joins, whereas Artemis deficiency does not (18, 19). Although the nature of this DNA-PKcs RS joining function is unknown, it might reflect in vitro ability of DNA-PKcs to synapse or protect DNA ends (20–22).
DNA-PKcs belongs to a family of proteins known as PI3-kinase-like kinases, which include ataxia-telangiectasia-mutated (ATM) protein (23). ATM is activated rapidly upon generation of a DSB and then functions to activate downstream DSB response proteins that effect cell-cycle checkpoints and DNA repair (24). Several DSB response proteins that are ATM substrates, including H2AX and 53BP1, are required for CSR and may function at the level of S-region synapsis (13, 25–27). In this regard, ATM-deficient mice (29, 30) and humans (28) also have a defect in CSR, but these defects appear more modest than that of H2AX- or 53BP1-deficient mice, perhaps reflecting the redundancy of other PI3-kinase-like kinases in the DSB response (25, 29). Inactivation of ATM is synergistically lethal with DNA-PKcs-deficiency (31, 32) but not with DNA ligase IV deficiency (31), arguing that overlapping developmental functions of ATM and DNA-PKcs may lie outside of NHEJ per se.
To further define the role of DNA-PKcs in CSR and normal development, we now compare the requirement for Artemis versus DNA-PKcs in CSR in HL-reconstituted mice. To provide further insight into DNA-PKcs functions, we test whether Artemis also has synergistic function with ATM in normal development. Our findings demonstrate Artemis-independent DNA-PKcs functions in CSR and normal development.
Methods
Generation of ArtN/NHL, DP-T/HL, and ArtN//N/ATMy/y Mice. The ArtN/NHL and DP-T/HL mice were made by a three-way cross of mice containing a productively rearranged B1–8 VH(D)JH variable region exon inserted (“knocked-in”) into the endogenous JH locus (34), with mice containing a κ3–83 VκJκ variable region exon knocked-in to the Jκ region of the Igk locus (33), and with ArtN/N (18) or DP-T mice (33). The various genotypes were identified by Southern blotting and PCR analyses of tail-derived genomic DNA as described in refs. 34 and 35. ArtN/NHL and DP-T/HL mice were crossed on a 129SvEvTac background for at least six generations to allow comparison in the same genetic background. Analyses of the ArtN/NHL mice were performed on progeny derived from Artemis-deficient mothers to exclude transplacental transfer of lymphocytes or Ig. To elucidate potential synergistic effects of Artemis- and ATM-deficiency, we used the previously described mice harboring the ATMy knockout allele (36). For the breedings, either Art+/N/ATM+/Y or ArtN/NATM+/Y mice were intercrossed and progeny were analyzed for Artemis and ATM genotypes (see Table 1).
Table 1. Mice doubly-deficient for ATM and Artemis are viable.
| Genotype | ATM+/+ | ATM+/y | ATMy/y |
|---|---|---|---|
| Art+/+ | 8 (4) | 7 (5) | 2 (4) |
| Art+/N | 8 (9) | 22 (17) | 9 (9) |
| ArtN/N | 3 (8) | 13 (10) | 5 (8) |
The progeny yielded from Art+/N ATM+/y × Art+/N ATM+/y and ArtN/N ATM+/y × ArtN/N ATM+/y breedings. The numbers listed represent the actual number of pups observed, and the numbers in parentheses represent the numbers theoretically expected for Mendelian segregation of the various genotypes.
Splenic B Cell Cultures. Single-cell suspensions of spleen cells were cultured at 5 × 105 or 1 × 106 cells per ml in RPMI medium supplemented with 10% FCS and either 25 ng/ml LPS (to assay for switching to IgG2a, IgG3, and IgG2b) or anti-CD40 (Pharmingen) using 500 ng/ml antibody with 25 ng/ml mouse recombinant IL-4 (R & D Systems) (for CSR to IgG1 and IgE) as described in ref. 15. Cultured cells were maintained daily at a density of 1 × 106 per ml. Cells were sampled on various days for flow cytometry analyses, DNA was prepared, and culture supernatants were harvested for ELISA analysis as described in ref. 15.
