Abstract
T-wave alternans (TWA) is a potent arrhythmia substrate under the conditions of acute myocardial ischemia. Abnormal intracellular calcium cycling contributes to the genesis of cardiac alternans. Ryanodine receptor (RyR) is a pivotal Ca2+ cycling protein central to Ca2+ signaling in the heart. Here, we investigated the potential role of RyR in cardiac alternans and ventricular arrhythmias in acute myocardial ischemia. Transmembrane action potentials were simultaneously recorded from epicardium and endocardium together with a transmural ECG and isometric contraction force in the arterially perfused left ventricular wedge preparations. Calcium alternans were induced by incremental frequency of field stimulation in rat ventricular myocytes. TWA, mechanical alternans and ventricular arrhythmias were reproducibly induced by rapid pacing in the acute ischemic wedge preparations. Compared with control group, calcium alternans ratio and spontaneous calcium release were increased in acute ischemic myocytes. Verapamil, a phenylalkylamine calcium channel blocker, can successfully abolish spontaneous calcium release, TWA, and ventricular arrhythmias. The inhibition effect of verapamil could be diminished by low concentration of ryanodine (10 nmol/L). However, nifedipine, a dihydropyridine calcium channel blocker, could not block TWA or arrhythmias. Moreover, verapamil, but not nifedipine, significantly decreased ROS production in ischemic myocytes. Collectively, our results indicate that verapamil can significantly inhibit the development of cardiac alternans and ventricular arrhythmias in acute myocardial ischemia, and the mechanism was related to the inhibition of RyR and the protective function to oxidative stress.
Keywords: Verapamil, cardiac alternans, arrhythmias, myocardial ischemia, ryanodine receptor
Introduction
T wave alternans (TWA), defined as periodic beat-to-beat variation in the amplitude or shape of the T wave in an electrocardiogram, has long been observed in different settings including acute myocardial ischemia (myocardial infarction, unstable angina, coronary artery spasm) [1-3]. TWA has been found to precede the onset of life-threatening ventricular arrhythmias, and may serve as an important prognostic indicator of ventricular tachycardia and sudden cardiac death in acute myocardial ischemia [2-4].
TWA is the manifestation of beat-to-beat alternation in the action potential duration (APD) and intracellular calcium transient [5,6]. Alternation of intracellular calcium cycling between the plasma lemma and sarcoplasmic reticulum (SR) leads to corresponding changes in action potential morphology and alternated contraction force mechanistically via sarcolemmal calcium cycling ion channels [7,8]. Regulation of Ca2+ release from the SR via ryanodine receptor (RyR), has been recognized as a key factor in the genesis of electromechanical alternans [9,10].
Altered redox balance and redox-mediated changes in Ca2+ handling have been proved to be important pathogenic factors in different cardiac diseases, including ischemic heart disease [11-15]. Reactive oxygen species (ROS) could affect RyR due to its sensitivity to reactive oxygen [16-18]. Multiple clinical trials have found that verapamil has a positive influence on main end-point events in patients with myocardial infarction [19,20]. However, the potential role of redox-mediated alterations of Ca2+ signaling in the genesis of cardiac alternans and arrhythmias remains to be investigated. The present study investigated the role of RyR and ROS in cardiac alternans and ventricular arrhythmias in acute myocardial ischemia.
Materials and methods
Ethics statement
All experiments involving animals conforms to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Tongji Medical College.
Arterially perfused rabbit left ventricular wedge preparations
The methods used for isolation, perfusion, and recording of transmembrane activity from the arterially perfused rabbit ventricular wedge preparation were detailed in previous studies [21-24]. In brief, a transmural wedge preparation was dissected from rabbit left ventricle, and cannulated via the left circumflex branch of the coronary artery and perfused with cardioplegic solution. The cannulated preparation was then placed in a small heated tissue bath and arterially perfused with Tyrode’s solution containing 129 NaCl, 4 KCl, 0.9 NaH2PO4, 20 NaHCO3, 1.8 CaCl2, 0.5 MgSO4, and 5.5 glucose (in mmol/L), buffered with 95% O2 and 5% CO2 (temperature: 35.7 ± 0.2°C, perfusion pressure: 35-45 mmHg).
Transmembrane action potentials in the rabbit left ventricular wedge preparations were recorded simultaneously from epicardial and endocardial sites using 2 separate intracellular floating microelectrodes. A transmural ECG signal was recorded with a pair of silver/silver chloride electrodes positioned in the bath at 1.0 to 1.5 cm from the epicardial and endocardial surfaces of the preparation and along the same vector as the transmembrane ECG recordings. The isometric contraction force (ICF) was recorded by fixing one upper end of the wedge and connecting another opposite end with a silk wire to a force transducer. APD was measured at 90% repolarization (APD90). Transmural dispersion of repolarization (TDR) was defined as the difference between the endocardial and the epicardial repolarization times across the left ventricular wall.
