Abstract
Background
Glycosylation of natural compounds increases the diversity of secondary metabolites. Glycosylation steps are implicated not only in plant growth and development, but also in plant defense responses. Although the activities of uridine-dependent glycosyltransferases (UGTs) have long been recognized, and genes encoding them in several higher plants have been identified, the specific functions of UGTs in planta remain largely unknown.
Methods
Spatial and temporal patterns of gene expression were analyzed by quantitative reverse transcription (qRT)-polymerase chain reaction (PCR) and GUS histochemical assay. In planta transformation in heterologous Arabidopsis was generated by floral dipping using Agrobacterium tumefaciens (C58C1). Protein localization was analyzed by confocal microscopy via fluorescent protein tagging.
Results
PgUGT72AL1 was highly expressed in the rhizome, upper root, and youngest leaf compared with the other organs. GUS staining of the promoter: GUS fusion revealed high expression in different organs, including axillary leaf branch. Overexpression of PgUGT72AL1 resulted in a fused organ in the axillary leaf branch.
Conclusion
PgUGT72AL1, which is phylogenetically close to PgUGT71A27, is involved in the production of ginsenoside compound K. Considering that compound K is not reported in raw ginseng material, further characterization of this gene may shed light on the biological function of ginsenosides in ginseng plant growth and development. The organ fusion phenotype could be caused by the defective growth of cells in the boundary region, commonly regulated by phytohormones such as auxins or brassinosteroids, and requires further analysis.
Keywords: abiotic stress, organ fusion, Panax ginseng, UDP-dependent glycosyltransferase
1. Introduction
To overcome their sessile lifestyle, plants produce a large amount of natural compounds that are not directly involved in plant growth and development but play crucial roles in plant defense responses to various adverse environmental conditions. These compounds contribute to plant–biosphere interactions and are referred to as plant secondary metabolites [1]. They exhibit considerable structural diversity, which is the result of various skeleton modification steps throughout their biosynthetic processes including oxidation/hydroxylation, substitution, and glycosylation. Glycosylation is considered to have a prominent role and is mainly involved in the final modification steps of secondary metabolite biosynthesis. These glycosylation processes are mediated by members of a multigene glycosyltransferase (GT) superfamily, which catalyze the transfer of single or multiple activated sugars to a wide range of substrates [2]. Although their activities have long been recognized, the specific function of GTs in planta remains largely unknown.
The system of GT classification is mainly based on amino acid sequence similarities, catalytic mechanisms, and the presence of a conserved sequence motif [3], [4]. In total, 101 GT families from different species have been classified (http://www.cazy.org/GlycosylTransferases.html). Most GTs belong to family 1 of the uridine diphosphate (UDP)-glycosyltransferases, which catalyze the transfer of a UDP-activated sugar to wide-ranging acceptors, and are therefore referred to as UDP-dependent GTs (UGTs) [5], [6]. Hundreds of different UGT genes exist in each plant genome, which is a reflection of the diversity of these secondary metabolites. More than 120 UGTs have been identified from Arabidopsis thaliana even though this species does not possess a large number of secondary metabolites [7].
Terpenoids represent a large group of natural secondary products with more than 40,000 different compounds identified [8]. Triterpenoid saponins from ginseng, called ginsenosides, may account for the well-known pharmacological efficacies of these compounds, including their antitumor, antistress, antiaging, and immune system-enhancing actions [9]. Thus, their utilization by humans has been attractive for many decades. Glycosylation plays an important role in the modification of ginsenoside skeletons leading to structural diversity [10] via the addition of monosaccharides to triterpene aglycones mainly at C-3 and/or C-20 for protopanaxadiol (PPD)-type ginsenosides, and at C-6 and/or C-20 for PPT-type ginsenosides [11]. Therefore, the biological activities of ginsenosides are regulated mainly by UGTs. The removal of sugar residues from saponins, terpene glycosides, often results in the loss of antifungal properties [12]. Although sequence analysis has revealed that numerous UGTs exist in ginseng, only a few have been functionally characterized [13], [14], [15], [16]. In the present study, molecular cloning and functional characterization of PgUGT72AL1, a UDP-dependent GT in ginseng, were performed.
2. Materials and methods
2.1. Plant materials and growth conditions
Korean ginseng (Panax ginseng Meyer) cv. Chun-Poong was used to analyze gene expression. Different ginseng organs (stem, leaf, root, petiole, and rhizome) were harvested from 2-year-old ginseng plants grown in soil at 25°C under a 16-h photoperiod. The Columbia ecotype (Col-0) of A. thaliana was used as a heterologous system for functional analysis. Seeds were sown on 1/2 MS medium (Duchefa Biochemie, Haarlem, The Netherlands) containing 1% sucrose, 0.5 g/L MES (2-[N-morpholino] ethanesulfonic acid), pH 5.7 adjusted with 1M KOH, and 0.8% phytoagar. Seeds that were cold-treated for 2 days were germinated under long-day conditions of 16 h light/8 h dark at 23°C.
2.2. DNA analysis
Polymerase chain reaction (PCR) products were checked by nucleotide sequencing. Amino acid sequences were analyzed to predict and identify conserved domains or active sites using online programs (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Multiple sequence alignment was performed using the BioEdit program (version 7.1.9, Copyright ©1997–2017 Tom Hall). A phylogenetic tree was generated by the neighbor-joining method using the MEGA6 (version 6.06, Copyright 1993–2013) program.