Flow Cytometry Analysis. Single-cell suspensions from spleen were prepared according to standard methods from 6- to 10-week-old mice. Cells from day-4 cultures were washed twice in PBS/2% FCS and stained with various antibodies conjugated with fluorescein (IgM, IgG1, IgG2b, and Igκ), phycoerythrin (IgM, IgG3, and Igλ), or CyChrome (B220) (Pharmingen). The cells were analyzed by using a FACSCalibur (BD Biosciences), and data were interpreted by using flowjo software (Tree Star, Ashland, OR). The FACS profiles representing 10,000 events were gated for live, lymphoid cells as determined by forward scatter versus side scatter.
ELISA. ELISA to detect IgM, the various IgG isotypes, and IgA in serum and/or in culture supernatants was done as described in ref. 15. For all analyses, we used isotype-specific antibodies purchased from Southern Biotechnology Associates. Mice ranged in age from 6–12 weeks, and culture supernatants were assayed after 5 days of stimulation.
Nucleotide Sequence Analyses. Splenic B cells from homozygous ArtN/NHL mice were activated with anti-CD40 plus IL-4 for 4 days, and genomic DNA was prepared by using standard techniques. Sμ–Sγ1 junctions were PCR-amplified as described in ref. 37. At least two independent reactions were used to obtain reproducible products that hybridized with the Sγ1 probe. The products were cloned into the Topo-TA (Invitrogen), sequenced, and analyzed by using sequencher (Gene Codes, Ann Arbor, MI) software and the NCBI database.
Results
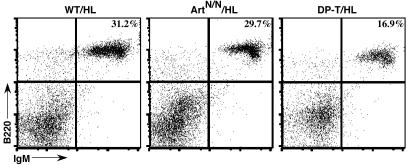
Knock-In IgH and Ig Light Chain Alleles Rescue B Cell Development in Artemis-Deficient Mice. To assay for potential roles of Artemis in CSR, we crossed ArtN/N mice with B1–8-HC (H) (34) and 3–83k-LC (L) (33) knock-in mice to generate ArtN/NHL mice on a 129S6/SvEvTac mouse strain background. The H and L alleles contain preassembled IgH and κ Ig light chain variable region gene exons, which obviate the need for V(D)J recombination to generate mature B lymphocytes. To exclude any transplacental transfer of maternal Ig or mature lymphocytes, mouse breeding strategies with ArtN/NHL mothers or ArtN/N mothers were used to generate all of the Artemis-deficient/HL mice for these studies. As expected, the knock-in H and L alleles did not rescue T cell development in ArtN/N mice, because we detected no substantial numbers of CD4+ or CD8+ cells in the spleens of six independent ArtN/NHL mice (data not shown). In contrast to ArtN/N mice, which lack mature B cells because of their V(D)J recombination defect (18), 5- to 8-week-old ArtN/NHL mice had substantial populations of B220+/IgM+ mature B cells in their spleens as detected by FACS analyses (Fig. 1). The absolute numbers of splenic B cells in ArtN/NHL mice approached those of Art+/+HL or Art+/NHL mice (WT/HL), with equivalent surface IgM expression on Art+/NHL and ArtN/NHL B cells (Fig. 1 and data not shown).
Fig. 1.
Reconstituted B cells in ArtN/NHL mice. Single-cell suspensions from 8-week-old ArtN/NHL, DP-T/HL, and WT/HL mice were stained for surface expression of B220 and IgM, and analyzed by FACS. Percentage of B220/IgM double-positive lymphocytes is depicted in the upper right corner of the FACS plot.
Normal CSR in ArtN/NHL B Cells. To determine whether ArtN/NHL B cells were capable of undergoing CSR in vivo, we used ELISA to measure the concentrations of the various IgH isotypes in sera from mice ranging in age from 6 to 8 weeks of age. All IgG isotypes and IgA were detected in serum isolated from ArtN/NHL mice, although we observed variability in the actual amount of antibody among individual mice (data not shown). Both the variability and the reduced concentration of serum IgH isotypes has been observed previously in other models in which Ig knock-in alleles were used to rescue V(D)J recombination defects (12, 16, 17, 38) and may reflect a lack of substantial numbers of peripheral T cells. In addition, serum levels of IgH isotypes are influenced by additional factors including mouse age and infection status (6). Therefore, to more directly look for potential CSR defects, we assayed purified B cells from the various lines for ability to undergo CSR in vitro.