Study protocol and grouping
The ventricular wedge preparations were allowed to equilibrate in the tissue bath for one hour (cycle length = 1000 ms) prior to electrical recordings. After baseline data acquisition, TWA was induced by rapid pacing (cycle length = 200 ms). Then preparations were divided into nine groups (n = 10 per group, Figure 1). Control group: wedge preparations were kept perfusion with Tyrode’s solution. Acute myocardial ischemia group: before rapid pacing, perfusion was cut down for 15 min to mimic acute ischemia. Drug pretreatment groups: prior to acute myocardial ischemia induction, wedge preparations were pretreated with Tyrode’s solution containing seven different drugs (1 μM verapamil, 1 μM nifedipine, 10 nM ryanodine, 3 μM ryanodine, 1 μM verapamil plus 10 nM ryanodine, 1 μM nifedipine plus 3 μM ryanodine, 750 µM mercaptopropionyl glycine).
Figure 1.
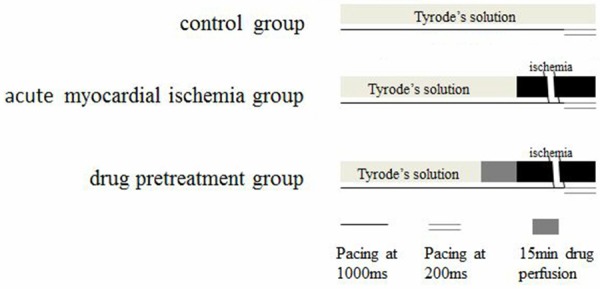
Study protocol and grouping of wedge preparations. Wedge preparations in all groups were allowed to equilibrate in the tissue bath for one hour (cycle length = 1000 ms) prior to electrical recordings. After baseline data acquisition, TWA was induced by rapid pacing (cycle length = 200 ms). Drug pretreatment groups including: verapamil (1 μM) group, nifedipine (1 μM) group, ryanodine in low level (10 nmol/L) or ryanodine-L group, ryanodine in high level (3 μM) or ryanodine-H group, verapamil-ryanodine-L mixed (verapamil 1 μM plus ryanodine 10 nmol/L) group, nifedipine-ryanodine-H (nifedipine 1 μM plus ryanodine 3 μmol/L) mixed group, MPG (750 µM) group. MPG: mercaptopropionyl glycine.
Recording of calcium transient in rat ventricular myocytes
The hearts were removed, mounted on a Langendorff apparatus and retrogradely perfused with 37°C Krebs-Ringer HEPES (KRH) buffer containing 125 NaCl, 5 KCl, 1.8 CaCl2, 11 glucose, 1.2 MgCl2, 0.5 NaH2PO4 and 25 NaHCO3 (in mmol/L, saturated with O2, pH = 7.4) for 3 min. And then, the hearts were perfused with KRH solution supplemented with collagenase Type 2 (Worthington Biochemical Corporation) and proteinase type XIV (Sigma) for 10-15 min. The free wall of the ventricle was removed and gently triturated with a large bore Pasteur pipette. Single myocytes were then collected and resuspended in normal solution supplemented with 0.1% bovine serum albumin (Sigma). Single myocytes were allowed to settle onto the coverslip at the bottom of the custom made perfusion chamber and then were loaded with 5 µmol/LFluo-4/AM (Molecular Probes) for 30 min at dark. Cells were then continuously perfused with normal KRH solution or ischemia injury solution for 20 min at 0.5 Hz.
For imaging, laser light from a xenon lamp was passed through monochromators to provide excitation (488 nm) into the fluorescent port of an inverted Leica microscope equipped with a 200 objective. The fluorescent light (520 nm) was passed through a filter located in the emission path into a CCD camera (Hamamatsu). Field stimulation was delivered between parallel silver electrodes at the edges of the perfusion chamber, at a voltage at least twice the stimulation threshold. Fluorescence intensity of myocytes was recorded at baseline and during increment field stimulation at 1-4 Hz. Between different stimulation frequency, there were 5 seconds’ interval for observation of spontaneous calcium release (SCR). Effects of verapamil (1 µM), nifedipine (1 µM) and ryanodine (3 µM) on calcium transient and SCR were studied.
Measurements of ROS production
Changes in ROS production were measured with the fluorescent indicator 5-(and-6) chloromethyl-20, 70-dichlorodihydrofluorosceindiacetate (DCFDA, 10 µM, Molecular Probes). After subtraction of background fluorescence, the signal (DF) was normalized to maximum fluorescence attained by application of 10 mM H2O2 (FMAX). Cells were incubated in KRH solution with verapamil (1.5 µM), nifedipine (1.5 µM), or mercaptopropionyl glycine (750 µM) for one hour. Myocytes were allowed to settle onto the coverslip at the bottom of the custom made perfusion chamber and continuously perfused with normal KRH solution or ischemia injury solution or drugs mentioned above for 20 min. The fluorescent intensity was measured every 4 seconds, after experiment the initial and ultimate value were compared.
Statistical analysis
Data are presented as mean ± SEM unless otherwise indicated. Statistical analysis of the data was performed using Student t-test for paired data or 1-way ANOVA coupled with Scheffé’s test as appropriate. Fisher’s exact test was used as appropriate for non-parametric data [25]. Significance was defined as a value of P-values < 0.05.