2.3. Abiotic stresses and hormone treatment
Three-week-old ginseng plantlets (cv. Chun-Poong) were stimulated with various abiotic treatments and two defense-modulating plant hormones. The plantlets were placed on a petri dish by dipping the root in 60 mL solution containing the following concentrations of chemicals; 5mM salicylic acid (SA), 0.2mM jasmonic acid (JA), 100μM abscisic acid (ABA), 10mM H2O2, and 100mM NaCl. Methanol, for JA, and distilled water, for SA, ABA, NaCl, and H2O2, were used as solvents. Chilling stress was induced by placing the roots in tap water at 4°C. Each treated plantlet sample, with the corresponding control sample, was collected at intervals of 1 h, 4 h, 8 h, 12 h, 24 h, and 48 h after treatment. The sampled materials were immediately frozen in liquid nitrogen and stored at –70°C.
2.4. RNA isolation and (quantitative) real-time reverse transcription-PCR
Total RNA was isolated using the RNeasy plant mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions, with modifications. Contaminating genomic DNA was removed by treatment with DNase I (Takara, Japan) in a total 100-μL reaction volume for 1 h just prior to the washing step. The concentration of RNA was determined using a NanoDrop 2000 UV–Vis spectrophotometer (Thermo, Wilmington, DE, USA). To synthesize first-strand cDNA, 3 μg of total RNA was reverse transcribed using RevertAid Reverse transcriptase (Thermo, USA). RT-PCR was performed in a 25-μL total reaction volume composed of 1–2 μL of cDNA, 20 pmol of each primer, and AmpONE Taq DNA polymerase (GeneAll, Seoul, Korea) using a T100 thermal cycler (Bio-Rad, Hercules, CA, USA) PCR machine under the following conditions: 95°C for 2 min, followed by 28 cycles of 95°C for 30 s, 60°C for 20 s, 72°C for 40 s, and extension at 72°C for 10 min. The RT-PCR products were visualized on 1.0% agarose gel. Quantitative PCR was carried out using the Thermal Cycle Dice real-time PCR system (Takara, Shiga, Japan) according to the manufacturer's instructions. The thermal cycler conditions recommended by the manufacturer were used as follows: initial denaturation at 95°C for 30 s followed by 45 cycles of 95°C for 5 s and 56°C for 10 s, and 72°C for 20 s in a 25-μL total reaction volume. At the end of the PCR, a dissociation curve was generated to evaluate the generation of by-products. To determine the absolute fold differences in expression for each sample, the threshold cycle (Ct) value of each sample was normalized to that of β-actin and calculated relative to a calibrator using the 2–△△Ct method. Three independent experiments were conducted. The gene-specific primers for PgUGT72AL1 were 5ʹ-ATG GAT ACC GAA AAG CTT-3′ (forward) and 5′-CAG ATC TTC CCA CGT GTT-3′ (reverse). Control primers for ginseng β-actin (DC03005B05) were 5′-AGA GAT TCC GCT GTC CAG AA-3′ (forward) and 5′-ATC AGC GAT ACC AGG GAA CA-3′ (reverse).
2.5. Vector construction and in planta transformation
To characterize the function of ginseng PgUGT72AL1, genomic sequences of the N-terminal half of PgUGT72AL1 (PgNΔUGT72AL1, 648 nucleotides) and the C-terminal half of PgUGT72AL1 (PgCΔ UGT72AL1, 627 nucleotides) were expressed under the control of the cauliflower mosaic virus (CaMV) 35S promoter. PgNΔUGT72AL1 genomic DNA was amplified using primers containing KpnI and AvrII sites (underlined) as follows: 5′-TC GGT ACC ATG GAT ACC GAA AAG CTT-3′ and 5′-GT CCT AGG CAG ATC TTC CCA CGT GTT-3′. PgCΔUGT72AL1 genomic DNA was amplified using primers containing SalI and AvrII sites (underlined) as follows: 5′-GA GTC GAC ATG TAT GTA TCG TTT GGG-3′ and 5′-GC CCT AGG AAT TAA TTT TTT TAA CCT CCT-3′. PgUGT72AL1 genomic DNA was amplified using primers containing ApaI and AvrII sites: 5′-TT GGG CCC ATG GAT ACC GAA AAG CTT-3′ and 5′-GC CCT AGG AAT TAA TTT TTT TAA CCT CCT-3′. Individual PCR products were subsequently cloned into cloning sites of pCAMBIA1300 vectors containing Pro35S and yellow fluorescent protein (YFP) fusion proteins. The promoter::GUS fusion construct was generated based on the obtained upstream intergenic region (952 bp) of PgUGT72AL1. The promoter region was amplified using primers containing PstI and SalI sites (underlined) as follows: 5′-TC CTG CAG CTG CAA CAC ATT TAA ATT-3′ (forward) and 5′-TC GTC GAC TGA GTG AAT AGA AAC TAT-3′ (reverse). The amplified PCR product was subsequently cloned into a pCAM1300 vector containing a gusA reporter gene. All transgene constructs were confirmed by nucleotide sequencing prior to transformation in planta. These constructs were transformed into Arabidopsis Col-0 using Agrobacterium tumefaciens C58C1 (pMP90) [17]. Transformants were selected on hygromycin-containing plates (50 μg/mL), and more than 15 T1 independent lines for each construct were used for further analyses. Homozygous transgenic lines carrying one copy of the insertion and following a Mendelian segregation ratio were further characterized.
2.6. GUS histochemical staining
GUS staining was performed by incubating samples in staining solution containing 1mM 5-bromo-4-chloro-3-indoyl-β-d-GlcA cyclohexylammonium salt (X-Gluc; Duchefa Biochemie, Haarlem, The Netherlands), 0.1M of NaH2PO4, 0.1% (w/v) Triton-X, and 0.5mM potassium ferricyanide and ferrocyanide, respectively, at 37°C until a blue color appeared (1–3 h). Stained samples were sequentially cleared in 70% (v/v) ethanol and then in 100% ethanol for 2 h. Samples were further cleared by incubation in 10% (v/v) glycerol/50% (v/v) ethanol and 30% (v/v) glycerol/30% (v/v) ethanol stepwise with a final dehydration step. Samples were photographed using a digital camera (Nikon D80; Nikon, Tokyo, Japan) and under a microscope (Leica EZ4HD, Ltd. Korea).
