Abstract
Background/Objective: IFNs induce potent antiviral and antitumor activities. β-catenin pathway is a surviving pathway adapted by carcinogenetic mechanisms of various cancers. Crosstalk between these pathways has not been well described in lung cancer cells. Methods: Lung cancer cell lines, A549 and Calu-3, were used in this study. β-catenin protein levels and signaling activities were tested by flow cytometry and luciferase assay. Cell proliferation was measured by counting viable cells under microscope, and apoptosis by TUNEL assay and caspase 3 activation. DKK1 and GSK3β levels were tested by flow cytometry. Secreted DKK1 was measured by ELISA. αDKK1 , FLUD and S3I were to inhibit DKK1, STAT1 and STAT3 activities, respectively. Results: All of IFNα, IFNγ and IFNλ1 suppressed β-catenin signaling in A549 and Calu-3 cells, where IFNγ was the strongest (P<0.05). They inhibited cellular proliferation and promoted apoptosis. IFNγ gave greater induction ability compared to IFNα and IFNλ1 (P<0.05). All tested IFNs promoted DKK1 activation but not GSK3β in A549 and Calu-3 cells. IFNs activated STAT1 and STAT3. But only STAT3 was vital for IFN-mediated DKK1 activation and apoptosis. Plus, DKK1 antagonist abrogated IFN-mediated apoptosis. The degree of STAT3 activation was corresponding to the level of apoptosis induced by different IFNs (P<0.05). Conclusions: In lung cancer cells, all three types of IFNs can induce apoptosis via suppressing β-catenin signaling by a STAT3- and DKK1-dependent manner. This findings demonstrate a link between IFNs and β-catenin signaling, which may possess potentials on the development of novel therapeutic measures against lung cancer.
Keywords: β-catenin, interferon, lung cancer, crosstalk
Introduction
Lung cancer is known as the most common cause of cancer-related death in the world. Its prevalence is rising rapidly compared to other cancers due to air pollution, smoking and other possible causes [1].
Interferons (IFNs) are pleiotropic cytokines that are known to be involved in various physiological and pathological processes, such as anti-microbial and anti-tumor immunity [2-8]. These activities are believed to be mediated by regulating a variety of genes, and facilitating pre-apoptotic responses of infected cells [9,10].
Downstream activities induced by IFNs are mostly mediated by the JAK-STAT (Janus Kinase-Signal Transducer and Activator of Transcription) signaling pathway [11,12]. The activation of JAK-STAT signaling pathway requires binding of IFN and its receptor, leading to phosphorylation and activation of different JAK kinases and STAT family members. Activated STATs will then form dimers, which is translocated into the nucleus, where they bind interferon-stimulated response element (ISRE) or γ-activated sequences (GAS) in the promoter regions of IFN-regulated genes, which interacts with other transcriptional factors and finally regulates IFN-responsive genes. About 500 genes are regulated by IFN-JAK-STAT pathway, including GTPase and suppressor of cytokine signaling I (SOCS-1) [11,12].
β-catenin plays key roles in the canonical pathway of Wnt-β-catenin signaling, which regulates cell proliferation, differentiation, and carcinogenesis [13,14]. The canonical β-catenin pathway is initiated by the binding of Wnt proteins to their receptor, which consequently inhibits the β-catenin destruction complex (GSK3β, axin, APC, and Ck1), leading to accumulation of activated β-catenin. Together with other co-activators, activated β-catenin activates transcription factors such as T-cell factor/lymphoid enhancer (TCF/LEF), and aims at the regulation of target gene [13].
Induction of apoptosis is important in anti-tumor immunity, therefore, interruption of apoptosis is always crucial in the development of cancer, and enhancement or activation of apoptosis could be a potential anti-tumor therapeutic strategy [15]. There are almost four hundred genes that could be regulated by IFNs and are involved in apoptosis [16-19]. Prior publications have demonstrated potent antitumor/apoptotic potential of IFNs in many cancer cells including lung cancers [16,20,21]. Even the latest IFNλs, especially IFNλ1, have been proven capable of activating downstream anti-proliferative/apoptotic signaling in selected cancer cells [20-22]. But detailed underlying mechanisms on how IFNs promote apoptosis, and the roles of β-catenin pathway in it in lung cancer cells have not yet been discovered.
In our study, we tried to discover and compare the effects of IFNα, IFNγ and IFNλ1 in inhibiting β-catenin signaling and promoting apoptosis induction in lung cancer cells. Critical signaling components in this signaling network have also been investigated and revealed. The data of our study may help develop novel IFN-based anti-tumor medications against lung cancer or related therapeutic approaches.
