Abstract
Gastric cancer is the most common malignant tumor and globally the third leading cause of cancer-related deaths. Therefore, there exists an urgent need to identify new effective gastric cancer treatments. Given the important roles in tumorigenesis and progression, p21-activated kinase 4 (PAK4) has been regarded as an attractive high-value druggable target. In this study, we examined the effects and molecular mechanisms of action of the small molecular compound LC-0882 on gastric cancer cells in vitro. LC-0882 was found to significantly inhibit the proliferation of human gastric cancer cells by repressing phospho-PAK4/cyclin D1 and CDK4/6 expression. In addition, LC-0882 was found to attenuate cell invasion by blocking the PAK4/LIMK1/cofilin signaling pathway. Finally, analysis of immunofluorescence revealed that LC-0882 exposure decreased filopodia formation and induced cell elongation in BGC823 and SGC7901 gastric cancer cells. These findings suggest that targeting PAK4 with the novel compound LC-0882 may provide a new chemotherapeutic approach in gastric cancer treatment.
Keywords: LC-0882, small molecular compound, PAK4, gastric cancer
Introduction
Gastric cancer is a common malignancy worldwide. According to the World Health Organization (WHO), gastric cancer is the third leading cause of global cancer-related deaths, accounting for about 700,000 deaths in 2012 [1]. Despite major advances in chemotherapy and surgical treatment, long-term outcomes among gastric cancer patients remain poor, due to disease progression, tumor invasion and metastasis [2,3]. Therefore, there exists an urgent need to characterize new pharmaceutical treatment targets within gastric cancer cells and to identify effective compounds that can improve survival outcomes and prevent gastric cancer progression.
P21-activated kinase 4 (PAK4) is one of the PAK family members which are downstream effectors of Rho GTPases Rac and Cdc42 as serine/threonine protein kinases [4,5]. It is the first identified member of the Group II division of the PAK family and is the most extensively studied member in this group [6]. Genetic amplification and overexpression of PAK4 have been observed in a variety of tumors, including breast cancer, pancreatic cancer, prostate cancer and in particular, gastric cancer [7-9]. More recently, studies have further characterized PAK4 is overexpressed in metastatic gastric cancer, and the level of activated PAK4 expression has been found to correlated with worse prognosis [10-12].
PAK4 has been proposed to serve multiple critical roles in the regulation of cancer cell signaling networks. In ovarian cancer cells, overexpression and activation of PAK4 have been found to significantly promote cell proliferation, while depletion of PAK4 has been found to suppress cell proliferation [13]. Other studies have reported that PAK4 promotes cell proliferation by regulating expression of cyclin D1, CDC25A, Smad2/3, and CDK6 [14-16]. In addition, through its regulation of actin assembly, PAK4 has been strongly implicated in cytoskeletal dynamics contributing to cell migration [17,18]. In further support of the critical role in cell migration, PAK4 has been identified to be involved in regulating the HGF/LIMK1/cofilin pathway, interacting with DGCR6L and phosphorylating SCG10 [9,19,20]. Other studies have also reported that PAK4 could inhibit cell adhesion [21], promote anchorage-independent growth [8,21,22], and mediate the induction of filopodia formation [18]. Consequently, these important roles in key tumorigenesis and progression processes make PAK4 an attractive candidate drug target.
In this study, we investigated the effects and potential mechanisms of action of the small molecule compound LC-0882 on the proliferation and invasion of human gastric cancer cells. Our observations suggested that LC-0882 might target PAK4 and its related signaling pathways, thereby presenting a potential new therapy for gastric cancer treatment.
Material and methods
Cell lines and culture
Human gastric cancer cell lines (SGC7901, BGC823, and MKN-45) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Life) with 10% fetal calf serum (Life) in a humidified incubator with 5% CO2 at 37°C.
Test compound
LC-0882 (C25H20ClNO5, Mass: 449.89) was purchased from the SPECS database and the structure of this compound is shown in Figure 1A. LC-0882 was dissolved in dimethyl sulfoxide (DMSO) at 20 mM concentration and stored as small aliquots at -20°C. Aliquots were thawed and diluted in cell culture medium to the desired concentrations immediately before use.
Figure 1.
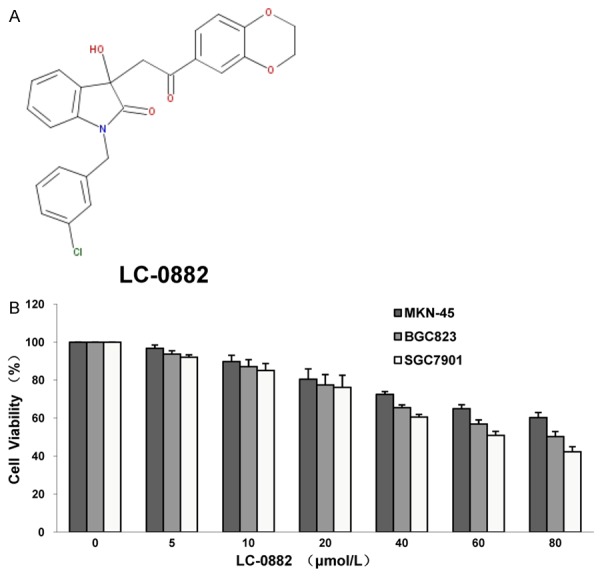
Chemical structure and the inhibitory effect of LC-0882 on human gastric cancer cell proliferation. A: Chemical structure of LC-0882 (C25H20ClNO5). B: The inhibitory effect of LC-0882 on human gastric cancer cell proliferation by MTT assay. MKN-45, BGC823 and SGC7901 cells were cultured with indicated concentrations of LC-0882 for 24 h, after which MTT assay was performed.