To directly compare CSR in the context of Artemis-deficiency versus DNA-PKcs-deficiency, we backcrossed our original DP-T/HL mice (which were on a mixed C57B6/129/OLA background) onto the same 129S6/SvEvTac mice (DP-T/HL/129) as the ArtN/NHL mice. For in vitro studies, B220+/CD43- mature splenic B cells were purified from ArtN/NHL and DP-T/HL/129 mice, as well as from ArtN/+HL and DP+/T HL control mice, and stimulated in vitro either with LPS to stimulate CSR to IgG3 and IgG2b or anti-CD40 plus IL-4 to stimulate CSR to IgG1 and IgE. Trypan-blue-excluded live cells were counted on days 2, 3, and 4 of culture, with no significant differences in the total number of live cells observed for any of the three genotypes, either in LPS or anti-CD40 plus IL-4 conditions (LPS, P = 0.23; anti-CD40 plus IL-4, P = 0.18; for comparison of WT and ArtN/NHL). These findings indicate lack of a major proliferation defect in Artemis- and DNA-PKcs-deficient B cells after stimulation for CSR.
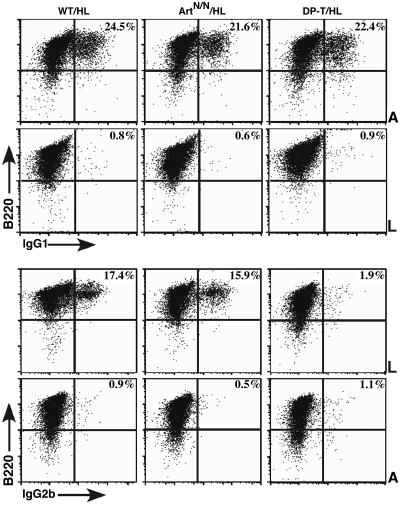
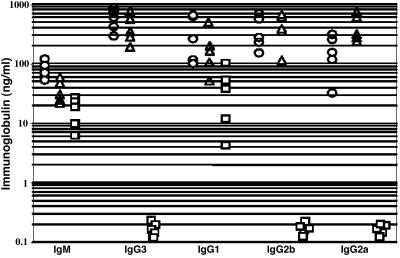
In-vitro-activated B cells were harvested on day 4 or 5 of culture and analyzed by FACS for surface IgH isotype expression. Robust switching was observed in ArtN/NHL and ArtN/+HL B cells, with no obvious difference from WT-HL with respect to the percentage of B cells that switched to IgG1, IgG3, or IgG2b (Fig. 2 and data not shown). Preliminary assays also indicated switching to IgE (data not shown). By comparison, DP-T/HL/129SvEv B cells switched only to IgG1 (Fig. 2 and data not shown), consistent with our previous studies of C57B6/129/OLA DP-T/HL mice (15). We also measured secreted IgH-isotype concentrations in supernatants from each splenic B cell culture. No substantial difference was observed in the amount of each IgH isotype produced by ArtN/NHL compared with WT/HL control cultures (Fig. 3). On the other hand, no IgH isotype except IgG1 was detected in supernatants from DP-T/HL/129 B cells cultures, consistent with our earlier findings of CSR in DP-T/HL mice on a mixed background (Fig. 3) (15). Together, these findings indicate that there is no substantial block for CSR in vitro in ArtN/NHL B cells, in contrast to the major defect of DP-T/HL B cells to switch to any IgH isotypes except IgG1.
Fig. 2.
IgH class switching to IgG1 or IgG2b is unaffected by Artemis-deficiency. Purified splenic B cells were activated in vitro for 4.5 days with anti-CD40 plus IL-4 (A) to assay switching to IgG1 (Upper) or with LPS (L) to assay switching to IgG2b (Lower). The percentage of IgG1- or IgG2b-positive cells that also stained for B220 is shown in the upper right corner of each FACS plot.
Fig. 3.
Normal secretion of different IgG isotypes in activated ArtN/N/HL B cells. Supernatants from cultures of stimulated splenic B cells from the indicated sources were analyzed by ELISA for secreted IgM and IgG isotypes at day 5 after treatment in vitro. Circles, WT/HL; triangles, ArtN/N/HL; squares, DP-T/HL.