Materials and methods
Cell lines and reagents
The hepatocellular carcinoma cell lines A549 and Calu-3 were obtained from PriCell Research Institute. They were propagated in DMEM (Gibco Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated FBS (Sigma, St. Louis, MO) and 1% penicillin-streptomycin (Gibco Invitrogen). The cells were used at approximately 80% confluence. Human recombinant IFNγ, GSK3β antibody (pY216), pSTAT1 (S727)-AF647 mAb, pSTAT3 (pY705)-AF488, caspase 3-AF488 were purchased from BD Pharmingen (San Jose, CA). APC and FITC-conjugated goat anti-mouse antibodies and FITC-bovine anti-goat IgG (H+L) antibody were purchased from Jackson ImmunoResearch Lab Inc (West Grove, PA). Human recombinant IFNα, IFNλ (IFNλ1, IL29) and Fludarabine (FLUD) were purchased from Sigma-Aldrich (St. Louis, MO). STAT3 inhibitor V, Stattic, STAT5 inhibitor, and GSK3β inhibitor IX were purchased from CalBiochem/EMD biosciences (Gibbstown, NJ). hDKK1 neutralizing antibody was purchased from R&D Systems (Minneapolis, MN) while DKK1 detection antibody was purchased from Abcam (Cambridge, MA). DKK1 ELISA was purchased from RayBiotech (Norcros, GA) and used as recommended.
All inhibitors were tittered starting with the reported most effective concentration according to the publications and manufacturers’ instructions, and in the range of 500 times of it.
DNA constructs and transfection
A549 and Calu-3 lung cancer cell lines were transiently transfected using the LT-1 transfection reagent (Mirus Bio LLC; Madison, WI) as recommended by the manufacturer. To measure β-catenin-dependent signaling activity, 5×106 cells were transfected with 10 μg TOPflash reporter construct (Millipore; Billerica, MA). TOPflash construct consists of two sets of three TCF/LEF binding sites linked to a luciferase reporter. The cells were also co-transfected with 1 ng Renilla construct (Promega, Madison, WI) to normalize for transfection efficiency and green fluorescent protein (GFP) (pMaxGFP, Lonza, Biologics, Portsmouth, NH) to equalize the amount of total DNA used per transfection condition. Firefly and Renilla luciferase activity were measured using dual luciferase assay reporter system (Promega, Madison, WI).
Immunofluorescence staining and flow cytometry analysis
To detach cells without cleaving surface proteins, cells were incubated with 1 mM EDTA for 5 min then washed and suspended in PBS. Cells were stained with appropriate target antibodies and isotype antibodies using conventional surface and/or intracellular staining methods. When both surface and intracellular staining were prepared, cells were first fixed and made permeable using BD Cytofix/Cytoperm, Fixation and Permeability Solution (BD Pharmingen, San Jose, CA), followed by staining for intracellular proteins. Cells were washed extensively with 1×PBS to remove excess antibodies, stained for extracellular targets, and fixed with 2% formaldehyde. Fluorescence was evaluated with a FACSCalibur flow cytometry and data analyzed using FlowJo software (TreeStar, Ashland, OR).
Proliferation and cell viability assays
Cell viability assays were performed as described previously [3]. Briefly, to determine cell viability, equal amount of cells (105/well) were plated in wells of 6-well plates and transfected and/or treated, as indicated in the text. Dead cells lost their attachment and were washed away by 1×PBS. Viable (adherent) cells were released from the wells by trypsinization before cell counting.
TUNEL assay
TUNEL assay was performed according to the manufacturer’s suggested protocols (Promega). 3-6×106 cells were briefly trypsinized, washed twice with cold PBS, fixed in 4% paraformaldehyde at 4.0°C for 15 minutes, and then washed again with PBS and made permeable with 0.5 ml 0.5% saponin at 22.0°C for 10 minutes. The cells were washed with PBS, incubated with 80 μl equilibration buffer at 22.0°C for 5 minutes, washed with PBS, re-suspended in 50 μl Nucleotide Mix and incubated in the dark at 37.0°C for 1 hr. Cells were washed with PBS and analyzed by fluorescence microscopy.
Statistical analysis
Statistical analyses were performed using Prism software (GraphPad Prism, San Diego, CA). Untreated and treated groups were compared using the Student’s t-Test with the data normally distributed. Abnormally distributed data had the two groups compared using the non-parametric Mann-Whitney test. All tests were two-tailed and a p-value <0.05 was considered significant.
Results
All three types of IFNs suppress β-catenin level and activity in A549 and Calu-3 cells
In our study, IFNα, IFNγ and IFNλ1 were shown to be able to down-regulate β-catenin in lung cancer cell lines, A549 and Calu-3. These cell lines were co-transfected with a TCF/LEF firefly luciferase construct (TOPflash) and a control reporter (Renilla luciferase). Transfected A549 and Calu-3 cells were left untreated or treated with IFNα (100 ng/ml), IFNγ (100 ng/ml) and IFNλ1 (100 ng/ml), respectively. The TOPflash reporter construct is to show basal and induced levels of β-catenin-related signaling. At day 1 post-IFN stimulation, IFNα, IFNγ and IFNλ significantly reduced β-catenin signaling by around 50%, 60% and 40%, respectively. The reduction in A549 and Calu-3 cells was similar (Figure 1A, 1C). Suppression of β-catenin signaling was consistent with active β-catenin inhibition in A549 and Calu-3 cells, by intracellular flow cytometry (Figure 1B, 1D). These data indicated that all types of IFNs can inhibit β-catenin signaling in lung cancer cell lines, among which IFNγ is the most potent (P<0.05).