Cell viability assay
Cell viability was evaluated using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. Gastric cancer cells were seeded in 96-well Corning plates at a density of 1×104 cells/well and incubated overnight. Then the medium was removed and replaced with 100 μl of medium containing indicated concentrations of LC-0882 for 24 hours (h). At the end of incubation, 10 μl MTT solution (5 mg/ml in phosphate-buffered saline (PBS)) was added to each well and incubated for 4 h at 37°C. The medium was then replaced with 150 μl DMSO for 15 minutes (min) at 37°C. Finally, optical densities at 595 nm were detected using a microplatereader (BIO-RAD).
Cell cycle analysis using flow cytometry
SGC7901 and BGC823 cells were treated with the indicated concentrations of LC-0882 for 24 h. Cells were then collected, washed with PBS, fixed with 70% ethanol, and stored for 2 h at -20°C. Finally, cells were suspended in a staining buffer (0.1% RNase, 0.5% Tween 20, 10 µg/ml propidium iodide in PBS). Analysis of the proportion of cells in different cell cycle phases was performed using flow cytometer (FACSVantage, Becton Dickinson and Co., San Jose, CA). Gating was set to exclude cell debris, doublets and clumps.
Transwell assay
The invasive capability of the cultured and treated cells was assessed using Boyden chambers with polycarbonate Nucleopore membrane. Matrigel pre-coated membranes (8-μm pore size, 6.5 mm in diameter, matrigel 100 μg/cm2) were first rehydrated with 100 μl medium. Next, gastric cancer cells (1×105) in 100 μl serum-free DMEM were placed in the upper part of each chamber, while the lower part of each chamber contained 600 μl DMEM including 10% serum. Chambers were incubated for 18 h at 37°C. Following incubation, cells which had not invaded through the membrane pores were discarded using a cotton swab. Cells adherent to the lower surface of the filter were then fixed, stained, and counted microscopically.
Lentivirus production and transduction
Recombinant lentiviruses (PAK4-RNAi-Lentivirus and PAK4-Lentivirus) and NC-GFP-LV and pGC FU-GFP-LV vectors were purchased from the Shanghai GeneChem Company. To generate a stable PAK4 knockdown model, SGC7901 cells were infected with lentivirus carrying shPAK4, and infected cells were then selected out with puromycin (1.5 µg/ml). The pGC NC-GFP-LV vector was used as a control. To stably overexpress PAK4, MKN-45 cells were infected with lentivirus carrying PAK4, and selected with puromycin. NC-GFP-LV and pGC FU-GFP-LV vectors were used as controls for this experiment.
Real time monitoring of cell invasion
Invasion assays were conducted on CIM-16 plates with 8 μm pore membranes (Roche). Plate wells were pre-coated with 20 μl matrigel at 37°C for 4 hours. Next, 160 μl medium with 10% serum was added to the bottom chamber of each well, and the top and bottom portions of the plates were assembled. After adding 50 μl serum-free medium to the top chamber wells, the assembled CIM-16 plates were allowed to equilibrate at 37°C for 2 h. Finally, gastric cancer cells were seeded onto the top chambers of the plates at a density of 8×104 cells/well and these plates were incubated for 30 min. Samples were then analyzed using anxCELLigence data collection system.
Kinase assay
Exogenous MBP was used as a substrate to assess the activity of PAK4 kinase. Commercially available PAK4 kinase (life) was co-incubated with the indicated concentrations of LC-0882 to investigate the influence of this compound on PAK4 kinase activity. The kinase reaction was launched by combining MBP and a mixture of 1 mM ATP and [γ-32P] ATP. Kinase activity was detected in 40 μl kinase buffer containing 10 µCi [γ-32P] ATP (5,000 Ci/mmol) at 30°C for 30 min. The kinase reaction was stopped by adding 6×SDS buffer and loading on 12% SDS-PAGE. 32P-labelled proteins were transferred onto PVDF membranes and visualized through autoradiography. To assure equal loading amounts, MBP was detected by Ponceau stain and PAK4 by western blot analysis.
Molecular modeling
Docking simulation was conducted by Glide in Schrödinger (version 2014). The non-bonded interactions between LC-0882 and PAK4 were visualized using Discovery Studio 4.0 Visual.
Immunofluorescence and confocal microscopy
BGC823 and SGC7901 cells grown on glass coverslips were pre-treated with indicated concentrations of LC-0882 or DMSO (control) for 24 h. Cells were then fixed in methanol for 15 min and blocked with goat serum for 1 h. Cells were then exposed to FITC-conjugated phalloidin (Sigma) for 1 h and washed three times in PBS with 1% TritonX-100 (PBT). DAPI (4’,6-diamidino-2-phenylindole) was used to costain the DNA. Cells were viewed and photographed using a Leica laser confocal scanning microscope.
Western blot analysis
Proteins were extracted from 1×106 cells in RIPA buffer (150 mM NaCl, 50 mM Tris/HCL pH 7.4, 1 mM EDTA, 1% Nonidet P-40, 0.25% Na-deoxycholate and protease inhibitors). Denatured protein samples were separated by SDS-PAGE and transferred to a PVDF membrane (Millipore). The membrane was blocked for 3 h in 5% skimmed milk in TBS-T (137 mM NaCl, 20 mM Tris, pH 7.4, 0.05% Tween-20), and proteins were probed with specific antibodies: LIMK1, phospho-LIMK1 Thr508/LIMK2 Thr505, cofilin, phospho-cofilin Ser3, PAK4, phospho-PAK4 Ser474/PAK5 Ser602/PAK6 Ser560, CDK4, CDK6 (Cell signal); cyclin D1 (Neomarker). All PVDF membranes were assayed by chemiluminescence (ECL, Pierce Technology). To assure equal loading, membranes were stripped and reprobed with an antibody against GAPDH (ShangHaiKangchen).