2.7. Confocal microscopy
The fluorescence from reporter proteins was viewed using an Olympus Fluoview 500 confocal laser scanning microscope (Olympus, Tokyo, Japan). YFP was detected using the 514/>530 nm excitation/emission filter set. Roots of 4-day-grown seedlings were used to observe the subcellular localization of proteins. Fluorescence images were digitized with the Olympus FV10-ASW 4.0 Viewer (Copyright OLYMPUS CORPORATION).
3. Results and discussion
3.1. Isolation and identification of ginseng PgUGT72AL1 genes
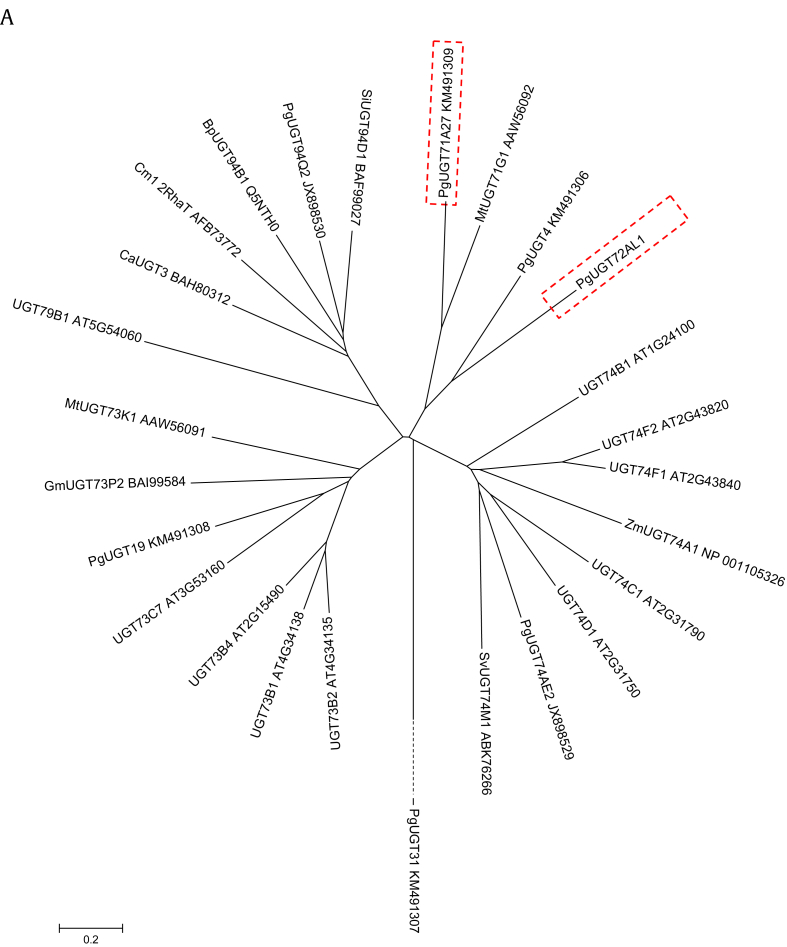
Total expressed sequence tags (ESTs) from previously constructed EST libraries, from 14-year-old ginseng and hairy roots [18], were used as a starting material to obtain full-length coding sequences. Consequently, putative genes encoding UGTs were identified [19]. Thereafter, full-length genomic DNA sequences of PgUGT72AL1 were obtained by a basic local alignment search tool (BLAST) search against the ginseng genome database constructed at Seoul National University, Korea. PgUGT72AL1 has a length of 1437 bp encoding 478 amino acids with no introns. Amino acid sequences of well-characterized UGTs in plants were collected from the National Center for Biotechnology Information bank database and used to generate the phylogenic tree (Fig. 1A). Among the identified ginseng PgUGTs, PgUGT72AL1 clustered into the same group as PgUGT71A27 (UGTPg1), which is reported to be involved in the production of ginsenoside compound K (C-K) [16]. Overall, the N-terminal part of the amino acid sequence was diverse among the selected UGTs, with the exception of the C-terminal plant secondary product GT (PSPG) box (Fig. 1B). The important active binding domain in plant UGTs, referred to as the PSPG box that is responsible for the binding activity of UGTs to donor sugar moieties nucleotide [5], [20], is well conserved in PgUGT72AL1 and in other characterized UGTs in plants (Fig. 1B). Therein, several crucial residues for catalytic activity [21], including cysteine and arginine/serine/asparagine, are depicted with opened triangles (Fig. 1B).
Fig. 1.
Phylogenetic tree and sequence alignment of PgUGT72AL1 with other characterized UGTs in plants. (A) The phylogenetic tree was constructed using the ClustalX program (neighbor-joining method). The GenBank accession numbers are: UGT74C1 (AT2G31790), UGT74D1 (AT2G31750), UGT74B1 (AT1G24100), UGT74F2 (AT2G43820), UGT74F1 (AT2G43840), UGT73C7 (AT3G53160), UGT73B4 (AT2G15490), UGT73B2 (AT4G34135), UGT73B1 (AT4G34138), UGT79B1 (AT5G54060), BpUGT94B1 (Q5NTH0), CaUGT3 (BAH80312), GmUGT73P2 (BAI99584), MtUGT71G1 (AAW56092), MtUGT73K1 (AAW56091), SvUGT74M1 (ABK76266), ZmUGT74A1 (NP_001105326), PgUGT4 (KM491306), PgUGT19 (KM491308), PgUGT31 (KM491307), PgUGT71A27 (KM491309), PgUGT74AE2 (JX898529), and PgUGT94Q2 (JX898530). The Genbank ID abbreviations are as follows; At, Arabidopsis thaliana; Bp, Bellis perennis; Ca, Catharanthus roseus; Gm, Glycine max; Mt, Medicago truncatula; Pg, Panax ginseng; Sv, Vaccaria hispanica; Zm, Zea mays. The bar represents 0.2 substitutions per amino acid position. (B) Sequence alignment of PgUGT72AL1 with other close homologs. The underlined domain represents the PSPG consensus motif. The triangles indicate important residues for catalytic activity and substrate binding. PSPG, plant secondary product glycosyltransferase; UGTs, uridine-dependent glycosyltransferases.