Normal Processing of Sμ–Sγ1 Switch Junctions in IgG1+ ArtN/NHL B Cells. Analysis of the sequence composition of S-region junctions can provide insights into the mechanistic pathways used to resolve CSR-associated breaks. For example, CSR junctions from B cells deficient for the mismatch-repair factors Mlh1 or Pms2 have longer stretches of sequence microhomology (37, 39–41). To evaluate the sequence at CSR junctions formed in the absence of Artemis, DNA was isolated from day-4 cultures stimulated with anti-CD40 plus IL-4, and Sμ–Sγ1 junctions were amplified by PCR, cloned into a plasmid vector without size selection, and sequenced. Thirteen junctions were obtained from ArtN/NHL, and the resultant sequences were compared with germ-line sequences deposited in the NCBI public database, as well as those obtained from previously published studies (15). Most junctions were joined either directly (6/13) or with 1 or 2 nucleotide homology overlap (average overlap = 1.2 nt; Fig. 4, which is published as supporting information on the PNAS web site), similar to the junctions in normal mice (15). Moreover, there was no obvious alteration of nucleotide substitutions, mutation load, or general location of S-region junctions relative to the constant region genes (data not shown). These findings indicate that Artemis-deficiency does not alter substantially the nucleotide sequence pattern of S-region junctions.
Artemis and ATM Double-Deficient Mice Are Born in a Mendelian Fashion. The profound defect in CSR in DNA-PKcs-deficient B cells, as compared with Artemis-deficient B cells, indicates that DNA-PKcs has functions in CSR that are independent of Artemis. To further elucidate potential Artemis-independent functions of DNA-PKcs, we asked whether DNA-PKcs- and Artemis-deficiency were similar in the context of ATM-deficiency. For this purpose, we crossed ArtN/N mice to ATM-deficient mice (ATMy/y mice) (36). In contrast to the embryonic lethality associated with DNA-PKcs/ATM-deficiency, ArtN/N/ATMy/y mice were born alive, and (based on limited numbers of breedings) at close to the expected frequency (Table 1). Furthermore, the ArtN/N/ATMy/y mice appeared healthy, and some lived for up to 9 months with no gross phenotypic defects, other than immunodeficiency due to impaired NHEJ and smaller size associated with ATM deficiency (data not shown). These findings indicate that the synergistic lethality of the ATM- and DNA-PKcs-deficiencies does not involve Artemis-related functions of DNA-PKcs.
Discussion
IgH CSR has been assumed to involve DNA DSBs as intermediates and to employ the NHEJ pathway for their resolution. For NHEJ, in general, and V(D)J recombination, in particular, Artemis functions downstream of DNA-PK (4). Because all three DNA-PK components are required for normal CSR (5), it was reasonable to assume that Artemis may also play a major role in the generation and/or processing of CSR-associated DNA breaks via its DNA-PK-activated endonuclease and exonuclease activities (42, 43). Yet, we found that Artemis is not required for efficient CSR. Thus, if end-processing is required for CSR, the dispensability of Artemis for the process suggests that such activities may be provided by alternative factors or pathways (44, 45). On the other hand, Artemis is not required for the NHEJ-mediated ligation of DNA ends that do not require processing, such as blunt RS ends in V(D)J recombination (46) or noncomplex ends generated by etoposide (47). Therefore, a major role for NHEJ in CSR still could be rationalized if most S-region DSBs do not require processing. In this regard, our studies do not rule out some role for Artemis in CSR. For example, if the blunt DNA ends that constitute a subset of S-region DNA breaks (10) are efficiently joined, they might contribute to relatively normal cellular CSR levels. Finally, although classic NHEJ has been strongly implicated in CSR, as discussed in more detail below, it remains possible that other repair pathways function in CSR and that DNA-PK may have roles other than, or in addition to, potential roles in NHEJ.