Results
TWA, APD alternans and TDR in acute myocardial ischemia wedge preparations
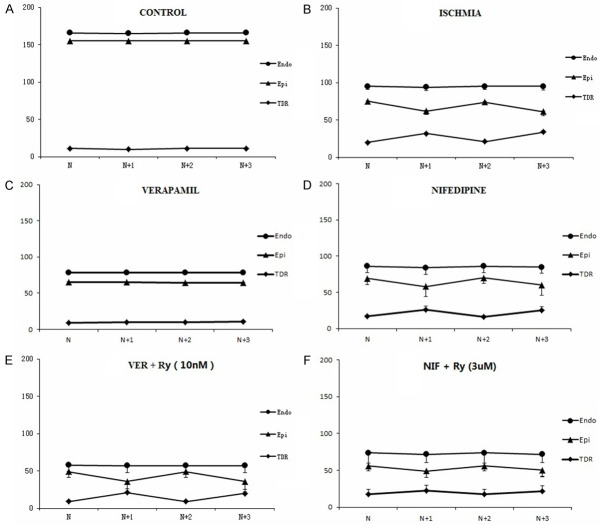
During rapid pacing (cycle length = 200 ms), there were no noticeable alternans of APD or T wave in control group (Figure 2). The alteration of APD in epicardium in every other two beats, together with the development of TWA in acute ischemic wedge preparations are shown in Figure 3. A more marked beat-to-beat change in epicardial APD than endocardial APD resulted in an alternated change in TDR (Figure 6B). Discordant T Wave and mechanical alternans (a beat with larger T wave was associated with weaker ICF) occurred in all the wedges (n = 10) in acute myocardial ischemia group. Ventricular arrhythmias were reproducibly induced in 5 wedges in acute myocardial ischemia group (Figure 4).
Figure 2.
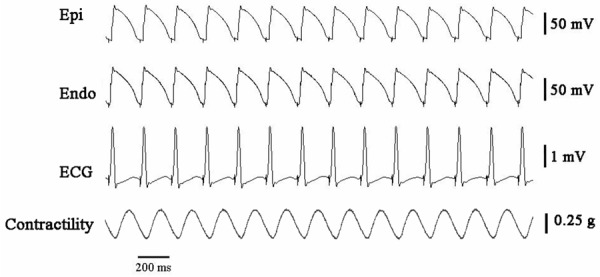
There were no obvious alterations of action potential duration in epicardium (Epi), endocardium (Endo), T waveor isometric contractile force (ICF) in a normal rabbit left ventricular wedge preparation paced at 5 Hz stimulation.
Figure 3.
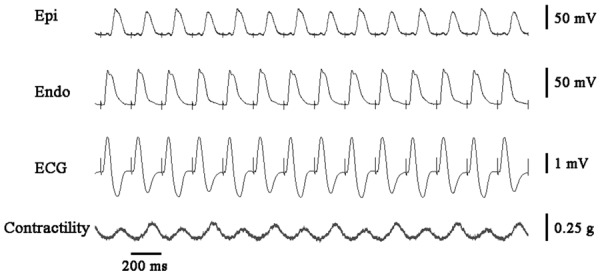
The alteration of action potential duration in epicardium in every other two beats, together with the development of discordant T Wave and mechanical alternans in acute ischemic wedge preparations. ICF: isometric contractile force; Epi: epicardium; Endo: endocardium.
Figure 6.
Beat-to-beat changes in action potential duration (APD) and transmural dispersion of repolarization (TDR) in wedge preparations at 5 Hz stimulation. A: There were no noticeable alternans of APD or TDR in control group. B: Beat-to-beat changes in APD were more marked in the epicardium (Epi) than the endocardium (Endo) in acute myocardial ischemia wedges. C: Verapamil at 1 μM abolished beat-to-beat changes in APD and TDR in acute myocardial ischemia wedges. D: Nifedipine (1 μM) could not block alternans of APD or TDR. E: Low concentration of ryanodine (10 nM) diminished the inhibition effect on alternans of APD and TDR by verapamil. F: High concentration of ryanodine (3 μM) enhanced the inhibition effect on alternans of APD and TDR by nifedipine. Nif: nifedipine; Ry: ryanodine; Ver: verapamil.
Figure 4.
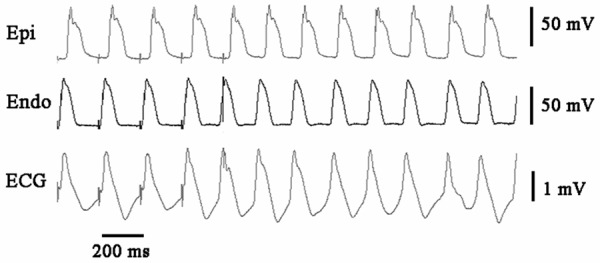
Ventricular arrhythmia developed after the cessation of rapid ventricular pacing by which TWA was induced steadily in acute ischemic wedge preparations. Epi: epicardium; Endo: endocardium.