3.2. Organ-specific expression patterns of PgUGT72AL1
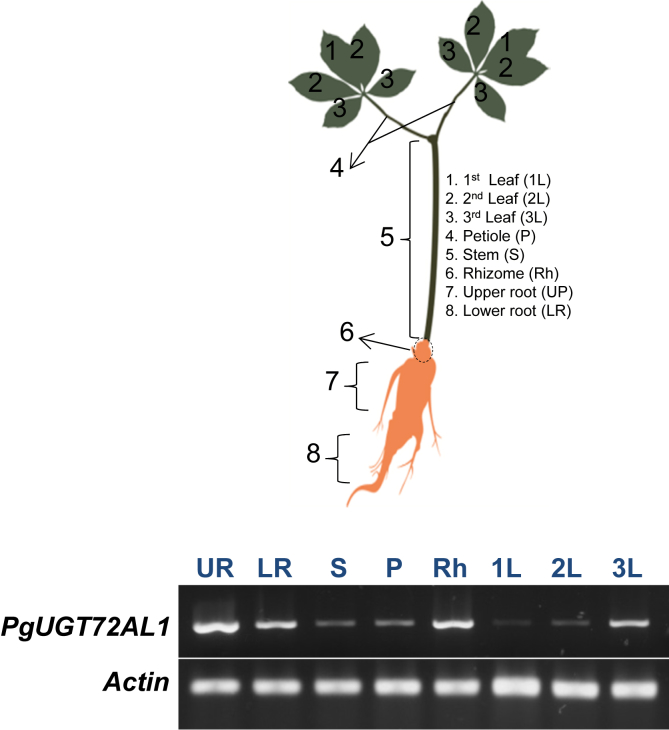
Ginseng plants display a distinct anatomical structure at different ages [11]. Ginseng plantlets show only three organs—the root, stem, and a compound leaf with three leaflets—until it reaches 1 year of age. From the 2nd year, compound leaves with five leaflets appear with the same number of petioles, which are connected to each compound leaf, with the number correlated to the year of cultivation [11]. In order to investigate the gene expression patterns of PgUGT72AL1, 2-year-old ginseng plants, which possess a full anatomical structure, were selected as the source material. In general, PgUGT72AL1 transcripts are ubiquitously expressed in all organs, with predominant expression in the roots and rhizomes rather than in the leaves (Fig. 2). However, notably, it is also highly expressed in youngest leaflets as compared with older leaflets.
Fig. 2.
Organ-specific expression patterns of PgUGT72AL1. Differential expression patterns of PgUGT72AL1 in ginseng were evaluated in 2-year-old plant by reverse transcription-polymerase chain reaction (RT-PCR) using cDNA from stem, leaf, root, rhizome, and petiole. The names for each organ are indicated on the right side of the plant diagram.
3.3. Differential transcript levels of PgUGT72AL1 in response to biotic and abiotic stresses
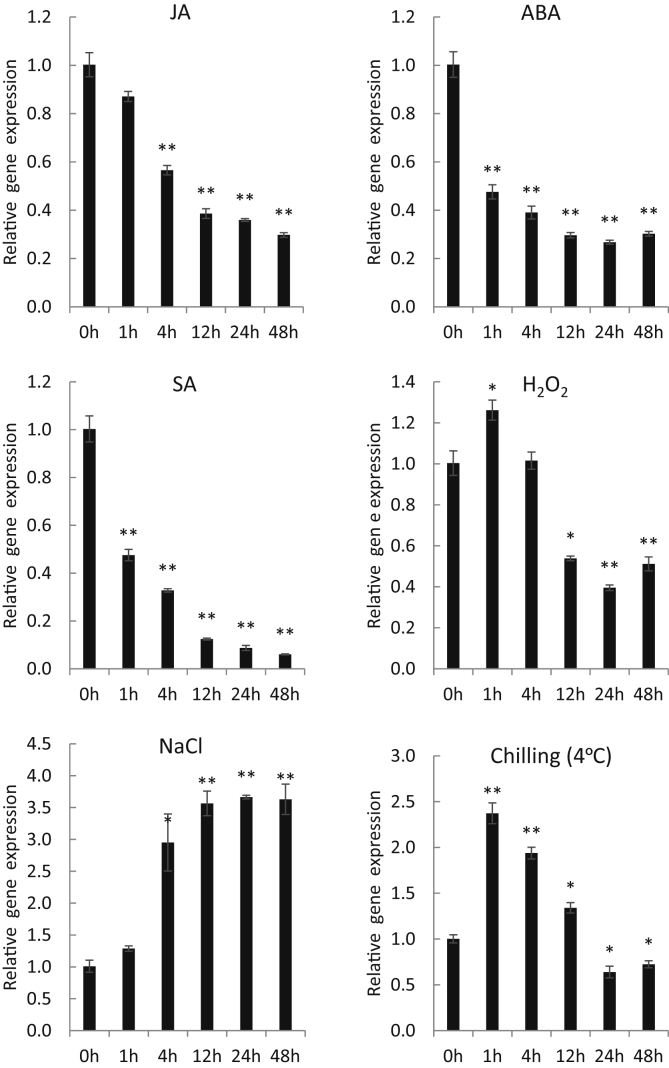
The involvement of UGTs in the modification of secondary metabolites is associated with differences in their expression levels in response to various external conditions. Several studies have documented the involvement of UGTs in plant immune systems [22], [23], [24] as well as phytohormone production [25], [26]. Thus, in order to provide basic knowledge regarding the function of PgUGT72AL1, changes in mRNA levels were analyzed during different exposure times to several abiotic stresses such as chilling stress (4°C), NaCl (100 mM), H2O2 (10 mM), and phytohormones such as ABA (100 µM), SA (5 mM), and JA (0.2 mM). SA and JA are key components of signal transduction pathways involved in plant defense and resistance. Following treatment with phytohormones, PgUGT72AL1 transcripts were gradually reduced to 30 and 25% by JA and ABA, respectively, and to 6% by SA after 48 h of treatment (HAT) (Fig. 3). In the case of H2O2 treatments, PgUGT72AL1 was initially up-regulated at 1 HAT and subsequently down-regulated from 12 to 48 HAT, in average 48%, compared to that observed at 0 HAT (Fig. 3). Cold treatment increased PgUGT72AL1 mRNA levels up to 2.4-fold at 1 HAT and maintained transcripts at higher levels up to 12 HAT, they were then down-regulated from 24 to 48 HAT (Fig. 3). Interestingly, NaCl treatment increased gene expression by 3-fold at 4 HAT and by an average 3.5-fold from 12 to 48 HAT (Fig. 3). These data suggest that PgUGT72AL1 may play a role in salt-tolerance.