Statistical analysis
Comparison between two groups was performed by Student’s t test using SPSS 16.0 software. Values of P<0.05 were considered statistically significant.
Results
LC-0882 suppresses proliferation and induces G1 cell cycle arrest in human gastric cancer cells
Effects of LC-0882 on proliferation of MKN-45, BGC823 and SGC7901 cells were evaluated by MTT assay. LC-0882 exposure significantly inhibited proliferation of human gastric cancer cells in a dose-dependent manner (Figure 1B). To further elucidate the mechanisms by which LC-0882 might have this effect, cell cycle progression was investigated using flow cytometry. Cell cycle analyses for SGC7901 and BGC823 cells treated with LC-0882 for 24 h revealed significant concentration-dependent increases in the number of cells in G1 phase and a remarkable decrease in S phase cells, compared with controls not exposed to LC-0882 (Figure 2). These findings indicate that LC-0882 induces G1 phase cell cycle arrest in SGC7901 and BGC823 cells.
Figure 2.
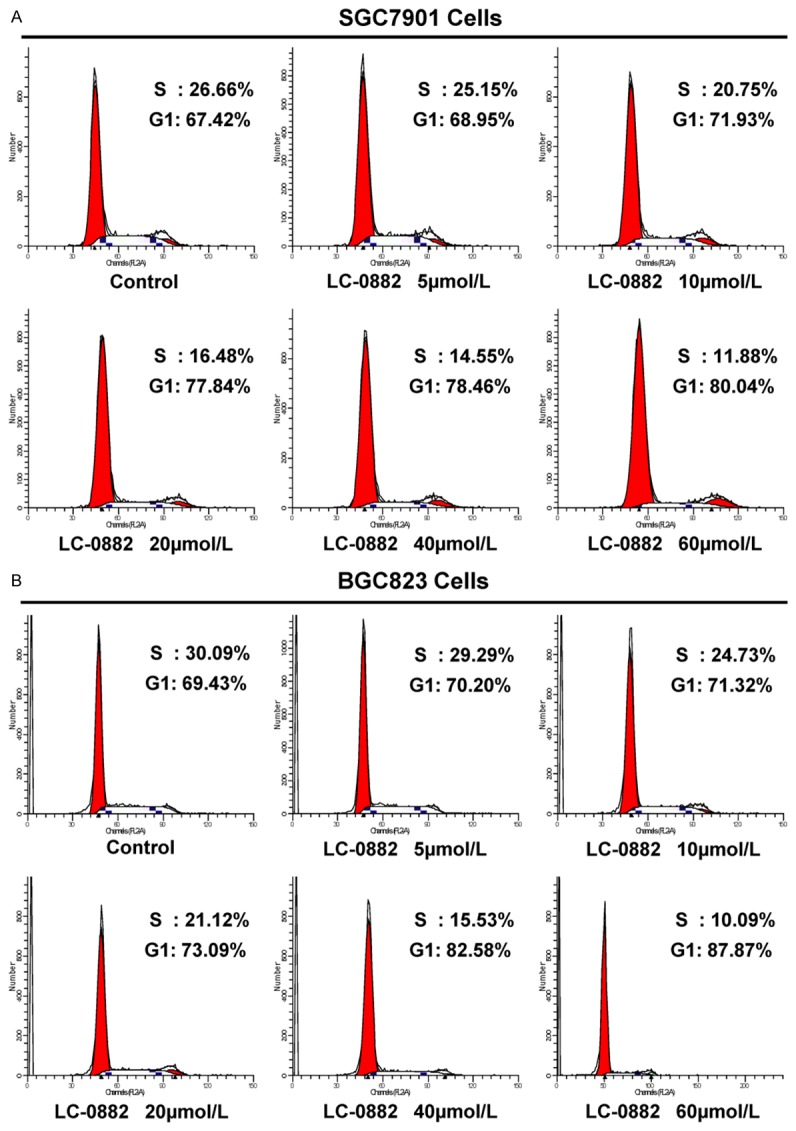
LC-0882 suppresses the transition of gastric cancer cells from G1 to S phase. SGC7901 and BGC823 cells were incubated with indicated concentrations of LC-0882 for 24 h. Cells were then collected, washed with PBS, fixed with 70% ethanol and stained. Analysis of the proportion of cells in different cell cycle phases was performed using flow cytometry. A: LC-0882 suppresses the transition of SGC7901 cells from G1 to S phase. B: LC-0882 suppresses the transition of BGC823 cells from G1 to S phase.
LC-0882 attenuates the invasive capacity of gastric cancer cells
Transwell migration assays were performed to evaluate the impact of LC-0882 on the invasive capacity of gastric cancer cells. To control for the effects of cell proliferation inhibition on cell invasion, we counted the same number of cells pre-incubated with indicated concentrations of LC-0882 or DMSO. LC-0882 was observed to prominently decrease the invasive capacity of SGC7901 and BGC823 in a dose-dependent manner (Figure 3A, 3B). Consistent with these findings, real-time invasion monitoring data from the xCELLigence system indicated a dose-dependent decrease in cell invasiveness after treatment with LC-0882 in MKN-45 cells (Figure 3D). Thus, these results strongly suggest that LC-0882 could play an important clinical role in decreasing the invasive potential of gastric cancer cells.
Figure 3.