Figure 1.
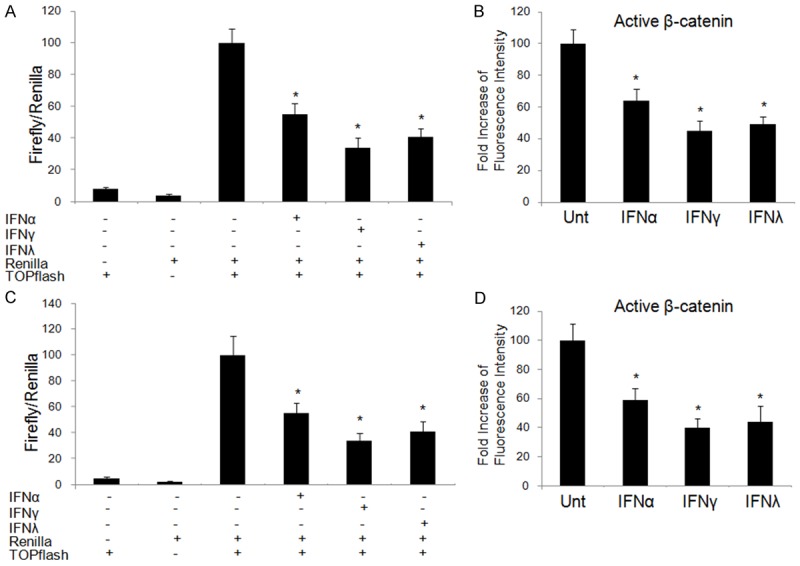
IFNs down-regulate β-catenin activation and signaling activity. Lung cancer cell lines, A549 (A) and Calu-3 (C), were left untreated or treated with IFNα (100 ng/ml), IFNγ (100 ng/ml) or IFNλ1 (100 ng/ml) for 24 hrs before transfection with TOPflash luciferase and Renilla luciferase constructs. After 4 hrs, the cells were cultured with or without initial treatment of different IFNs. Dual luciferase activity was measured 24 hrs later. Data shown is normalized to Renilla activity. A549 (B) and Calu-3 (D) were treated with or without IFNα (100 ng/ml), IFNγ (100 ng/ml) or IFNλ1 (100 ng/ml) for 48 hrs and expression of hypophosphorylated/active β-catenin level was measured by flow cytometry. Data represent a minimum of three experiments and asterisks denote P<0.05 in comparison to untreated samples.
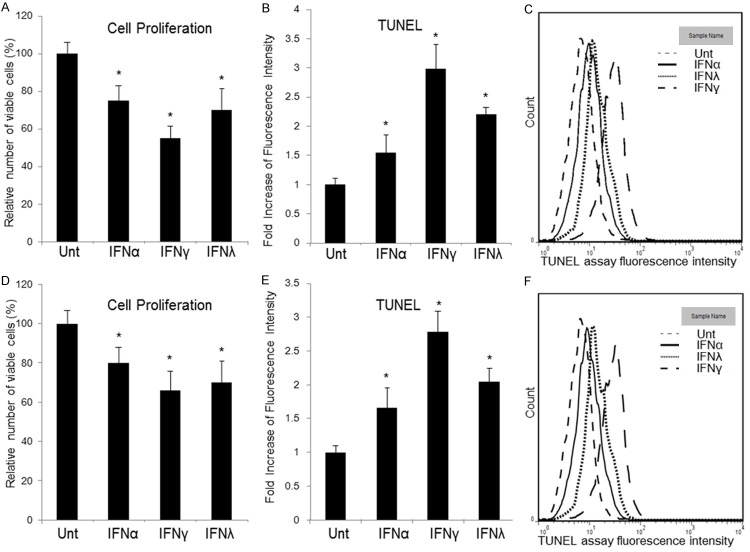
IFNs inhibit lung cancer cell line proliferation and induce apoptosis
Proliferation of lung cancer cells was investigated. A549 and Calu-3 cells were untreated or treated with IFNα (100 ng/ml), IFNγ (100 ng/ml) and IFNλ1 (100 ng/ml) for 3 days, and viable cells were counted. We found that, compared with untreated controls, all three types of IFNs reduced viable A549 cell counts by around 25%, 45%, and 25%, respectively, where IFNγ was the most potent (P<0.05) (Figure 2A). Same pattern was found in Calu-3 cells (Figure 2D).