Fig. 3.
Temporal expression patterns of the PgUGT72AL1 gene in response to abiotic stresses. Three-week-old ginseng plantlets were exposed to jasmonic acid (JA, 0.2 mM), abscisic acid (ABA, 100 μM), salicylic acid (SA, 5 mM), H2O2 (10 mM), NaCl (100 mM), and chilling conditions (4°C), for the time intervals indicated. Data represent the mean ± standard error (SE) for three independent replicates. Means for treated samples were significantly different from the control at *p < 0.05 and **p < 0.01.
3.4. Spatial and temporal expression of PgUGT72AL1 in heterologous Arabidopsis
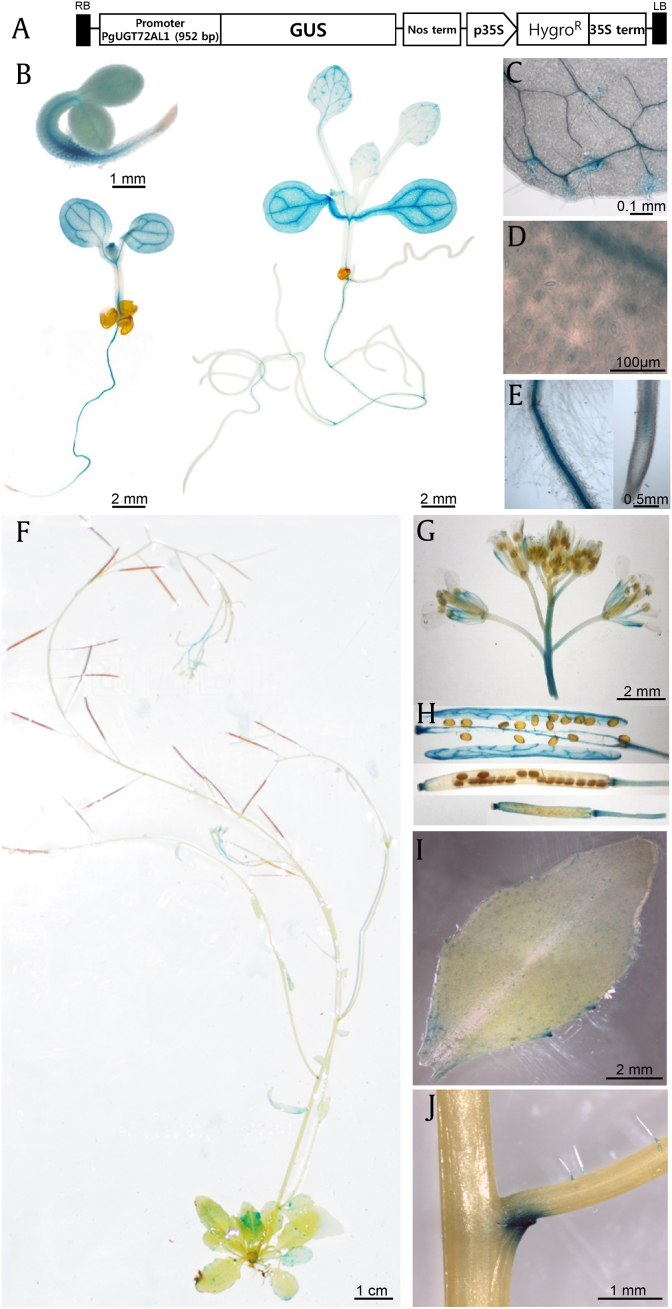
To further investigate the tissue- and cell-specific expression patterns of PgUGT72AL1, Arabidopsis was selected as the target host for further characterization. A vector harboring a ∼1-kb promoter region of PgUGT72AL1 transcriptionally fused with the gusA reporter gene (Fig. 4A) was then introduced into Arabidopsis via the A. tumefaciens-mediated floral dipping method. The T2 home line was histochemically analyzed for GUS activity. GUS staining was visible throughout the vasculature of the root and hypocotyls of 2-day-old seedlings, with the exception of the root tip (Fig. 4B). The expression pattern was maintained in 5-day-old seedlings and was enhanced in the cotyledon vasculature. In 12-day-old seedlings, in which the true leaf is emerged, the GUS signal in roots was weakened, and was restricted to the vasculature of cotyledons, and extended to the tip-side of leaf veins (Fig. 4B). GUS staining in trichomes and the inner membrane of stomata was also observed in 12-day-old seedlings (Figs. 4C and D). Overall, GUS staining was restricted to the vasculatures in the main roots except for the lateral roots and root hairs (Fig. 4E), and in leaf veins. In adult plants, a signal was detected in the youngest rosette leaves (Fig. 4F), sepals, stigmas, filaments in inflorescences (Fig. 4G), fully matured and young siliques (Fig. 4H), trichomes, and tips of cauline leaves (Fig. 4I). It is noteworthy that the GUS signal could also be detected in the axillary branch organs between the main stem and lateral stem, and in the tips of cauline leaves (Fig. 4J). Taken together, these GUS staining data suggested that PgUGT72AL1 is involved in the development of young tissue and specific axillary organs in adult plants.