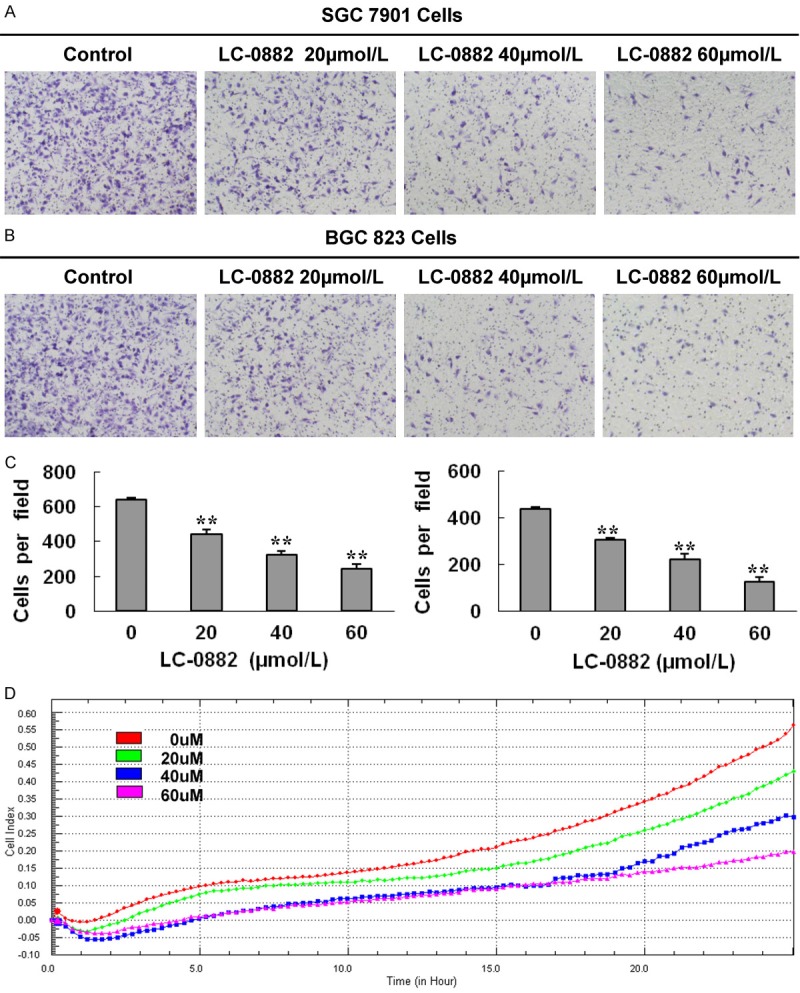
LC-0882 suppresses invasion of human gastric cancer cells. SGC7901, BGC823 and MKN-45 cells were incubated with indicated concentrations of LC-0882 for 24 h. A: Invasive capacity of SGC7901 cells were evaluated using a Boyden chamber matrigel invasion assay. B: Invasive capacity of BGC823 cells were evaluated using a Boyden chamber matrigel invasion assay. C: Number of cells invading is shown as bar diagram ± SEM, the left one is for SGC7901, and the right one is for BGC823, **P<0.01. D: The effect of LC-0882 on MKN-45 cell invasive ability was detected using real-time invasion monitoring.
LC-0882 inhibits PAK4 kinase activity
Previous studies have reported that PAK4 plays important roles in the regulation of cell proliferation, invasion and morphology of various cancer cell types. Most of these functions for PAK4 in cancer cell biology have been attributed to its kinase activity. To better assess the inhibitory potency of LC-0882 on PAK4, a kinase assay was performed at a range of compound concentrations. Kinase assay results indicated that LC-0882 potently inhibited the kinase activity of PAK4 in a dose-dependent manner (Figure 4A). The results of docking simulations performed using Glide in Schrödinger to detect the modes in which LC-0882 interacts with PAK4 are shown in Figure 4B. In these simulations, LC-0882 forms three conventional hydrogen-bonding (H-bonding) interactions, a weak carbon H-bonding interaction, a π-sigma interaction, and three π-alkyl interactions with receptor PAK4.
Figure 4.
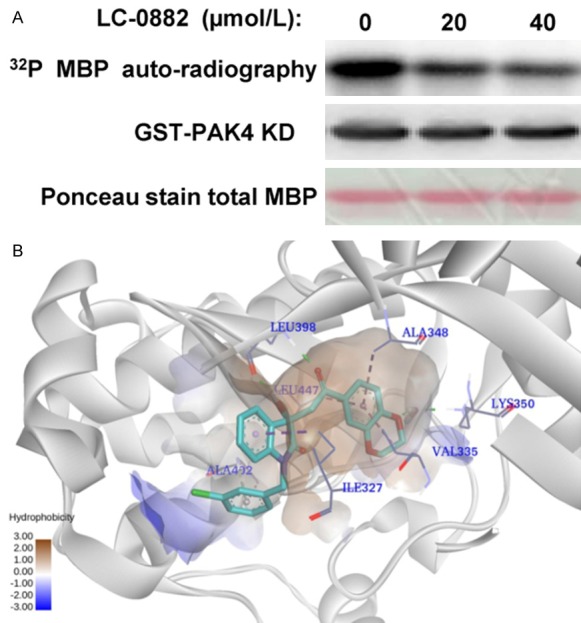
LC-0882 inhibits PAK4 kinase activity. A: The effect of LC-0882 on PAK4 kinase activity was detected by kinase assay. Different concentrations of LC-0882 was co-incubated with PAK4 kinase for 45 min in kinase buffer. Kinase assays were then performed to detect the effect of LC-0882 on PAK4 kinase activity. B: Proposed binding mode of LC-0882 within the PAK4 binding site. Docking simulation was conducted using Glide, Version 2014 (Schrödinger). The non-bonded interaction between LC-0882 and PAK4 was visualized using Discovery Studio 4.0 Visual.