Figure 7 summarized the occurrence of TWA and VT/VF in each group. Verapamil (1 μM), a non-dihydropyridine L-type calcium channel (LTCC) blocker, abolished electromechanical alternans (0/10 vs. 10/10, P < 0.001) and ventricular arrhythmias (0/10 vs. 5/10, P < 0.05, Figure 5). Meanwhile, the beat-to-beat alternation change in epicardial APD and TDR were suppressed by verapamil (Figure 6C). Nifedipine (1 μM), one type of dihydropyridine LTCC blocker, could not block electromechanical alternans and arrhythmias. Low concentration of ryanodine (10 nM), which activated RyR and increased calcium release from SR, diminished the antiarrhythmic effect of verapamil. High concentration of ryanodine (3 μM), which inhibited RyR and decreased calcium release from SR, enhanced the inhibition effect on electromechanical alternans by nifedipine. The anti-oxidant mercaptopropionyl glycine (MPG) could not suppress TWA or ventricular arrhythmias.
Figure 7.

The occurrence of T wave alternans (TWA) and ventricular tachycardia/fibrillation (VT/VF) in each group. Isc: ischemic group; MPG: mercaptopropionyl glycine; Nif: nifedipine; Ry: ryanodine; Ver: verapamil.
Figure 5.
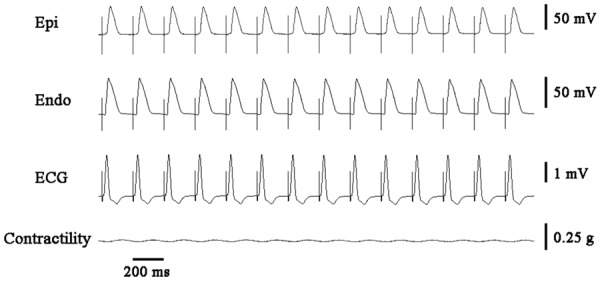
Verapamil (1 μM) inhibited the development of cardiac alternans and ventricular arrhythmias in acute ischemic wedge preparations. ICF: isometric contractile force; Epi: epicardium; Endo: endocardium.
Calcium alternans in acute ischemic myocytes
Calcium alternans ratio of each group was shown in Figure 8. Compared with control group, calcium alternans ratio and SCR increased significantly in the ischemia group. Verapamil (1 μM) significantly decreased calcium alternans ratio and SCR in acute ischemic myocytes. Neither nifedipine nor ryanodine decreased calcium alternans ratio and SCR.
Figure 8.

Calcium transient measurements in fluo-4 loaded myocytes during field stimulation. Calcium alternans and spontaneous calcium release (SCR) were elicited at stimulation at faster pacing rate in ischemia group. Verapamil significantly reduced calcium alternans ratio and SCR in acute ischemic myocytes. AR: alternans ratio; Isc: ischemic group; MPG: mercaptopropionyl glycine; Nif: nifedipine; Ver: verapamil.
Effect of verapamil on ROS production in acute ischemic myocytes
Compared with controls (2.9 ± 0.3 F/F 0, n = 22), ROS production increased approximately 1.2-fold in ischemic myocytes (3.5 ± 0.3 F/F o, n = 18, Figure 9). MPG (2.7 ± 0.5 F/F 0, n = 20) and verapamil (3.1 ± 0.5 F/F o, n = 28) significantly decreased ROS levels in ischemic myocytes.
Figure 9.
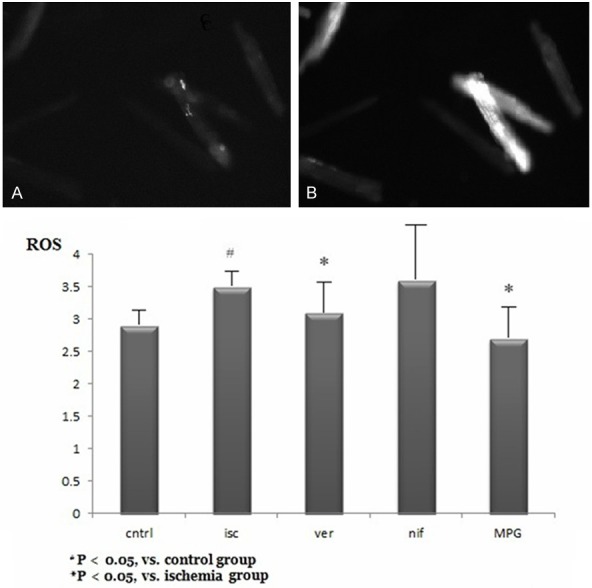
Reactive oxygen species (ROS) production in each group. A. ROS imaging of myocytes in control group. B. ROS imaging of myocytes in ischemia group. Isc: ischemic group; MPG: mercaptopropionyl glycine; Nif: nifedipine; Ver: verapamil.
Discussion
In the present study, using arterially-perfused rabbit left ventricular wedge, the ECG, APD and ICF were simultaneous recorded. The results of this study indicated that action potential alternans were more likely originating from epicardium than endocardium, accompanying with TWA, mechanical alternans, and ventricular arrhythmias.