Potential Roles of DNA-PKcs in CSR. In contrast to Artemis, all DNA-PK components (Ku70/80 and DNA-PKcs) are required for normal CSR. Ku70- and Ku80-deficient B cells fail to switch detectably to any IgH isotype (12, 14), whereas DP-T/HL B cells are defective in CSR to all but IgG1 (15). To be certain that the differential impairment of CSR observed for DNA-PKcs-deficient versus Artemis-deficient mice was not due to a strain-specific modifier, we backcrossed DP-T/HL mice into the same genetic background as the ArtN/NHL mice (129/SvEv). Our analyses of these mice confirmed that DNA-PKcs-deficiency still resulted in a severely impaired CSR to all IgH isotypes except IgG1. Therefore, we conclude that DNA-PKcs functions independently of Artemis in CSR. In this regard, DNA-PK might have an Artemis-independent NHEJ function in CSR and other processes, for example, by acting as scaffold for the XRCC4/ligase IV complex (20, 48–52). In addition, DNA-PK also may have functions outside of NHEJ in CSR (15, 16).
How might DNA-PK function outside of NHEJ in CSR? CSR relies on cellular DSB-response factors, such as H2AX and 53BP1, for its joining phase (13, 25). In this regard, DNA-PKcs, and related PI3-kinase-like kinases, initiate the DSB response by phosphorylating these factors (26, 53). Thus, a potential DNA-PKcs function in CSR, not directly related to NHEJ, may be in the activation of the DSB response. However, DNA-PKcs kinase activity is not absolutely required for CSR, as evidenced by CSR (at reduced levels) to all isotypes in mice homozygous for the scid mutation, which generates a kinase-null form of DNA-PKcs (16, 17, 54). One interpretation of the latter finding, when compared with the more dramatic effects of the complete DNA-PKcs knockout on CSR, is that DNA-PKcs may have a CSR function independent of kinase activity. In this regard, DNA-PKcs has been suggested to synapse DNA ends based on in vitro studies (20–22), although there is no direct in vivo evidence for such a role. However, indirect support for a DNA-PKcs role in the synapsis portion of the CSR reaction came from the finding that intra-Sμ-region deletions occur normally in DP-T/HL B cells (15). Finally, Ku and DNA-PKcs may have additional functions in regulation of apoptosis and telomere maintenance (55, 56). Thus, until potential CSR roles of XRCC4 or ligase IV, which are not known to have roles outside of NHEJ, are assessed, evidence that classical NHEJ is fully responsible for repair of DSBs during CSR remains indirect.
Differential Effects of Artemis-Deficiency Versus DNA-PKcs-Deficiency in the Context of ATM-Deficiency. DSBs activate cell-cycle checkpoints to prevent unrepaired lesions from being carried into the next phase of the cell cycle (23), and ATM is central to activation of the DSB response. Notably, inactivation of any of the DNA-PK holoenzyme components in mice results in synergistic lethality with ATM-deficiency (32). In contrast, we have found that mice with combined deficiency for Artemis and ATM are viable, with some living up to at least 9 months. In this regard, recent studies suggest that Artemis may, in fact, be epistatic to ATM in the IR-induced ATM signaling pathway (47, 57, 58). The viability of Artemis/ATM double-deficient mice suggests that synergistic function of DNA-PKcs with ATM in preventing embryonic lethality does not involve Artemis. Moreover, the finding that DNA-PKcs deficiency, but not Artemis-deficiency, synergizes with ATM-deficiency in generating embryonic lethality provides additional evidence that DNA-PKcs has functions separable from those of Artemis in processes other than CSR. Some of these synergistic functions may lie outside of NHEJ. Thus, ATM-deficiency actually rescues the neuronal apoptosis and embryonic lethality associated with ligase-IV-deficiency (32), indicating that it is not loss of NHEJ per se that leads to the synergistic lethality of ATM and DNA-PK deficiencies. The finding that this synergism does not appear to involve Artemis supports this notion. As speculated previously (32), synergistic developmental effects of ATM and DNA-PKcs may involve various functions, ranging from activation of the DSB response to potential roles in telomere maintenance. It will be of interest to determine whether such DNA-PKcs functions overlap with the role of DNA-PKcs in CSR.
Supplementary Material
Acknowledgments
We thank Y. Fujiwara, T. Borjeson, S. Whitlow, and L. Kaylor for technical assistance, K. Rajewsky for providing the B1–8 and κ3–83 knock-in mouse lines, and, especially, Drs. M. Lieber, A. Nussenzweig, and K. Rajewsky for critical review of the manuscript. This work was supported by National Institutes of Health Grants AI31541 and AI35714 (to F.W.A.). J.P.M. was supported by a Lymphoma Research Foundation Fellowship. J.S. was a Special Fellow of the Leukemia and Lymphoma Society. F.W.A. is an Investigator of Howard Hughes Medical Institute.