The inhibitory effect of LC-0882 on invasion is achieved by targeting PAK4
Given that PAK4 is known to promote tumor invasion, and the findings in this investigation that LC-0882 treatment inhibited PAK4 kinase activity, we then sought to determine if the inhibitory effects of LC-0882 on cell invasion were due to modulation of PAK4 kinase activity. Transwell migration assays were performed to compare the invasive capability of SGC7901 cells exposed to LC-0882 with PAK4 knockdowns. The results of these comparisons demonstrated that the inhibitory effect of LC-0882 was comparable to the effect of shRNA targeted to silence PAK4 in SGC7901 cells (Figure 5). Furthermore, in MKN-45 cells overexpressing PAK4, LC-0882 exposure dramatically inhibited invasiveness in a dose-dependent manner, resulting in behavior similar to that of MKN-45 cells infected only with lentivirus carrying the control vector (Figure 6).
Figure 5.
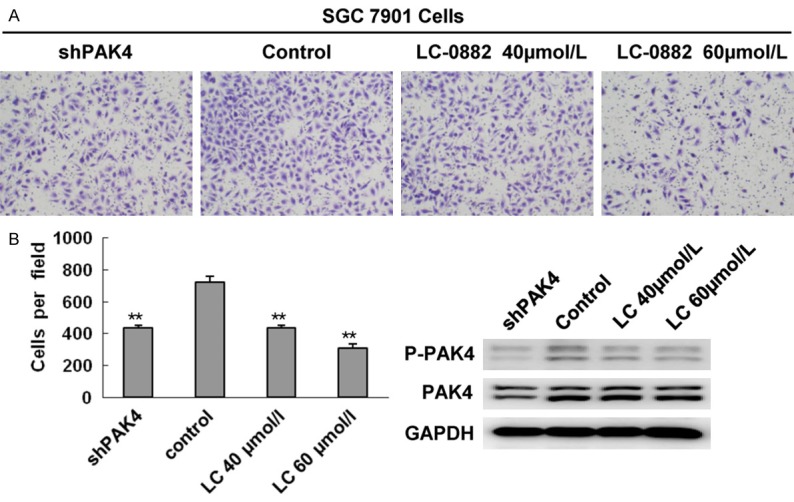
LC-0882 treatment resulted in inhibitory effects on cell invasion comparable to PAK4 knockdown. SGC7901 cells were incubated with indicated concentrations of LC-0882 for 24h. A: The invasive ability of SGC7901 treated with LC-0882 and PAK4 knockdowns were evaluated using a Boyden chamber matrigel invasion assay. B: The number of cells invading is shown as bar diagram ± SEM (left), **P<0.01. Western blot analysis was used to detect cellular protein levels of PAK4 (right).
Figure 6.
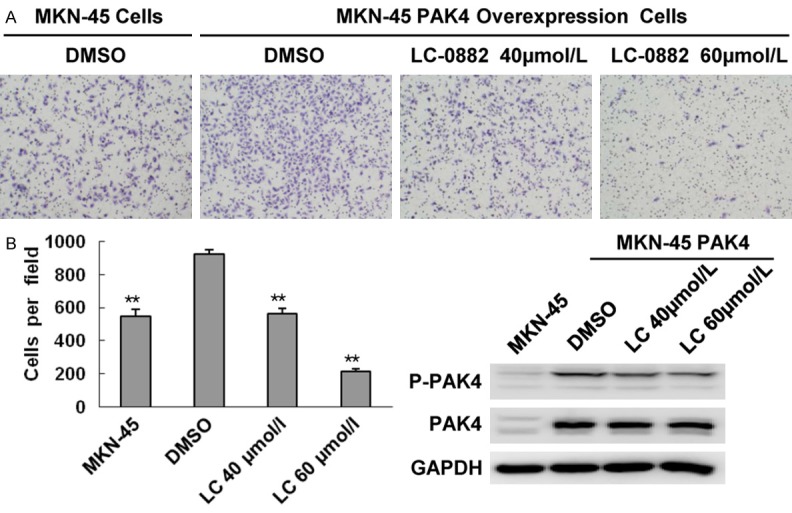
LC-0882 treatment exhibited the same inhibitory effect on cell invasion in PAK4-overexpressing MKN-45 cells and control MKN-45 cells. PAK4-overexpressing MKN-45 cells were incubated with indicated concentrations of LC-0882 for 24 h. A: The invasive ability of PAK4-overexpressing and control MKN-45 cells was evaluated using a Boyden chamber matrigel invasion assay. B: The number of cells invading is shown as bar diagram ± SEM (left), **P<0.01. Western blot analysis was used to detect cellular protein levels of PAK4 (right).
LC-0882 suppresses filopodia formation of gastric cancer cells
Among the significant functions of PAK4 is induction of filopodia formation in response to the Rho GTPase Cdc42. Given the observed inhibitory effects of LC-0882 on PAK4 kinase activity, we used FITC-phalloidin staining to investigate how LC-0882 impacts filopodia formation. LC-0882 was found to suppress the formation of filopodia in a dose-dependent manner in SGC7901 and BGC823 cells (Figure 7).
Figure 7.