In the clinical setting, the optimal detection of TWA requires a heart rate in the range 90-110 bpm, which may be achieved by exercise testing, pharmacological intervention, or rapid atrial pacing [3,23]. The present study showed that TWA and ventricular arrhythmias were reproducibly induced by rapid pacing (cycle length = 200 ms) in the acute ischemic wedge preparations. It is a reliable model to study the mechanism underlying TWA and ventricular arrhythmias in ischemic condition.
TWA, APD alternans and mechanical alternans in acute myocardial ischemia
Previous studies showed that action potential alternans generated TWA in cellular level [26,27]. Selvaraj reported that a greater number of epicardial sites exhibited alternans than endocardial sites at cycle length 600 ms in patients with cardiomyopathy [28]. In the present study, a significant beat-to-beat change in epicardial APD created beat-to-beat alteration in transmural voltage gradient that manifested as TWA on the ECG. A more noteworthy beat-to-beat change in epicardial APD than endocardial APD resulted in an alternated change in TDR (Figure 6B). Interestingly, contraction force alternated in an opposite phase (“out of phase”) with APD and T-wave. Larger epicardial APD and greater T wave was associated with smaller contraction force.
Electromechanical cardiac alternans has been closely associated with susceptibility to ventricular arrhythmias in acute myocardial ischemia. TWA has been shown to be an effective tool for identifying high-risk patients in the presence of myocardial ischemia. The risk of cardiac arrhythmia in TWA positive patients is close to 4 times than in TWA negative [29]. Repolarization alternans at the level of the single cell could trigger ventricular fibrillation [30]. Previous studies showed that TWA could lead to reentry and triggered activity, resulting in the genesis of ventricular fibrillation [31-33]. In the present study, 5 of 10 acute myocardial ischemia wedges developed ventricular tachycardia and/or fibrillation after TWA, which was consistent with clinical manifestations and preceded experiments.
Verapamil and RyR in intracellular calcium transient in acute myocardial ischemia
Recent study has shown that intracellular Ca2+ oscillation plays a critical role in the genesis of APD alternans and TWA [34]. Previous studies indicated that RyRs are critical in excitation contraction coupling [34,35]. Ryanodine activates the open probability of RyR at low concentration (~10 nM) while has inhibitory effects at high concentration (~10 μM) [36].
Our study proved that verapamil (1 μM), a phenylalkylamine ICa-L blocker, might have blocking effect on RyR and electromechanical alternans by improving discordant repolarization alternans and abnormal intracellular calcium homeostasis. Nifedipine (1 μM), a dihydropyridine calcium channel blocker, had no effect on RyR and electromechanical alternans. Inhibition of RyR (3 μM ryanodine) enhanced the antiarrhythmic effect of nifedipine. Moreover, enhance of RyR function (10 nM ryanodine) reduced the antiarrhythmic effect of verapamil. The nature of the beat-to-beat response of T wave and contraction may depend on the activity of the LTCC and SR function.
Intracellular calcium overload and spontaneous calcium wave of SR could induce arrhythmias in ischemic heart disease [37]. Previous clinical trials showed that verapamil had a positive influence on main end-point events in patients with myocardial infarction [19,20]. Verapamil has been reported to effectively suppress ventricular arrhythmias in rats subjected to myocardial ischemia [38]. Apart from L-type calcium channel blockade, it also occupies functions such as reducing sarcoplasmic reticulum calcium content [34], protecting energy metabolism, maintaining mitochondrion function [13], stabilizing connexin 43 at the gap junction and promoting electrophysiological property [38]. In vitro studies have suggested that verapamil may bind to RyR2 and inhibit its channel activity [39].
Oxidative stress, redox modification of RyR and cardiac alternans in acute myocardial ischemia
Cardiac alternans is highly dependent on SR Ca2+ regulatory proteins and their redox status [40-42]. Sulfhydryl oxidation of reactive cysteine molecules could increase RyR open time probability, thus producing ‘leaky’ RyR channels [43,44]. Previous studies showed that ROS production and oxidative stress increased in ischemic cardiac diseases [45-51]. Superoxide generation occurred during ischemia from the ubisemiquinone site of the mitochondrial electron transport chain [52-54]. The present study showed that ROS production increased approximately 1.2 fold in ischemic myocytes. We also found that verapamil could decrease ROS level and cardiac alternans. MPG, a powerful anti-oxidant with the ability to reduce ROS, could not suppress TWA or arrhythmias. These results addressed the significance of oxidative/anti-oxidative balance in ischemic cardiac disease.
Clinical implications
Acute myocardial ischemia and associated electromechanical instability predispose to malignant ventricular arrhythmias, leading to sudden cardiac death. Several interesting electrocardiographic and mechanical manifestations observed in the present study may enhance our understanding of arrhythmogenic markers in acute myocardial ischemia.
Verapamil inhibits cardiac alternans with multiple role including L-type calcium channel blockade, RyR inhibition, and anti-oxidative effects, thus making it a prospective drug for ischemic ventricular arrhythmia.