Author contributions: S.R., F.W.A., J.S., and J.P.M. designed research; S.R., J.S., and J.P.M. performed research; F.W.A., S.R., J.S., and J.P.M. analyzed data; and S.R., F.W.A., J.S., and J.P.M. wrote the paper.
Abbreviations: ATM, ataxia-telangiectasia-mutated; CSR, class switch recombination; DNA-PK, DNA-dependent protein kinase; DNA-PKcs, DNA-PK catalytic subunit; DP-T/HL, HL-reconstituted DNA-PKcs-null; DSB, double strand break; HL, IgH and κ-light chain knock-in; IgH, Ig heavy chain; NHEJ, nonhomologous end-joining; RS, recombination signal; S, switch.
References
- 1.Jung, D. & Alt, F. W. (2004) Cell 116, 299-311. [DOI] [PubMed] [Google Scholar]
- 2.Rooney, S., Chaudhuri, J. & Alt, F. W. (2004) Immunol. Rev. 200, 115-131. [DOI] [PubMed] [Google Scholar]
- 3.Ma, Y., Pannicke, U., Schwarz, K. & Lieber, M. R. (2002) Cell 108, 781-794. [DOI] [PubMed] [Google Scholar]
- 4.Jeggo, P. & O'Neill, P. (2002) DNA Repair (Amsterdam) 1, 771-777. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri, J. & Alt, F. W. (2004) Nat. Rev. Immunol. 4, 541-552. [DOI] [PubMed] [Google Scholar]
- 6.Manis, J. P., Tian, M. & Alt, F. W. (2002) Trends Immunol. 23, 31-39. [DOI] [PubMed] [Google Scholar]
- 7.Muramatsu, M., Kinoshita, K., Fagarasan, S., Yamada, S., Shinkai, Y. & Honjo, T. (2000) Cell 102, 553-563. [DOI] [PubMed] [Google Scholar]
- 8.Revy, P., Muto, T., Levy, Y., Geissmann, F., Plebani, A., Sanal, O., Catalan, N., Forveille, M., Dufourcq-Labelouse, R., Gennery, A., et al. (2000) Cell 102, 565-575. [DOI] [PubMed] [Google Scholar]
- 9.Chen, X., Kinoshita, K. & Honjo, T. (2001) Proc. Natl. Acad. Sci. USA 98, 13860-13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rush, J. S., Fugmann, S. D. & Schatz, D. G. (2004) Int. Immunol. 16, 549-557. [DOI] [PubMed] [Google Scholar]
- 11.Yu, K., Huang, F. T. & Lieber, M. R. (2004) J. Biol. Chem. 279, 6496-6500. [DOI] [PubMed] [Google Scholar]
- 12.Manis, J. P., Gu, Y., Lansford, R., Sonoda, E., Ferrini, R., Davidson, L., Rajewsky, K. & Alt, F. W. (1998) J. Exp. Med. 187, 2081-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reina-San-Martin, B., Difilippantonio, S., Hanitsch, L., Masilamani, R. F., Nussenzweig, A. & Nussenzweig, M. C. (2003) J. Exp. Med. 197, 1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casellas, R., Nussenzweig, A., Wuerffel, R., Pelanda, R., Reichlin, A., Suh, H., Qin, X. F., Besmer, E., Kenter, A., Rajewsky, K. & Nussenzweig, M. C. (1998) EMBO J. 17, 2404-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manis, J. P., Dudley, D., Kaylor, L. & Alt, F. W. (2002) Immunity 16, 607-617. [DOI] [PubMed] [Google Scholar]
- 16.Bosma, G. C., Kim, J., Urich, T., Fath, D. M., Cotticelli, M. G., Ruetsch, N. R., Radic, M. Z. & Bosma, M. J. (2002) J. Exp. Med. 196, 1483-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook, A. J., Oganesian, L., Harumal, P., Basten, A., Brink, R. & Jolly, C. J. (2003) J. Immunol. 171, 6556-6564. [DOI] [PubMed] [Google Scholar]
- 18.Rooney, S., Sekiguchi, J., Zhu, C., Cheng, H. L., Manis, J., Whitlow, S., DeVido, J., Foy, D., Chaudhuri, J., Lombard, D. & Alt, F. W. (2002) Mol. Cell 10, 1379-1390. [DOI] [PubMed] [Google Scholar]