Figure 2.
Cell proliferation and apoptosis can be regulated by IFNs via β-catenin signaling in HCC. A549 (A) and Calu-3 (D) cells were untreated or treated with IFNα (100 ng/ml), IFNγ (100 ng/ml) or IFNλ1 (100 ng/ml) for 72 hours. Viable cells were then counted. The abscissa represents different types of stimulation. The ordinate represents percentage of viable cells relative to mock cells. Apoptosis in A549 (B, C) and Calu-3 (E, F) was measured by TUNEL assay. The ordinate represents fold increase of fluorescence intensity relative to untreated cells. Data represent a minimum of three experiments and asterisks denote P<0.05 in comparison to untreated samples.
To test whether apoptosis was induced by IFNs in lung cancer cells, TUNEL assay was used to detect DNA fragmentation in apoptosis, and was performed on A549 cells, treated with or without different IFNs for 3 days, respectively. Apoptosis was induced by 1.8-, 3.0-, and 2.0-folds by TUNEL assay when treated with IFNα, IFNγ and IFNλ1, respectively, among which IFNγ was the most potent (P<0.05) (Figure 2B, 2C). Caspase 3 activation was also tested to confirm apoptosis. The results matched the patterns of TUNEL assay (Figure 3A). Anti-proliferative effects and apoptosis induced by different IFNs in A549 cells were similar to those in Calu-3 cells (Figures 2D-F, 3B). IFNγ was always the most potent one, compared with IFNα and IFNλ1 in both cell lines (P<0.05). It is clear that IFNs induces anti-proliferative effects and apoptosis lung cancer cell lines.
Figure 3.
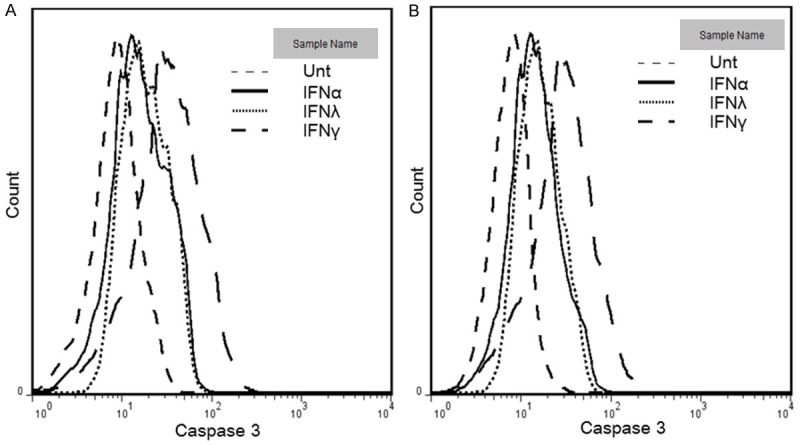
Apoptosis can be regulated by IFNs via β-catenin signaling in HCC. A549 (A) and Calu-3 (B) cells were untreated (dashed line) or treated with IFNα (100 ng/ml, solid line), IFNγ (100 ng/ml, long dashed line) or IFNλ1 (100 ng/ml, dotted line) for 72 hours. Active caspase 3 was measured by flow cytometry. Data represent a minimum of three experiments and asterisks denote P<0.05 in comparison to untreated samples.
IFNs induced DKK1, but not GSK3β in lung cancer cells
To find out the mechanisms on how IFNs suppress β-catenin signaling activity, we evaluated the correlation between IFN stimulation and levels of two potent endogenous antagonists of the canonical β-catenin pathway, DKK1 and GSK3β [23]. DKK1 expression was significantly induced by IFNs in A549 and Calu-3 lung cancer cells, showed by both flow cytometry and ELISA. The levels of DKK1 induction was the higher in cells stimulated by IFNγ treatment if compared with those by IFNα and IFNλ1 (P<0.05, respectively) (Figure 4A, 4C). GSK3β expression was not induced by any IFN stimulation in either lung cancer cell lines (Figure 4B, 4D).
Figure 4.