Study limitations
Although our present study and other previous studies have demonstrated that increased ICa-L can cause abnormal fluctuating of intracellular Ca2+ and cardiac alternans in acute myocardial ischemia. The exact linking mechanism of the beat-to-beat electromechanical abnormalities in acute myocardial ischemia is unclear. Remolding of ICa, IK and INa/Ca may also be involved in the beat-to-beat cardiac alternans.
Conclusions
In summary, our study shows that verapamil inhibits discordant cardiac alternans and ventricular arrhythmias via the suppression of RyR and ROS in acute myocardial ischemia. Our data suggests that SR function may contribute importantly to cardiac alternans and arrhythmias in acute myocardial ischemia.
Acknowledgements
This work is supported by the grant from the National Natural Science Foundation of China (81400255).
Disclosure of conflict of interest
None.
References
- 1.Yang L, Xie P, Wu J, Yu J, Yu T, Wang H, Wang J, Xia Z, Zheng H. Sevoflurane postconditioning improves myocardial mitochondrial respiratory function and reduces myocardial ischemia-reperfusion injury by up-regulating HIF-1. Am J Transl Res. 2016;8:4415–4424. [PMC free article] [PubMed] [Google Scholar]
- 2.Salerno-Uriarte JA, De Ferrari GM, Klersy C, Pedretti RF, Tritto M, Sallusti L, Libero L, Pettinati G, Molon G, Curnis A, Occhetta E, Morandi F, Ferrero P, Accardi F ALPHA Study Group Investigators. Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: results of the ALPHA Study. J Am Coll Cardiol. 2007;50:1896–1904. doi: 10.1016/j.jacc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner DV, Hohnloser SH, Ikeda T, Martinez JP, Narayan SM, Nieminen T, Rosenbaum DS. Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility--consensus guideline by international society for Holter and noninvasive electrocardiology. J Am Coll Cardiol. 2011;58:1309–1324. doi: 10.1016/j.jacc.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow T, Kereiakes DJ, Bartone C, Booth T, Schloss EJ, Waller T, Chung ES, Menon S, Nallamothu BK, Chan PS. Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2006;47:1820–1827. doi: 10.1016/j.jacc.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 5.Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am J Physiol Heart Circ Physiol. 2008;294:H1–H10. doi: 10.1152/ajpheart.00802.2007. [DOI] [PubMed] [Google Scholar]
- 6.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 7.Bayer JD, Narayan SM, Lalani GG, Trayanova NA. Rate-dependent action potential alternans in human heart failure implicates abnormal intracellular calcium handling. Heart Rhythm. 2010;7:1093–1101. doi: 10.1016/j.hrthm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kucerova D, Doka G, Kruzliak P, Turcekova K, Kmecova J, Brnoliakova Z, Kyselovic J, Kirchhefer U, Muller FU, Krenek P, Boknik P, Klimas J. Unbalanced upregulation of ryanodine receptor 2 plays a particular role in early development of daunorubicin cardiomyopathy. Am J Transl Res. 2015;7:1280–1294. [PMC free article] [PubMed] [Google Scholar]
- 9.Bers DM, Despa S. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J Pharmacol Sci. 2006;100:315–322. doi: 10.1254/jphs.cpj06001x. [DOI] [PubMed] [Google Scholar]
- 10.Laurita KR, Rosenbaum DS. Cellular mechanisms of arrhythmogenic cardiac alternans. Prog Biophys Mol Biol. 2008;97:332–347. doi: 10.1016/j.pbiomolbio.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail. 2007;9:219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Kajiyama K, Pauly DF, Hughes H, Yoon SB, Entman ML, McMillin-Wood JB. Protection by verapamil of mitochondrial glutathione equilibrium and phospholipid changes during reperfusion of ischemic canine myocardium. Circ Res. 1987;61:301–310. doi: 10.1161/01.res.61.2.301. [DOI] [PubMed] [Google Scholar]
- 14.Mostarda C, Rodrigues B, Medeiros A, Moreira ED, Moraes-Silva IC, Brum PC, Angelis KD, Irigoyen MC. Baroreflex deficiency induces additional impairment of vagal tone, diastolic function and calcium handling proteins after myocardial infarction. Am J Transl Res. 2014;6:320–328. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YI, Wei PC, Hsu JL, Su FY, Lee WH. NPGPx (GPx7): a novel oxidative stress sensor/transmitter with multiple roles in redox homeostasis. Am J Transl Res. 2016;8:1626–1640. [PMC free article] [PubMed] [Google Scholar]
- 16.Clusin WT. Calcium and cardiac arrhythmias: DADs, EADs, and alternans. Crit Rev Clin Lab Sci. 2003;40:337–375. doi: 10.1080/713609356. [DOI] [PubMed] [Google Scholar]