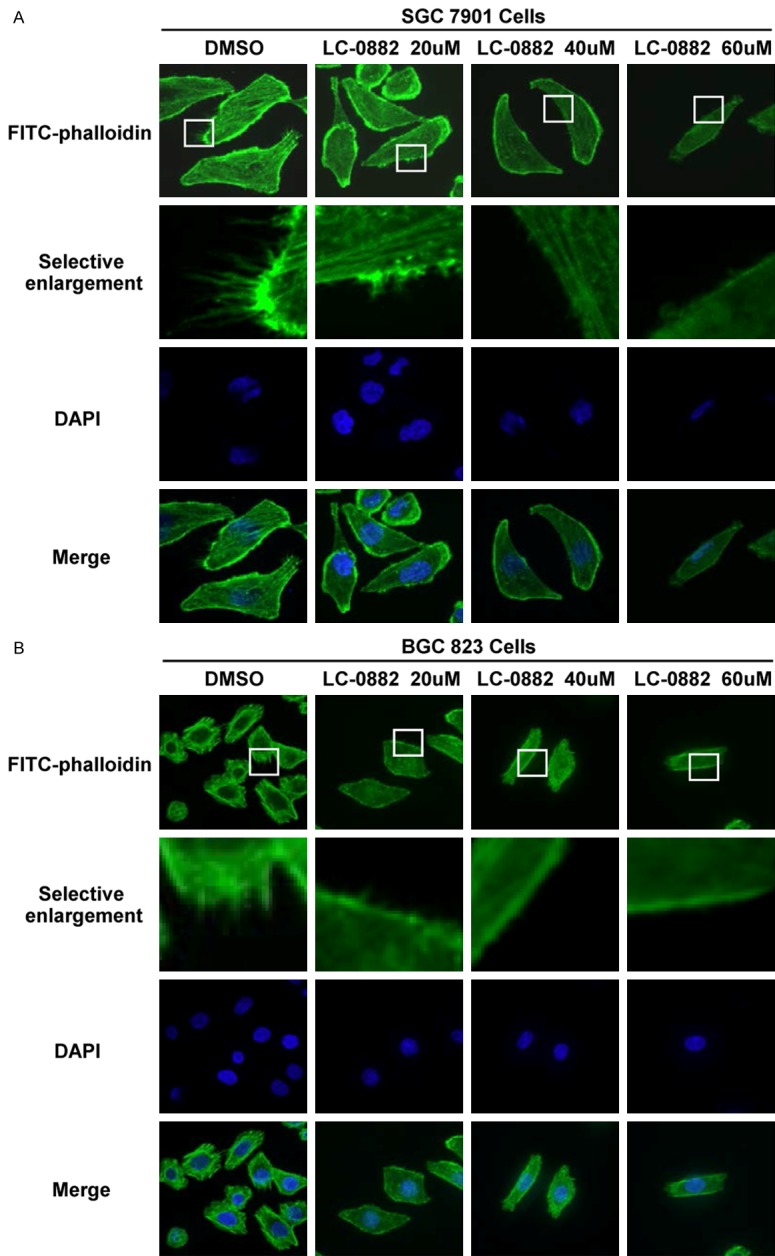
LC-0882 inhibits the formation of filopodia in SGC7901 and BGC823 cells. SGC7901 and BGC823 cells grown on glass coverslips were pre-treated with test concentrations of LC-0882 or DMSO (control) for 24 h. Cells were then fixed, blocked and stained with FITC-phalloidin. A: LC-0882 inhibits filopodia formation in SGC7901 cells. B: LC-0882 inhibits filopodia formation in BGC823 cells.
LC-0882 inhibits phosphorylation of PAK4 and its downstream signaling pathways
Cyclin D1 and CDK4/6 are well-known regulators in the G1 phase of the cell cycle, and PAK4 plays a vital role in the regulation of cyclin D1. We found that LC-0882 exposure could suppress proliferation and induce G1 cell cycle arrest in human gastric cancer cells. Thus, the expression levels of these regulators in cells treated with LC-0882 were further investigated by western blot. The results showed that phosphorylated PAK4, cyclin D1 and CDK4/6 were all significantly down-regulated after LC-0882 treatment (Figure 8A, 8B). In addition, it is noteworthy that the effects of LC-0882 on the PAK4/cyclin D1 pathway were similar to the effect of shRNA expression targeting PAK4. Taken together, these results indicate that LC-0882 exposure induces cell cycle arrest at the G1 phase and then suppresses cell proliferation via down-regulation of phosphor-PAK4/cyclin D1 and CDK4/6.
Figure 8.
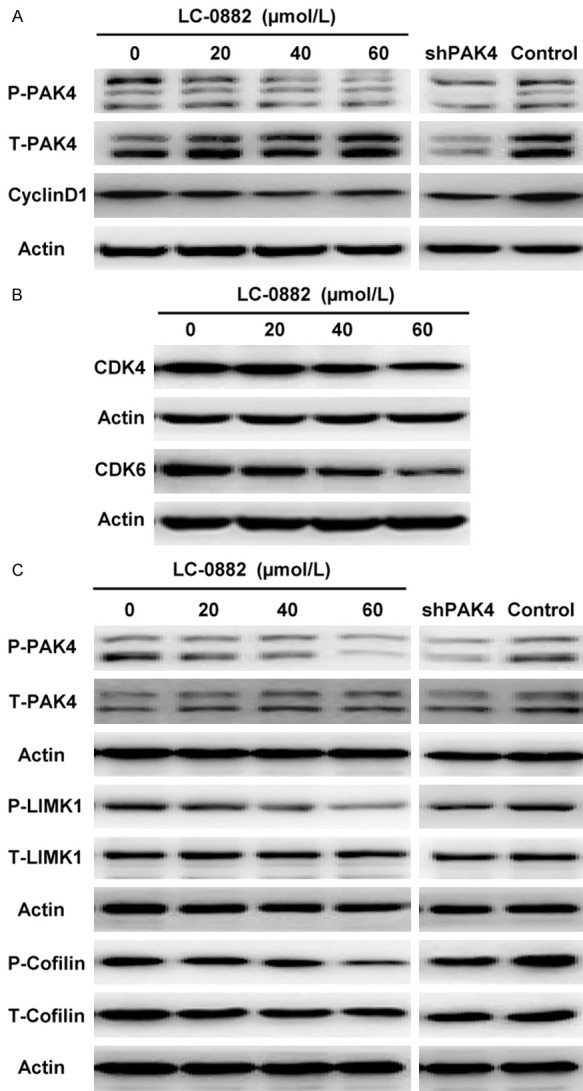
The mechanisms of LC-0882 underlying the inhibition of proliferation and invasion. SGC7901 cells were cultured with indicated concentrations of LC-0882 for 24 h. Cells with different treatments, including LC-0882 exposure and PAK4 knockdown, were collected and western blot was then performed. A: LC-0882 decreased the expression levels of phosphorylated PAK4 and cyclin D1. B: LC-0882 down-regulated the expression of CDK4/6. C: LC-0882 inhibited the PAK4/LIMK1/cofilin pathway.