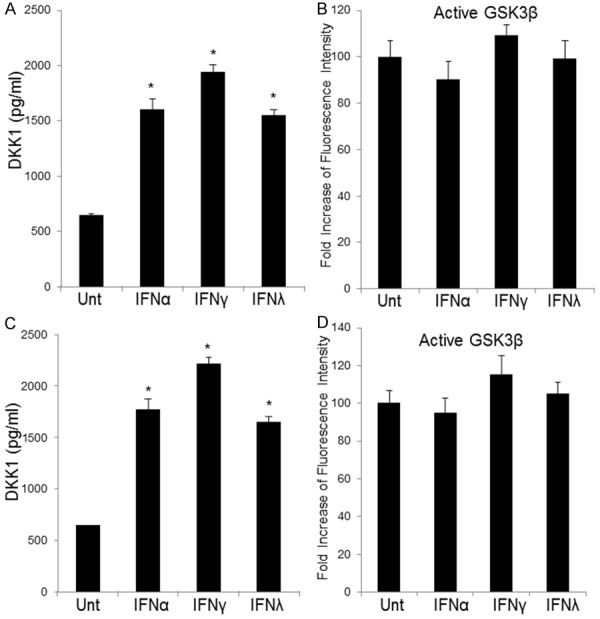
IFNs up-regulate DKK1 expression but show no effects on GSK3β. A549 and Calu-3 cells were treated with or without IFNα (100 ng/ml), IFNγ (100 ng/ml) or IFNλ1 (100 ng/ml), and levels of DKK1 were measured by ELISA (A, C) at 48 hours post-treatment. Activated GSK3β level was evaluated by flow cytometry in A549 and Calu-3 cells at 48 hours upon different IFN stimulation (B, D). Data represent a minimum of three experiments and asterisks denote P<0.05 in comparison to untreated samples.
IFN induced-apoptosis in lung cancer cells is dependent on DKK1 and STAT3 activation
JAK-STAT pathway could be induced by all IFNs and many other cytokines to conduct downstream signaling. STAT family members are induced selectively by various IFNs. Previous studies have shown that STAT1 and STAT3 could be activated by all IFNs [12]. First, we tested the activation of STAT1 and STAT3 by IFNα, IFNγ and IFNλ1 in A549 and found that all three IFNs activated STAT1 and STAT3, among which IFNα was strongest in STAT1 activation, and IFNγ was the most potent in STAT3, while IFNλ1 was weak in both (P<0.05, respectively). The pattern of STAT3 activation, but not STAT1, by different IFNs was consistent to the intensity of apoptosis induction with corresponding IFNs (Figure 5). Similar results were seen in Calu-3 cells (data not shown).
Figure 5.
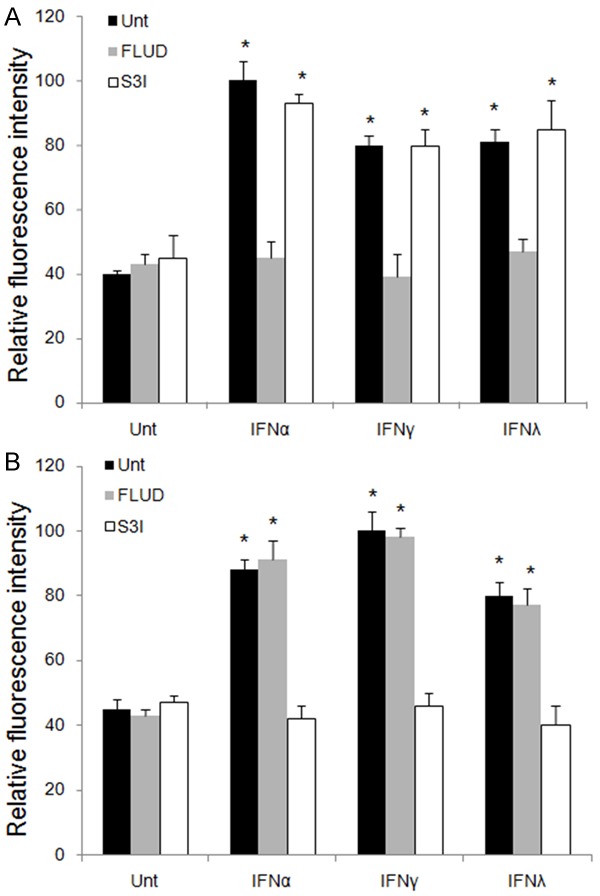
STAT activation induced by IFNs in HCC. A549 cells were untreated or treated with IFNα, IFNγ or IFNλ1 for 0.5 hour in the absence (black bars) or presence of STAT1 inhibitor (FLUD, grey bars) or STAT3 inhibitor (S3I, open bars). Active STAT1 (A) and STAT3 (B) levels were measured by flow cytometry. Data represent a minimum of three independent experiments. Asterisks denote P<0.05 in comparison to untreated samples.
To test the importance of DKK1, STAT1 and STAT3 in IFN-mediated signaling in lung cancer cells, A neutralizing antibody targeting DKK1 (αDKK1), STAT1 inhibitor (FLUD), and STAT3 inhibitor (S3I) were used. αDKK1 abrogated the ability of IFNs to induce DKK1 and reduced the level of DKK1 compared to untreated groups in both A549 and Calu-3 cells (Figure 6). These results imply that lung cancer cells constitutively express DKK1, and DKK1 is critical for IFN-mediated DKK1 activation and downstream suppression of β-catenin. We next investigated the inhibitory effects of FLUD and S3I on IFNα, IFNγ and IFNλ1 signaling pathways. Our data showed that FLUD and S3I effectively inhibited STAT1 and STAT3 activation, respectively, in A549 (Figure 5) and Calu-3 cells (data not shown). IFN-mediated DKK1 up-regulation in A549 and Calu-3 cells were blocked by S3I, but not FLUD, suggesting that STAT3, but not STAT1, is required for IFN-mediated DKK1 up-regulation (Figure 6). The results indicate that STAT3 should be upstream to DKK1 in IFN signaling in lung cancer cells and is critical for IFN-mediated DKK1 induction.