- 19.Fukumura, R., Araki, R., Fujimori, A., Tsutsumi, Y., Kurimasa, A., Li, G. C., Chen, D. J., Tatsumi, K. & Abe, M. (2000) J. Immunol. 165, 3883-3889. [DOI] [PubMed] [Google Scholar]
- 20.DeFazio, L. G., Stansel, R. M., Griffith, J. D. & Chu, G. (2002) EMBO J. 21, 3192-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weterings, E., Verkaik, N. S., Bruggenwirth, H. T., Hoeijmakers, J. H. & van Gent, D. C. (2003) Nucleic Acids Res. 31, 7238-7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merkle, D., Douglas, P., Moorhead, G. B., Leonenko, Z., Yu, Y., Cramb, D., Bazett-Jones, D. P. & Lees-Miller, S. P. (2002) Biochemistry 41, 12706-12714. [DOI] [PubMed] [Google Scholar]
- 23.Shiloh, Y. (2003) Nat. Rev. Cancer 3, 155-168. [DOI] [PubMed] [Google Scholar]
- 24.Bassing, C. H. & Alt, F. W. (2004) Cell Cycle 3, e119-e123. [DOI] [PubMed] [Google Scholar]
- 25.Manis, J. P., Morales, J. C., Xia, Z., Kutok, J. L., Alt, F. W. & Carpenter, P. B. (2004) Nat. Immunol. 5, 481-487. [DOI] [PubMed] [Google Scholar]
- 26.Ward, I. M., Reina-San-Martin, B., Olaru, A., Minn, K., Tamada, K., Lau, J. S., Cascalho, M., Chen, L., Nussenzweig, A., Livak, F., et al. (2004) J. Cell Biol. 165, 459-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celeste, A., Petersen, S., Romanienko, P. J., Fernandez-Capetillo, O., Chen, H. T., Sedelnikova, O. A., Reina-San-Martin, B., Coppola, V., Meffre, E., Difilippantonio, M. J., et al. (2002) Science 296, 922-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan, Q., Petit-Frere, C., Lahdesmaki, A., Gregorek, H., Chrzanowska, K. H. & Hammarstrom, L. (2002) Eur. J. Immunol. 32, 1300-1308. [DOI] [PubMed] [Google Scholar]
- 29.Reina-San-Martin, B., Chen, H. T., Nussenzweig, A. & Nussenzweig, M. C. (2004) J. Exp. Med. 200, 1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumsden, J. M., McCarty, T., Petiniot, L. K., Shen, R., Barlow, C., Wynn, T. A., Morse, H. C., III, Gearhart, P. J., Wynshaw-Boris, A., Max, E. E. & Hodes, R. J. (2004) J. Exp. Med. 200, 1111-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurley, K. E. & Kemp, C. J. (2001) Curr. Biol. 11, 191-194. [DOI] [PubMed] [Google Scholar]
- 32.Sekiguchi, J., Ferguson, D. O., Chen, H. T., Yang, E. M., Earle, J., Frank, K., Whitlow, S., Gu, Y., Xu, Y., Nussenzweig, A. & Alt, F. W. (2001) Proc. Natl. Acad. Sci. USA 98, 3243-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao, Y., Chaudhuri, J., Zhu, C., Davidson, L., Weaver, D. T. & Alt, F. W. (1998) Immunity 9, 367-376. [DOI] [PubMed] [Google Scholar]
- 34.Pelanda, R., Schwers, S., Sonoda, E., Torres, R. M., Nemazee, D. & Rajewsky, K. (1997) Immunity 7, 765-775. [DOI] [PubMed] [Google Scholar]
- 35.Sonoda, E., Pewzner-Jung, Y., Schwers, S., Taki, S., Jung, S., Eilat, D. & Rajewsky, K. (1997) Immunity 6, 225-233. [DOI] [PubMed] [Google Scholar]
- 36.Borghesani, P. R., Alt, F. W., Bottaro, A., Davidson, L., Aksoy, S., Rathbun, G. A., Roberts, T. M., Swat, W., Segal, R. A. & Gu, Y. (2000) Proc. Natl. Acad. Sci. USA 97, 3336-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrenstein, M. R., Rada, C., Jones, A. M., Milstein, C. & Neuberger, M. S. (2001) Proc. Natl. Acad. Sci. USA 98, 14553-14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lansford, R., Manis, J. P., Sonoda, E., Rajewsky, K. & Alt, F. W. (1998) Int. Immunol. 10, 325-332. [DOI] [PubMed] [Google Scholar]