Fig. 4.
Histochemical analysis of pPgUGT72AL1::GUS activity in Arabidopsis. (A) Schematic diagram of pPgUGT72AL1::GUS construct (B) GUS histochemical staining was observed from 2-, 5-, and 12-day-old seedlings, (C) trichomes, (D) stomata, (E) root vasculatures except root hairs, (F) matured 6-week-old plant, (G) inflorescences, (H) siliques, (I) mature cauline leaf, and (J) axillary branch organs in between the main stem and the lateral stem. Scale bars are provided in each image.
3.5. Over expression of PgUGT72AL1 in Arabidopsis resulted in axillary organ fusion
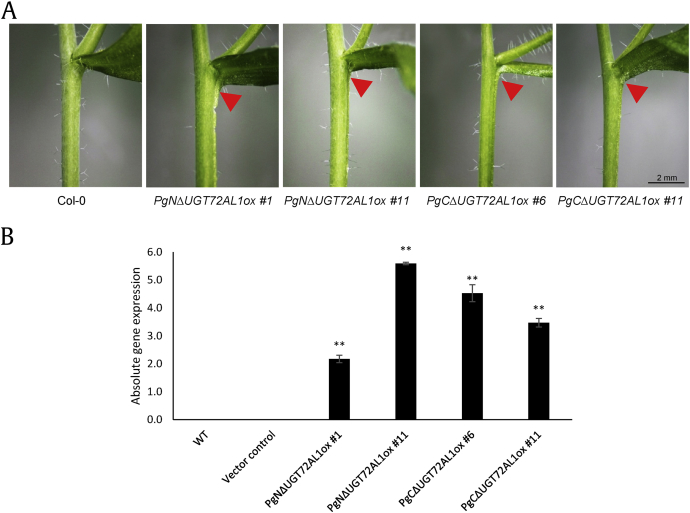
Plant UGTs share structural similarity with GT1 enzymes such as GtfA and GrfB, which are involved in vancomycin biosynthesis in Amycolatopsis orientalis, and OleD and OleI, which are involved in the glycosylation of macrolide antibiotics in Streptomyces. This indicates that there is structural similarity in the overall folding and in the core structures [21]. Generally, the C-terminal domains share higher similarity than the N-terminal domains; presumably, the C-terminal domains recognize the same or similar donors, whereas the N-terminal domains recognize different acceptors [21]. In order to determine the functional differences in plant growth and development, two halves of PgUGT72AL1 (PgNΔUGT72AL1 and PgCΔUGT72AL1) were introduced in planta via Agrobacterium-mediated transformation. Among 15 independent transgenic lines, at least two different lines, which were confirmed by quantitative PCR (Fig. 5A), were selected for phenotyping. No phenotypic defects were observed in plants at the young stage. However, as observed in the promoter::GUS expression patterns (Fig. 4I), an organ fusion phenotype was observed between the main stem and axillary cauline leaves (Fig. 5B). Organ fusion can occur as the result of an imbalance in hormones, such as brassinosteroid. Brassinosteroid hormone is well documented that plays important role in boundary organ formation [27]. It suggests that the possible involvement of PgUGT72AL1 in hormonal changes.
Fig. 5.
Overexpressing PgUGT72AL1 in Arabidopsis caused organ fusion in axillary leaves. (A) Overexpression of the N-terminal and C-terminal halves of PgUGT72AL1 resulted in organ fusion of the axillary branch in two 6-week-old transgenic lines, respectively. The scale bar represents 2 mm. (B) Overexpressed transcripts were confirmed by qPCR in two independent transgenic lines. Data represent the mean ± SE for three independent replicates. Averages for treated samples were significantly different compared to the control at *p < 0.05 and **p < 0.01. ABA, abscisic acid; JA, jasmonic acid; qPCR, quantitative polymerase chain reaction; SA, salicylic acid; SE, standard error.
3.6. Subcellular localization of PgUGT72AL1 in Arabidopsis
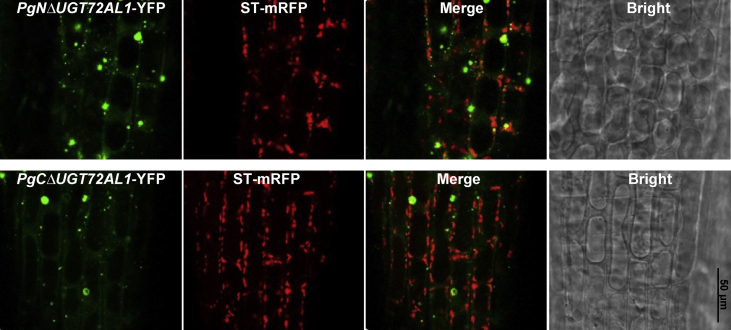
The subcellular location of two halves of PgUGT72AL1 was analyzed by generating C-terminal fusion with a fluorescent protein (YFP). Both the N-terminal and C-terminal halves of PgUGT72AL1 were localized to Golgi-like small vesicles (Fig. 6). To confirm whether these small vesicles are merged with Golgi, transgenic lines were crossed with the Golgi marker sialyl transferase (ST)-mRFP line [27]. Some of the fluorescent signals from PgNΔUGT72AL1-YFP were comerged with ST-GFP, indicating that the N-terminal domain of PgUGT72AL1 plays an important role in localization to the Golgi. Thus, the diverse N-terminal domain may possess a signal peptide that directs the protein to the correct subcellular location, and the C-terminal domain is involved in the recognition of appropriate donors.