Figure 6.
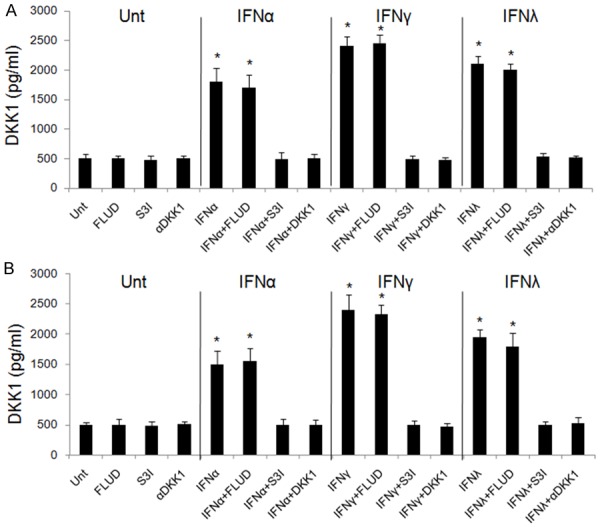
IFN-induced stimulation of DKK1 is STAT3-dependent. A549 (A) and Calu-3 (B) cells were untreated or treated with FLUD, S3I, or αDKK1 alone or combined with IFNα, IFNγ or IFNλ1, respectively for 48 hours. DKK1 levels were evaluated by ELISA. Data represent a minimum of three independent experiments. Asterisks denote P<0.05 in comparison to untreated samples.
Since DKK1, STAT1 and STAT3 could be induced by all types of IFNs, we next investigated the roles of DKK1, STAT1 and STAT3 to IFN-mediated apoptosis in lung cancer cell lines. Stimulation of αDKK1 or S3I abrogated the apoptosis induced by IFNα, IFNγ and IFNλ1 in A549 and Calu-3 cells, tested by TUNEL assay. STAT1 inhibitor, FLUD, on the contrary showed no impact on IFN-mediated apoptosis, indicating that STAT1 had no significant role in this regulation (Figure 7). These suggest that IFN-mediated apoptosis in lung cancer cell lines is DKK1- and STAT3-dependent.
Figure 7.
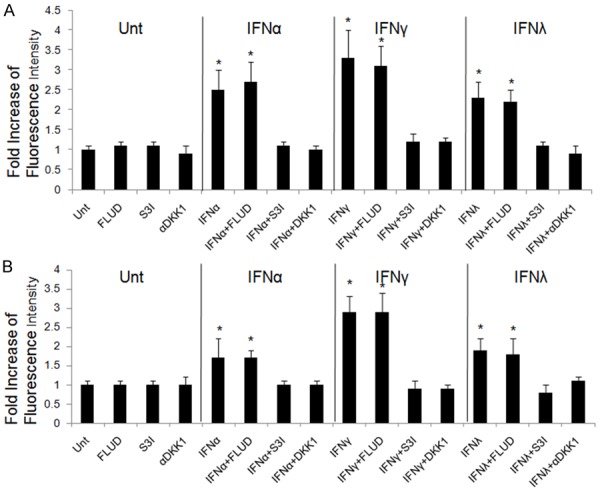
IFN-induced apoptosis in lung cancer cells is DKK1 and STAT3-dependent. A549 (A) and Calu-3 (B) cells were left untreated or treated with FLUD, S3I, or αDKK1 alone or in combination with IFNα, IFNγ or IFNλ1, respectively for 72 hrs. The levels of induced apoptosis were measured by TUNEL assay. Data represent a minimum of three independent experiments. Asterisks denote P<0.05 in comparison to untreated samples.
Discussion
There are three types of IFNs, type I, type II and type III IFNs. IFN signaling is a critical for the innate immunity and has a broad effect on the immune system. β-catenin signaling is involved in cell proliferation and differentiation. These two pathways have been reported to have a cross-regulation in previous publications [20,21,24,25]. Nava et al discovered that IFNγ exerted a proliferative and apoptotic effect on intestinal epithelial cell via DKK1 and β-catenin signaling [26]. Li et al reported the crosstalk between IFNγ signaling and β-catenin signaling via STAT3 and DKK1 in human astrocytes and its correlation to HIV replication [25]. The same research group also showed that β-catenin can also regulate IFN signaling [27]. Another publication indicated that IFNα treatment lead to export of nuclear β-catenin [28]. In rat livers, in vivo IFNα2b stimulation could attenuate Wnt/β-catenin pathway and promote programmed cell death [29]. A previous study showed that IFNα2 suppress β-catenin signal by down-regulating β-catenin and Frizzled 7 receptor proteins, and the interaction of β-catenin with TCF4, in liver cancer cells [30]. The crosstalk between IFN and β-catenin signaling has been confirmed, but the detailed mechanisms and in what kinds of cells or tissues still remain unclear.