Subsequently, to explore in further detail the mechanisms of LC-0882 underlying the inhibition of filopodia formation and cell invasion, we investigated the effects of LC-0882 on the PAK4/LIMK1/cofilin pathway, a pathway previously recognized for its role in cell invasion. As shown in Figure 8C, treatment with varying LC-0882 concentrations down-regulated the phosphorylation of PAK4, LIMK1 and cofilin in a dose-dependent manner. In addition, the effect of LC-0882 exposure on the PAK4/LIMK1/cofilin pathway was comparable to the impact of PAK4 knockdown (Figure 8C).
Discussion
In this investigation, we evaluated the anti-tumor activities of LC-0882, a small molecular compound selected for further evaluation after screening for inhibition efficacy in gastric cancer cells. Our results, for the first time, demonstrated that LC-0882 efficiently suppresses gastric cancer cell proliferation and induces G1 cell cycle arrest. Furthermore, we found dose-dependent inhibition of gastric cancer cell invasion behavior and filopodia formation by LC-0882.
PAK4 has been previously found to play a role in a number of cellular processes [23], including cell cycle progression [24], cell survival [25], and cytoskeletal organization [18]. Moreover, PAK4 dysregulation has been reported to promote tumorigenesis and disease progression in several types of cancers, in particular gastric cancer. PAK4 therefore offers promise as a potential high-value drug target. In this investigation, we confirmed that LC-0882 could suppress proliferation and invasion capability of gastric cancer cells. We then explored whether LC-0882 exerted these effects by targeting PAK4. As we had hypothesized, LC-0882 strikingly inhibited the kinase activity of PAK4 in a dose-dependent manner. Results from the docking simulations performed as part of this investigation provide further insights into the interactions between LC-0882 and PAK4.
In order to further confirm that LC-0882 inhibits cell invasion via targeting PAK4, we also compared the invasive capability of gastric cancer cells treated with LC-0882 and cells in which PAK4 expression was suppressed by targeted shRNA. The results from this experiment show analogous effects for LC-0882 treatment and PAK4 knockdown. These findings provide further indication that the suppressive effect of LC-0882 on cell invasion is achieved through targeting PAK4. Of note, PAK4 has also been previously reported to mediate filopodia formation through interaction with activated Cdc42, and activated PAK4 has been proposed to induce cell rounding [18]. Accordingly, we directly investigated the influence of LC-0882 on formation of filopadia. Our findings revealed that LC-0882 inhibited filopodia formation and induced elongation of gastric cancer cells in a dose-dependent manner. So it is intelligible that the effects of LC-0882 exposure on filopodia formation and cellular elongation are mediated by PAK4 kinase inhibition.
Multiple studies have established that cyclin D1 and CDK4/6 act to regulate G1 phase progression to control tumor proliferation [26,27]. Moreover, it has been previously demonstrated that PAK4 could up-regulate expression of cyclin D1 via the PAK4/c-Src/EGFR/cyclin D1 signaling pathway in ovarian cancer cells. Our finding that LC-0882 down-regulates expression levels of phospho-PAK4/cyclin D1 and CDK4/6 in a dose-dependent manner provides a powerful mechanistic connection between LC-0882 exposure and G1 cell cycle arrest. Based on the results of our series of experiments, we propose that LC-0882 exposure results in markedly decreased levels of phosphorylated PAK4, cyclin D1 and CDK4/6, subsequently inhibiting the transition of cells from G1 to S phase and finally leading to an antiproliferative effect on gastric cancer cells. In addition, to further elucidate the mechanisms by which LC-0882 suppresses gastric cancer cell invasion, we investigated the effects of LC-0882 on the PAK4/LIMK1/cofilin pathway, which had been previously reported to correlate with cancer cell migration and invasion. As previously described in prostate cancer cells [24], our data supported that LC-0882 suppressed the invasive capacity of gastric cancer cells via down-regulating the PAK4/LIMK1/cofilin signaling pathway.