- 17.Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu WJ, Wang T, Wang B, Liu XT, He XW, Liu YJ, Li ZX, Tan R, Zeng HS. CYP2C8-derived epoxyeicosatrienoic acids decrease oxidative stress-induced endothelial apoptosis in development of atherosclerosis: role of Nrf2 activation. J Huazhong Univ Sci Technolog Med Sci. 2015;35:640–645. doi: 10.1007/s11596-015-1483-5. [DOI] [PubMed] [Google Scholar]
- 19.Frommeyer G, Eckardt L, Milberg P. Calcium handling and ventricular tachyarrhythmias. Wien Med Wochenschr. 2012;162:283–286. doi: 10.1007/s10354-012-0104-1. [DOI] [PubMed] [Google Scholar]
- 20.Nagra B, Liu Z, Mehta R, Hart D, Kantharia BK. Verapamil-sensitive left posterior fascicular ventricular tachycardia after myocardial infarction. J Interv Card Electrophysiol. 2008;21:59–63. doi: 10.1007/s10840-007-9187-9. [DOI] [PubMed] [Google Scholar]
- 21.Quan XQ, Bai R, Lu JG, Patel C, Liu N, Ruan Y, Chen BD, Ruan L, Zhang CT. Pharmacological enhancement of cardiac gap junction coupling prevents arrhythmias in canine LQT2 model. Cell Commun Adhes. 2009;16:29–38. doi: 10.1080/15419060903118567. [DOI] [PubMed] [Google Scholar]
- 22.Quan XQ, Bai R, Liu N, Chen BD, Zhang CT. Increasing gap junction coupling reduces transmural dispersion of repolarization and prevents torsade de pointes in rabbit LQT3 model. J Cardiovasc Electrophysiol. 2007;18:1184–1189. doi: 10.1111/j.1540-8167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 23.Pu J, Zhang C, Quan X, Zhao G, Lv J, Li B, Bai R, Liu N, Ruan Y, He B. Effects of potassium aspartate and magnesium on ventricular arrhythmia in ischemia-reperfusion rabbit heart. J Huazhong Univ Sci Technolog Med Sci. 2008;28:517–519. doi: 10.1007/s11596-008-0506-x. [DOI] [PubMed] [Google Scholar]
- 24.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 25.Pu J, Mintz GS, Brilakis ES, Banerjee S, Abdel-Karim AR, Maini B, Biro S, Lee JB, Stone GW, Weisz G, Maehara A. In vivo characterization of coronary plaques: novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur Heart J. 2012;33:372–383. doi: 10.1093/eurheartj/ehr387. [DOI] [PubMed] [Google Scholar]
- 26.Burton FL, Cobbe SM. Dispersion of ventricular repolarization and refractory period. Cardiovasc Res. 2001;50:10–23. doi: 10.1016/s0008-6363(01)00197-3. [DOI] [PubMed] [Google Scholar]
- 27.Flore V, Claus P, Antoons G, Oosterhoff P, Holemans P, Vos MA, Sipido KR, Willems R. Microvolt T-wave alternans and beat-to-beat variability of repolarization during early postischemic remodeling in a pig heart. Heart Rhythm. 2011;8:1050–1057. doi: 10.1016/j.hrthm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Selvaraj RJ, Picton P, Nanthakumar K, Mak S, Chauhan VS. Endocardial and epicardial repolarization alternans in human cardiomyopathy: evidence for spatiotemporal heterogeneity and correlation with body surface T-wave alternans. J Am Coll Cardiol. 2007;49:338–346. doi: 10.1016/j.jacc.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 29.Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: a meta-analysis. J Am Coll Cardiol. 2005;46:75–82. doi: 10.1016/j.jacc.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 30.Janse MJ, Sosunov EA, Coronel R, Opthof T, Anyukhovsky EP, de Bakker JM, Plotnikov AN, Shlapakova IN, Danilo P Jr, Tijssen JG, Rosen MR. Repolarization gradients in the canine left ventricle before and after induction of short-term cardiac memory. Circulation. 2005;112:1711–1718. doi: 10.1161/CIRCULATIONAHA.104.516583. [DOI] [PubMed] [Google Scholar]
- 31.Fish JM, Antzelevitch C. Cellular mechanism and arrhythmogenic potential of T-wave alternans in the Brugada syndrome. J Cardiovasc Electrophysiol. 2008;19:301–308. doi: 10.1111/j.1540-8167.2007.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwofie MA, Chaudhary AK, Martins JB. Association among intracardiac T-wave alternans, ischemia, and spontaneous ventricular arrhythmias after coronary artery occlusion in a canine model. Transl Res. 2011;158:265–272. doi: 10.1016/j.trsl.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Morita H, Zipes DP, Lopshire J, Morita ST, Wu J. T wave alternans in an in vitro canine tissue model of Brugada syndrome. Am J Physiol Heart Circ Physiol. 2006;291:H421–428. doi: 10.1152/ajpheart.01259.2005. [DOI] [PubMed] [Google Scholar]
- 34.Stokke MK, Tovsrud N, Louch WE, Oyehaug L, Hougen K, Sejersted OM, Swift F, Sjaastad I. I(CaL) inhibition prevents arrhythmogenic Ca(2+) waves caused by abnormal Ca(2+) sensitivity of RyR or SR Ca(2+) accumulation. Cardiovasc Res. 2013;98:315–325. doi: 10.1093/cvr/cvt037. [DOI] [PubMed] [Google Scholar]