Our study showed that all three types of IFNs inhibit activation of β-catenin and β-catenin signaling activity in different lung cancer cell lines. Among IFNs used in this study, IFNγ was the strongest inhibitor compared with the other two IFNs (P<0.05) (Figure 1). Our results demonstrated that IFNα, IFNγ and IFNλ1 showed anti-proliferative and apoptotic effects in lung cancer cells (Figure 2). We also found that IFNγ was the strongest among all three types of IFNs in apoptosis induction (Figure 2). We investigated STAT1 and STAT3 activation by different IFNs and discovered that both could be activated by all tested IFNs. By using specific STAT antagonists, we showed that only STAT3 was indispensable for IFN-mediated apoptosis in lung cancer cells. In addition, the pattern of STAT3 activation, not STAT1, by different IFNs was consistent to the degree of apoptosis induced by corresponding IFNs (Figures 5, 7). Our data supports the increasing body of evidences on the interface between different IFNs and β-catenin signaling in different cell types [31,32]. To our knowledge, there have not been any prior reports on STAT1-independent activity mediated by IFNλ1 in lung cancer cells. Additionally, we linked the crosstalk between IFNs and β-catenin signaling to apoptosis induction, which is one of the dominant mechanisms used by anti-tumor medications.
β-catenin pathway is tightly regulated by a number of proteins, including DKK1, a potent secreted endogenous antagonist [13]. We found that in tested lung cancer cell lines, DKK1 was induced by all types of IFNs, among which IFNγ was the most potent one. IFN-mediated DKK1 elevation could be abrogated by STAT3 antagonist, but not by STAT1 antagonist (Figure 6). As a key signaling pathway in proliferation and differentiation, β-catenin pathway crosslinks extensively with other signaling cascades, including PI3K/Akt and p38/MAPK pathways, which converge on GSK3β [13]. In our study, IFNs showed zero effect on GSK3β in lung cancer cells (Figure 4). This is consistent with a previous publication in human brain astrocytes [25]. It appears clear that GSK3β is not a target for IFN signaling.
Currently, there are very few studies to compare the activities induced by these three types of IFNs in lung cancer cells, especially for type III IFNs (IFNλs), the latest group of IFNs [33]. β-catenin pathway is a proliferative signaling, adapted by the oncogenesis of various cancers, such as HCC [13,14]. Since IFNs have demonstrated antitumor and apoptotic activities in many cancer cells, there is a great chance that IFN may regulate β-catenin signaling, directly or not. The crosslink between IFN and β-catenin signaling in lung cancer cells has not been reported yet. We are the first to reveal this interaction. Further investigation on how IFNs regulate β-catenin signaling is required and may benefit the therapeutic application of IFNs on lung cancer patient management.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, Chen D. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. Biomed Res Int. 2015;2015:506872. doi: 10.1155/2015/506872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Lewis-Antes A, Huang J, Balan M, Kotenko SV. Regulation of apoptosis by type III interferons. Cell Prolif. 2008;41:960–979. doi: 10.1111/j.1365-2184.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao M, Wang X, Du J, Liu L, Jiao Y, Wu H, Zheng J, Li W. Cytokine levels are associated with the severity of varicella infections. J Infect Dev Ctries. 2015;9:190–196. doi: 10.3855/jidc.5255. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Zhu Y, Wu H, Jiao Y, Van Halm-Lutterodt N, Li W. IL-6 and IFNgamma are elevated in severe mumps cases: a study of 960 mumps patients in China. J Infect Dev Ctries. 2014;8:208–214. doi: 10.3855/jidc.3557. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Li W, Yang X, Zhang T, Wang Y, Zhong R, Jiao Y, Li T, Jiang T, Tian Y, Wu H. Interleukin-8 is elevated in severe hand, foot, and mouth disease. J Infect Dev Ctries. 2014;8:94–100. doi: 10.3855/jidc.3542. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Sun G, Yu Y, Li N, Chen M, Jin R, Jiao Y, Wu H. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis. 2014;58:225–232. doi: 10.1093/cid/cit675. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, Zhao X, Wang W, Wu H, Chen M, Hua W, Wang H, Wei T, Jiao Y, Sun G, Li W. Diagnostic performance of interferon-gamma releasing assay in HIV-infected patients in China. PLoS One. 2013;8:e70957. doi: 10.1371/journal.pone.0070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platanias LC. Introduction: interferon signals: what is classical and what is nonclassical? J Interferon Cytokine Res. 2005;25:732. doi: 10.1089/jir.2005.25.732. [DOI] [PubMed] [Google Scholar]