- 39.Ehrenstein, M. R. & Neuberger, M. S. (1999) EMBO J. 18, 3484-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrader, C. E., Edelmann, W., Kucherlapati, R. & Stavnezer, J. (1999) J. Exp. Med. 190, 323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrader, C. E., Vardo, J. & Stavnezer, J. (2002) J. Exp. Med. 195, 367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honjo, T., Kinoshita, K. & Muramatsu, M. (2002) Annu. Rev. Immunol. 20, 165-196. [DOI] [PubMed] [Google Scholar]
- 43.Yu, K. & Lieber, M. R. (2003) DNA Repair (Amsterdam) 2, 1163-1174. [DOI] [PubMed] [Google Scholar]
- 44.Kenter, A. L. & Tredup, J. (1991) Mol. Cell. Biol. 11, 4398-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faili, A., Aoufouchi, S., Weller, S., Vuillier, F., Stary, A., Sarasin, A., Reynaud, C. A. & Weill, J. C. (2004) J. Exp. Med. 199, 265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rooney, S., Alt, F. W., Lombard, D., Whitlow, S., Eckersdorff, M., Fleming, J., Fugmann, S., Ferguson, D. O., Schatz, D. G. & Sekiguchi, J. (2003) J. Exp. Med. 197, 553-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riballo, E., Kuhne, M., Rief, N., Doherty, A., Smith, G. C., Recio, M. J., Reis, C., Dahm, K., Fricke, A., Krempler, A., et al. (2004) Mol. Cell 16, 715-724. [DOI] [PubMed] [Google Scholar]
- 48.Hsu, H. L., Yannone, S. M. & Chen, D. J. (2002) DNA Repair (Amsterdam) 1, 225-235. [DOI] [PubMed] [Google Scholar]
- 49.Chen, L., Trujillo, K., Sung, P. & Tomkinson, A. E. (2000) J. Biol. Chem. 275, 26196-26205. [DOI] [PubMed] [Google Scholar]
- 50.Calsou, P., Delteil, C., Frit, P., Drouet, J. & Salles, B. (2003) J. Mol. Biol. 326, 93-103. [DOI] [PubMed] [Google Scholar]
- 51.Drouet, J., Delteil, C., Lefrancois, J., Concannon, P., Salles, B. & Calsou, P. (2004) J. Biol. Chem., in press. [DOI] [PubMed]
- 52.Izsvak, Z., Stuwe, E. E., Fiedler, D., Katzer, A., Jeggo, P. A. & Ivics, Z. (2004) Mol. Cell 13, 279-290. [DOI] [PubMed] [Google Scholar]
- 53.Stiff, T., O'Driscoll, M., Rief, N., Iwabuchi, K., Lobrich, M. & Jeggo, P. A. (2004) Cancer Res. 64, 2390-2396. [DOI] [PubMed] [Google Scholar]
- 54.Blunt, T., Gell, D., Fox, M., Taccioli, G. E., Lehmann, A. R., Jackson, S. P. & Jeggo, P. A. (1996) Proc. Natl. Acad. Sci. USA 93, 10285-10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith, J., Riballo, E., Kysela, B., Baldeyron, C., Manolis, K., Masson, C., Lieber, M. R., Papadopoulo, D. & Jeggo, P. (2003) Nucleic Acids Res. 31, 2157-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Downs, J. A. & Jackson, S. P. (2004) Nat. Rev. Mol. Cell Biol. 5, 367-378. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, X., Succi, J., Feng, Z., Prithivirajsingh, S., Story, M. D. & Legerski, R. J. (2004) Mol. Cell. Biol. 24, 9207-9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poinsignon, C., de Chasseval, R., Soubeyrand, S., Moshous, D., Fischer, A., Hache, R. J. & de Villartay, J. P. (2004) Eur. J. Immunol. 34, 3146-3155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.