Taken together, the results of the current study provide the first evidence that LC-0882 targets PAK4 and inhibits PAK4-related signaling pathways to suppress the proliferation and invasion of gastric cancer cells. Although these results warrant further testing research in vivo, our findings suggest that LC-0882 may be a promising anti-cancer drug candidate in gastric cancer therapy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81302127, 81230077, 31371424, 31571457) and the Specialized Research Fund for the Doctoral Program of Higher Education in China (Grant No. 20132104120018).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Du X, Chen Q, Kang Y, Xu C, Fan L, Ye H, Ying S, Shi L, Jin R, Wu J, Liang G, Li X. Peptidomimetic suppresses proliferation and invasion of gastric cancer cells by fibroblast growth factor 2 signaling cascade blockage. Anticancer Drugs. 2016;27:164–172. doi: 10.1097/CAD.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 3.Corso S, Ghiso E, Cepero V, Sierra JR, Migliore C, Bertotti A, Trusolino L, Comoglio PM, Giordano S. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol Cancer. 2010;9:121. doi: 10.1186/1476-4598-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar R, Vadlamudi RK. Emerging functions of p21-activated kinases in human cancer cells. J Cell Physiol. 2002;193:133–144. doi: 10.1002/jcp.10167. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 6.King H, Nicholas NS, Wells CM. Role of p-21-activated kinases in cancer progression. Int Rev Cell Mol Biol. 2014;309:347–387. doi: 10.1016/B978-0-12-800255-1.00007-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Auletta T, Dovirak O, Hutter C, Kuntz K, El-ftesi S, Kendall J, Han H, Von Hoff DD, Ashfaq R, Maitra A, Iacobuzio-Donahue CA, Hruban RH, Lucito R. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther. 2008;7:1793–1802. doi: 10.4161/cbt.7.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, Jallal B, Smeal T. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem. 2002;277:550–558. doi: 10.1074/jbc.M105732200. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Ke Q, Li Y, Liu F, Zhu G, Li F. DGCR6L, a novel PAK4 interaction protein, regulates PAK4-mediated migration of human gastric cancer cell via LIMK1. Int J Biochem Cell Biol. 2010;42:70–79. doi: 10.1016/j.biocel.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, Inokuchi M, Takagi Y, Otsuki S, Fujimori Y, Sato Y, Yanaka Y, Higuchi K, Aburatani T, Tomii C, Uetake H, Kojima K, Kawano T. Prognostic significance of PAK4 expression in gastric cancer. J Clin Pathol. 2016;69:580–5. doi: 10.1136/jclinpath-2015-203330. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Zhang Y, Li Z, Wang X, Qu X, Liu Y. Activated Pak4 expression correlates with poor prognosis in human gastric cancer patients. Tumour Biol. 2015;36:9431–9436. doi: 10.1007/s13277-015-3368-4. [DOI] [PubMed] [Google Scholar]
- 12.Ahn HK, Jang J, Lee J, Se Hoon P, Park JO, Park YS, Lim HY, Kim KM, Kang WK. P21-activated kinase 4 overexpression in metastatic gastric cancer patients. Transl Oncol. 2011;4:345–349. doi: 10.1593/tlo.11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siu MK, Wong ES, Chan HY, Kong DS, Woo NW, Tam KF, Ngan HY, Chan QK, Chan DC, Chan KY, Cheung AN. Differential expression and phosphorylation of Pak1 and Pak2 in ovarian cancer: effects on prognosis and cell invasion. Int J Cancer. 2010;127:21–31. doi: 10.1002/ijc.25005. [DOI] [PubMed] [Google Scholar]
- 14.Siu MK, Chan HY, Kong DS, Wong ES, Wong OG, Ngan HY, Tam KF, Zhang H, Li Z, Chan QK, Tsao SW, Stromblad S, Cheung AN. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci U S A. 2010;107:18622–18627. doi: 10.1073/pnas.0907481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang HJ, Siu MK, Yeung MC, Jiang LL, Mak VC, Ngan HY, Wong OG, Zhang HQ, Cheung AN. Overexpressed PAK4 promotes proliferation, migration and invasion of choriocarcinoma. Carcinogenesis. 2011;32:765–771. doi: 10.1093/carcin/bgr033. [DOI] [PubMed] [Google Scholar]
- 16.He PP, Ouyang XP, Tang YY, Liao L, Wang ZB, Lv YC, Tian GP, Zhao GJ, Huang L, Yao F, Xie W, Tang YL, Chen WJ, Zhang M, Li Y, Wu JF, Peng J, Liu XY, Zheng XL, Yin WD, Tang CK. MicroRNA-590 attenuates lipid accumulation and pro-inflammatory cytokine secretion by targeting lipoprotein lipase gene in human THP-1 macrophages. Biochimie. 2014;106:81–90. doi: 10.1016/j.biochi.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci. 2005;118:1861–1872. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- 18.Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, Belisle B, Minden A. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 1998;17:6527–6540. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem. 2001;276:32115–32121. doi: 10.1074/jbc.M100871200. [DOI] [PubMed] [Google Scholar]
- 20.Li RK, Guo J. Single nucleotide variances can account for loss of microRNA function: the emerging cross talk between genetics and epigenetics. J Am Coll Cardiol. 2014;64:278–280. doi: 10.1016/j.jacc.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Qu J, Cammarano MS, Shi Q, Ha KC, de Lanerolle P, Minden A. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol Cell Biol. 2001;21:3523–3533. doi: 10.1128/MCB.21.10.3523-3533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X, Lee HJ, Suh N, Yang CS, Minden A. The pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol Cancer Res. 2008;6:1215–1224. doi: 10.1158/1541-7786.MCR-08-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao YG, Ning K, Li F. Group II p21-activated kinases as therapeutic targets in gastrointestinal cancer. World J Gastroenterol. 2016;22:1224–1235. doi: 10.3748/wjg.v22.i3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells CM, Abo A, Ridley AJ. PAK4 is activated via PI3K in HGF-stimulated epithelial cells. J Cell Sci. 2002;115:3947–3956. doi: 10.1242/jcs.00080. [DOI] [PubMed] [Google Scholar]
- 25.Nekrasova T, Minden A. PAK4 is required for regulation of the cell-cycle regulatory protein p21, and for control of cell-cycle progression. J Cell Biochem. 2011;112:1795–1806. doi: 10.1002/jcb.23092. [DOI] [PubMed] [Google Scholar]
- 26.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 27.Bates S, Bonetta L, MacAllan D, Parry D, Holder A, Dickson C, Peters G. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene. 1994;9:71–79. [PubMed] [Google Scholar]