- 10.Parmar S, Platanias LC. Interferons. Cancer Treat Res. 2005;126:45–68. doi: 10.1007/0-387-24361-5_3. [DOI] [PubMed] [Google Scholar]
- 11.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 12.Schindler C, Plumlee C. Inteferons pen the JAK-STAT pathway. Semin Cell Dev Biol. 2008;19:311–318. doi: 10.1016/j.semcdb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvory-Sobol H, Sagiv E, Kazanov D, Ben-Ze’ev A, Arber N. Targeting the active beta-catenin pathway to treat cancer cells. Mol Cancer Ther. 2006;5:2861–2871. doi: 10.1158/1535-7163.MCT-06-0122. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 16.Borden EC. Review: Milstein award lecture: interferons and cancer: where from here? J Interferon Cytokine Res. 2005;25:511–527. doi: 10.1089/jir.2005.25.511. [DOI] [PubMed] [Google Scholar]
- 17.Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-pour F, Sivakumar P, Chan C, Birks C, Foster D, Clegg CH, Wietzke-Braun P, Mihm S, Klucher KM. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]
- 18.Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Kalvakolanu DV. The GRIMs: a new interface between cell death regulation and interferon/retinoid induced growth suppression. Cytokine Growth Factor Rev. 2004;15:169–194. doi: 10.1016/j.cytogfr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Huang X, Tong H, Wang Y, Zhang T, Wang W, Dai L, Li T, Lin S, Wu H. Comparison of the regulation of beta-catenin signaling by type I, type II and type III interferons in hepatocellular carcinoma cells. PLoS One. 2012;7:e47040. doi: 10.1371/journal.pone.0047040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Huang X, Liu Z, Wang Y, Zhang H, Tong H, Wu H, Lin S. Type III interferon induces apoptosis in human lung cancer cells. Oncol Rep. 2012;28:1117–1125. doi: 10.3892/or.2012.1901. [DOI] [PubMed] [Google Scholar]
- 22.Kelly C, Klenerman P, Barnes E. Interferon lambdas: the next cytokine storm. Gut. 2011;60:1284–1293. doi: 10.1136/gut.2010.222976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Tong H, Huang X, Wang W, Wu H, Lin S. High levels of beta-catenin promote IFNgamma-induced apoptosis in hepatocellular carcinoma cells. Oncol Lett. 2012;4:1092–1096. doi: 10.3892/ol.2012.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Henderson LJ, Major EO, Al-Harthi L. IFN-gamma mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the beta-catenin pathway (DKK1) in a STAT 3-dependent manner. J Immunol. 2011;186:6771–6778. doi: 10.4049/jimmunol.1100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I, Skerra A, Li L, Parkos CA, Nusrat A. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Tong H, Huang X, Wang W, Wu H, Lin S. High levels of β-catenin promote IFNγ-induced apoptosis in hepatocellular carcinoma cells. Oncol Lett. 2012;4:1092–1096. doi: 10.3892/ol.2012.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson MD, Dar MJ, Monga SP. Pegylated interferon alpha targets Wnt signaling by inducing nuclear export of beta-catenin. J Hepatol. 2011;54:506–512. doi: 10.1016/j.jhep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parody JP, Alvarez ML, Quiroga AD, Ceballos MP, Frances DE, Pisani GB, Pellegrino JM, Carnovale CE, Carrillo MC. Attenuation of the Wnt/beta-catenin/TCF pathway by in vivo interferon-alpha2b (IFN-alpha2b) treatment in preneoplastic rat livers. Growth Factors. 2010;28:166–177. doi: 10.3109/08977190903547863. [DOI] [PubMed] [Google Scholar]
- 30.Ceballos MP, Parody JP, Alvarez Mde L, Ingaramo PI, Carnovale CE, Carrillo MC. Interferon-alpha2b and transforming growth factorbeta1 treatments on HCC cell lines: are Wnt/beta-catenin pathway and smads signaling connected in hepatocellular carcinoma? Biochem Pharmacol. 2011;82:1682–1691. doi: 10.1016/j.bcp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerer JM, Lesinski GB, Radmacher MD, Ruppert A, Carson WE 3rd. STAT1-dependent and STAT1-independent gene expression in murine immune cells following stimulation with interferon-alpha. Cancer Immunol Immunother. 2007;56:1845–1852. doi: 10.1007/s00262-007-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 33.Abushahba W, Balan M, Castaneda I, Yuan Y, Reuhl K, Raveche E, de la Torre A, Lasfar A, Kotenko SV. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol Immunother. 2010;59:1059–1071. doi: 10.1007/s00262-010-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]