- 35.Amador FJ, Kimlicka L, Stathopulos PB, Gasmi-Seabrook GM, Maclennan DH, Van Petegem F, Ikura M. Type 2 ryanodine receptor domain A contains a unique and dynamic alphahelix that transitions to a beta-strand in a mutant linked with a heritable cardiomyopathy. J Mol Biol. 2013;425:4034–4046. doi: 10.1016/j.jmb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Satoh H. Electrophysiological actions of ryanodine on single rabbit sinoatrial nodal cells. Gen Pharmacol. 1997;28:31–38. doi: 10.1016/s0306-3623(96)00182-6. [DOI] [PubMed] [Google Scholar]
- 37.Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 38.Zhou P, Zhang SM, Wang QL, Wu Q, Chen M, Pei JM. Anti-arrhythmic effect of verapamil is accompanied by preservation of cx43 protein in rat heart. PLoS One. 2013;8:e71567. doi: 10.1371/journal.pone.0071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdivia HH, Valdivia C, Ma J, Coronado R. Direct binding of verapamil to the ryanodine receptor channel of sarcoplasmic reticulum. Biophys J. 1990;58:471–481. doi: 10.1016/S0006-3495(90)82392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipido KR. Understanding cardiac alternans: the answer lies in the Ca2+ store. Circ Res. 2004;94:570–572. doi: 10.1161/01.RES.0000124606.14903.6F. [DOI] [PubMed] [Google Scholar]
- 41.Merchant FM, Armoundas AA. Role of substrate and triggers in the genesis of cardiac alternans, from the myocyte to the whole heart: implications for therapy. Circulation. 2012;125:539–549. doi: 10.1161/CIRCULATIONAHA.111.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards JN, Blatter LA. Cardiac alternans and intracellular calcium cycling. Clin Exp Pharmacol Physiol. 2014;41:524–532. doi: 10.1111/1440-1681.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 44.Marengo JJ, Hidalgo C, Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J. 1998;74:1263–1277. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, von Birgelen C, Muramatsu T, Li Y, Holm NR, Reiber JH, Tu S. A novel four-dimensional angiographic approach to assess dynamic superficial wall stress of coronary arteries in vivo: initial experience in evaluating vessel sites with subsequent plaque rupture. EuroIntervention. 2017 doi: 10.4244/EIJ-D-16-01020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Xu L, Ding S, Lin N, Ji Q, Gao L, Su Y, He B, Pu J. Novel protective role of the circadian nuclear receptor retinoic acid-related orphan receptor-alpha in diabetic cardiomyopathy. J Pineal Res. 2017:62. doi: 10.1111/jpi.12378. [DOI] [PubMed] [Google Scholar]
- 47.He B, Zhao Y, Xu L, Gao L, Su Y, Lin N, Pu J. The nuclear melatonin receptor RORalpha is a novel endogenous defender against myocardial ischemia/reperfusion injury. J Pineal Res. 2016;60:313–326. doi: 10.1111/jpi.12312. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y, Xu L, Qiao Z, Gao L, Ding S, Ying X, Su Y, Lin N, He B, Pu J. YiXin-Shu, a ShengMai-San-based traditional Chinese medicine formula, attenuates myocardial ischemia/reperfusion injury by suppressing mitochondrial mediated apoptosis and upregulating liver-Xreceptor alpha. Sci Rep. 2016;6:23025. doi: 10.1038/srep23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao T, Ying X, Zhao Y, Yuan A, He Q, Tong H, Ding S, Liu J, Peng X, Gao E, Pu J, He B. Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy. Antioxid Redox Signal. 2015;22:633–650. doi: 10.1089/ars.2014.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Q, Pu J, Yuan A, Lau WB, Gao E, Koch WJ, Ma XL, He B. Activation of liver-X-receptor alpha but not liver-X-receptor beta protects against myocardial ischemia/reperfusion injury. Circ Heart Fail. 2014;7:1032–1041. doi: 10.1161/CIRCHEARTFAILURE.114.001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang Y, Bai J, Tang L, Zhang P, Pu J. Anti-CCL21 antibody attenuates infarct size and improves cardiac remodeling after myocardial infarction. Cell Physiol Biochem. 2015;37:979–990. doi: 10.1159/000430224. [DOI] [PubMed] [Google Scholar]
- 52.Pu J, Yuan A, Shan P, Gao E, Wang X, Wang Y, Lau WB, Koch W, Ma XL, He B. Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J. 2013;34:1834–1845. doi: 10.1093/eurheartj/ehs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Yu Z, Huang X, Gao Y, Wang X, Gu J, Xue S. Peroxisome proliferator-activated receptor gamma (PPARgamma) mediates the protective effect of quercetin against myocardial ischemia-reperfusion injury via suppressing the NF-kappaB pathway. Am J Transl Res. 2016;8:5169–5186. [PMC free article] [PubMed] [Google Scholar]
- 54.Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]