Fig. 6.
Subcellular localization of PgNΔUGT72AL1-YFP and PgCΔUGT72AL1-YFP. The N-terminal half of PgUGT72AL1 is partially merged with the Golgi marker (ST-mRFP) and the C-terminal half of PgUGT72AL1 is localized in unidentified vesicles.
4. Conclusion
Glycosylation of low molecular weight compounds can alter the properties of aglycones by increasing their solubility and accumulation, and regulating their subcellular localization and bioactivity [28], [29] The family 1 GTs, UGTs, are the most common enzymes involved in catalyzing these processes in the plant kingdom. Glycosylation by UGTs is required in various biological processes during plant growth and development, especially in the modulation of secondary metabolites and in plant hormone homeostasis. To date, only a few UGTs from plants have been characterized in detail. Members of the UGT71 family in Arabidopsis have been shown to recognize a range of substrates such as benzoates, flavonoids, terpenoids [30], [31], [32], ABA [33], cytokinins [34], and brassinosteroid [35]. The PgUGT72AL1 gene sequence is not clustered with any of the previously characterized UGTs. However, it is clustered with PgUGT71A27 (same with UGTPg1) among existing UGTs (Fig. 1), the only one UGT characterized from ginseng which has been shown to be involved in C-K production by transferring a glucosyl moiety to free C-20S-OH of PPD [16]. PgUGT72AL1 shares 25% amino acid identity with UGTPg1, whereas 84% with UGTPg2 which was not proven to produce C-K [16]. This suggests that PgUGT72AL1 may also function in the production of C-K or C-K-like compounds. C-K has never been found in Panax plants [36]. Thus, further characterization may shed light on the biological functions of C-K in ginseng plant. But it also needs to be considered that it may function in other plant growth and developmental processes.
Considering the ubiquitous and enhanced gene expression of PgUGT72AL1 in roots and young leaves (Fig. 2), and spatially distributed GUS staining in Arabidopsis, especially in the junction region of the main stem and the axillary stem (Fig. 4), PgUGT72AL1 may function in plant growth and development. As expected, an organ fusion phenotype between the main stem and axillary cauline leaf was observed (Fig. 5). PgUGT72AL1 mRNA was downregulated in response to treatment with three plant hormones, such as JA, SA, and ABA (Fig. 3). This suggests that the tested plant hormones might be modulated by the function of PgUGT72AL1, which remains to be elucidated in the future.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This study was financially supported by a grant from the Next-Generation BioGreen 21 Program (SSAC, grant: PJ011129), Rural Development Administration, Republic of Korea.
References
- 1.Gershenzon J., Engelberth J. Secondary metabolites and plant defense. In: Taiz L., Zeiger E., editors. Plant physiology. 5th ed. Sinauer Associates; Sunderland, MA: 2010. pp. 369–400. [Google Scholar]
- 2.Lairson L.L., Henrissat B., Davies G.J., Withers S.G. Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–555. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 3.Campbell J.A., Davies G.J., Bulone V., Henrissat B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem J. 1997;326:929–939. doi: 10.1042/bj3260929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutinho P.M., Deleury E., Davies G.J., Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J Mol Biol. 2003;328:307–317. doi: 10.1016/s0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie P.I., Owens I.S., Burchell B., Bock K.W., Bairoch A., Bélanger A., Fournel-Gigleux S., Green M., Hum D.W., Iyanagi T. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics. 1997;7:255–269. doi: 10.1097/00008571-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Lim E.K., Bowles D.J. A class of plant glycosyltransferases involved in cellular homeostasis. EMBO J. 2004;23:2915–2922. doi: 10.1038/sj.emboj.7600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gachon C.M., Langlois-Meurinne M., Saindrenan P. Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends Plant Sci. 2005;10:542–549. doi: 10.1016/j.tplants.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Roberts S.C. Production and engineering of terpenoids in plant cell culture. Nat Chem Biol. 2007;3:387–395. doi: 10.1038/nchembio.2007.8. [DOI] [PubMed] [Google Scholar]
- 9.Wee J.J., Park K.M., Chung A.S. Biological activities of ginseng and its application to human health. In: Benzie I.F.F., Wachtel-Galor S., editors. Herbal medicine: biomolecular and clinical aspects. 2nd ed. CRC Press/Taylor & Francis; Boca Raton, FL: 2011. pp. 157–174. [Google Scholar]
- 10.Luo H., Sun C., Sun Y., Wu Q., Li Y., Song J., Niu J., Cheng X., Xu H., Li C. Analysis of the transcriptome of Panax notoginseng root uncovers putative triterpene saponin-biosynthetic genes and genetic markers. BMC Genomics. 2011;12:S5. doi: 10.1186/1471-2164-12-S5-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y.J., Lee O.R., Oh J., Jang M.G., Yang D.C. Functional analysis of 3-hydroxy-3-methylglutaryl coenzyme A reductase encoding genes in triterpene saponin-producing ginseng. Plant Physiol. 2014;165:373–387. doi: 10.1104/pp.113.222596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osbourn A.E. Saponins in cereals. Phytochemistry. 2003;62:1–4. doi: 10.1016/s0031-9422(02)00393-x. [DOI] [PubMed] [Google Scholar]
- 13.Yue C.J., Zhong J.J. Purification and characterization of UDPG: ginsenoside Rd glucosyltransferase from suspended cells of Panax notoginseng. Process Biochem. 2005;40:3742–3748. [Google Scholar]
- 14.He Y.P., Yue C.J. Establishment of measurement system of ginsenoside Rh2 glycosyltransferase activity. Med Plant. 2010;1:58–60. [Google Scholar]
- 15.Jung S.C., Kim W., Park S.C., Jeong J., Park M.K., Lim S., Lee Y., Im W.T., Lee J.H., Choi G. Two ginseng UDP glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014;55:2177–2188. doi: 10.1093/pcp/pcu147. [DOI] [PubMed] [Google Scholar]
- 16.Yan X., Fan Y., Wei W., Wang P., Liu Q., Wei Y., Zhang L., Zhao G., Yue J., Zhou Z. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res. 2014;24:770–773. doi: 10.1038/cr.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bechtold N., Pelletier G. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. In: Martinez-Zapater J.M., Salinas J., editors. Arabidopsis protocols. Humana Press; Totowa, NJ: 1998. pp. 259–266. [DOI] [PubMed] [Google Scholar]
- 18.Kim M.K., Lee B.S., In J.G., Sun H., Yoon J.H., Yang D.C. Comparative analysis of expressed sequence tags (ESTs) of ginseng leaf. Plant Cell Rep. 2006;25:599–606. doi: 10.1007/s00299-005-0095-0. [DOI] [PubMed] [Google Scholar]
- 19.Khorolragchaa A., Kim Y.J., Rahimi S., Sukweenadhi J., Jang M.G., Yang D.C. Grouping and characterization of putative glycosyltransferase genes from Panax ginseng Meyer. Gene. 2014;536:186–192. doi: 10.1016/j.gene.2013.07.077. [DOI] [PubMed] [Google Scholar]
- 20.Paquette S., Møller B.L., Bak S. On the origin of family 1 plant glycosyltransferases. Phytochemistry. 2003;62:399–413. doi: 10.1016/s0031-9422(02)00558-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang X. Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett. 2009;583:3303–3309. doi: 10.1016/j.febslet.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Langlois-Meurinne M., Gachon C.M., Saindrenan P. Pathogen-responsive expression of glycosyltransferase genes UGT73B3 and UGT73B5 is necessary for resistance to Pseudomonas syringae pv tomato in Arabidopsis. Plant Physiol. 2005;139:1890–1901. doi: 10.1104/pp.105.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J.T., Koo Y.J., Seo H.S., Kim M.C., Choi Y.D., Kim J.H. Overexpression of AtSGT1, an Arabidopsis salicylic acid glucosyltransferase, leads to increased susceptibility to Pseudomonas syringae. Phytochemistry. 2008;69:1128–1134. doi: 10.1016/j.phytochem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Clay N.K., Adio A.M., Denoux C., Jander G., Ausubel F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science. 2009;323:95–101. doi: 10.1126/science.1164627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grubb C.D., Zipp B.J., Ludwig-Müller J., Masuno M.N., Molinshi T.F., Abel S. Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J. 2004;40:893–908. doi: 10.1111/j.1365-313X.2004.02261.x. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K., Hayashi K., Natsume M., Kamiya Y., Sakakibara H., Kawaide H., Kasahara H. UGT74D1 catalyzes the glycosylation of 2-oxindole-3-acetic acid in the auxin metabolic pathway in Arabidopsis. Plant Cell Physiol. 2014;55:218–228. doi: 10.1093/pcp/pct173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee O.R., Kim S.J., Kim H.J., Hong J.K., Ryu S.B., Lee S.H., Ganguly A., Cho H.T. Phospholipase A2 is required for PIN-FORMED protein trafficking to the plasma membrane in the Arabidopsis root. Plant Cell. 2010;22:1812–1825. doi: 10.1105/tpc.110.074211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer C.M., Prata R.T., Willits M.G., De Luca V., Steffens J.C., Graser G. Cloning and regiospecificity studies of two flavonoid glucosyltransferases from Allium cepa. Phytochemistry. 2003;64:1069–1076. doi: 10.1016/s0031-9422(03)00507-7. [DOI] [PubMed] [Google Scholar]
- 29.Bowles D., Isayenkova J., Lim E.K., Poppenberger B. Glycosyltransferases: managers of small molecules. Curr Opin Plant Biol. 2005;8:254–263. doi: 10.1016/j.pbi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Lim E.K., Doucet C.J., Li Y., Elias L., Worrall D., Spencer S.P., Ross J., Bowles D.J. The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J Biol Chem. 2002;277:586–592. doi: 10.1074/jbc.M109287200. [DOI] [PubMed] [Google Scholar]
- 31.Lim E.K., Ashford D.A., Hou B., Jackson R.G., Bowles D.J. Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotechnol Bioeng. 2004;87:623–631. doi: 10.1002/bit.20154. [DOI] [PubMed] [Google Scholar]
- 32.Caputi L., Malnoy M., Goremykin V., Nikiforova S., Martens S. A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 2012;69:1030–1042. doi: 10.1111/j.1365-313X.2011.04853.x. [DOI] [PubMed] [Google Scholar]
- 33.Dong T., Xu Z.Y., Park Y., Kim D.H., Lee Y., Hwang I. Abscisic acid uridine diphosphate glucosyltransferases play a crucial role in abscisic acid homeostasis in Arabidopsis. Plant Physiol. 2014;165:277–289. doi: 10.1104/pp.114.239210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou B., Lim E.K., Higgins G.S., Bowles D.J. N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. J Biol Chem. 2004;279:47822–47832. doi: 10.1074/jbc.M409569200. [DOI] [PubMed] [Google Scholar]
- 35.Husar S., Berthiller F., Fujioka S., Rozhon W., Khan M., Kalaivanan F., Elias L., Higgins G.S., Li Y., Schuhmacher R. Overexpression of the UGT73C6 alters brassinosteroid glucoside formation in Arabidopsis thaliana. BMC Plant Biol. 2011;24:11–51. doi: 10.1186/1471-2229-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]