Abstract
The aim of this study was to evaluate the effects of miR-1180 on pancreatic cancer. We sampled adjacent cancer and carcinoma tissues from 30 pancreatic cancer patients and measured miR-1180 expression by qRT-PCR and NF-κB protein expression by immunohistochemistry. To explore the effects of this miRNA in cell culture, we used pancreatic cancer (PANC-1) cells that received only vehicle (negative control, NC), miR-1180 mimic, or miR-1180-inhibitor. Cells were treated with cisplatin to induce apoptosis. Proliferation, apoptosis, cell cycle progression, cell invasion, and cell migration were assessed in the three cell groups. The expression levels of relevant proteins (TNIP2, NF-κB, MMP-2, MMP-9, Bax, Bcl-2, p21, and cyclin D1) in each cell group were determined by western blotting. Compared with healthy tissue adjacent to carcinoma tissues, miR-1180 expression in cancer tissues was significantly enhanced (P<0.05). NF-κB protein had a similar expression pattern to miR-1180; miR-1180 expression was positively correlated with NF-κB expression. The invasion and wound healing abilities of miR-1180-inhibited cells were significantly reduced compared with the NC or miR-1180-expressing cells (P<0.05). The cell proliferation rate of miR-1180-inihibited cells was also significantly lower than that of NC or miR-1180-expressing cells (P<0.05), while the cell apoptosis and G1 phase rates of miR-1180-inihibited cells were significantly higher than the NC or miR-1180-expressing cells (P<0.05). In conclusion, suppressing miR-1180 expression may exert anti-cancer effects on pancreatic cancer cells via regulation of TNIP 2/NF-κB signaling and the downstream MMP-2/-9, Bax, Bcl-2, p21, and cyclin D1 factors.
Keywords: miR-1180, PANC-1, NF-κB, MMP-2/-9, Bax, Bcl-2, P21, Cyclin D1
Introduction
Since early diagnosis of pancreatic cancer is rare, the rate of radical surgeries is low, the prognosis is poor, and the mortality is high [1,2]. A better understanding of the mechanisms underlying pancreatic cancer will ultimately improve clinical diagnosis and advance treatment options. Human microRNAs (miRNAs) are non-coding RNAs of approximately 22 nucleotides that interact with target genes to regulate gene expression. miRNAs play important roles in proliferation, apoptosis, and metastasis of tumor cells [3,4].
NF-κB is transcription factor expressed in many cell types in vivo. NF-κB can bind to nucleotide sequences in many gene promoters and activate gene transcription and promoter activities of these genes. Furthermore, NF-κB is involved in the pathogenesis of various diseases, not only by affecting cell proliferation, apoptosis, growth, differentiation, cell cycle progression, and immune responses, but also by affecting tumor invasion and metastasis [5-7]. Studies of NF-κB in pancreatic cancer, however, have been limited. In this study, we investigated the role of miR-1180 in the inhibition of pancreatic cancer cell activity via the NF-κB signaling pathway.
Materials and methods
Tissue specimens
Fresh pancreatic cancer tissues and normal pancreas tissues were collected from 30 pancreatic cancer patients, who were diagnosed by histopathology. All samples were frozen in liquid nitrogen and stored at -80°C until analysis.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Using SYBR Green I (Invitrogen) dye according to manufacturer’s instructions, qRT-PCR was performed and analyzed using a 7500 Fast Real-Time Sequence detection system and software (Applied Biosystems). The qRT-PCR reactions for miRNAs were performed as follows: 95°C for 3 min, followed by 40 cycles (each 30 s) at 95°C, 58°C, and 72°C. miR-1180 expression levels were normalized to the expression levels of U6 small nuclear RNA (snRNA). The qRT-PCR conditions for genes were set at 95°C for 10 min, followed by 40 cycles at 95°C for 20 s, 60°C for 30 s, and 72°C for 1 min.
Immunohistochemistry (IHC)
All specimens were fixed with 10% formaldehyde solution and embedded in paraffin. Samples were then sectioned to a thickness of 4 μm. IHC was performed on the samples to detect the expression of NF-κB in pancreatic cancer and adjacent normal tissues using the streptavidin-peroxidase method.
Cell culture and transfection
Human pancreatic cancer (PANC-1) cells were purchased from the American Type Culture Collection (ATCC, USA). Cells were divided into three groups for treatment: NC (negative control), miR-1180 mimic (miR-1180), and miR-1180 inhibitor (miR-1180-in). The miR-1180 mimic, miR-1180 inhibitor, and negative control were purchased from Kingsy Biotechnology Co., Ltd. Cells were grown in RPMI-1640 (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) as recommended. We used Lipofectamine 2000 reagent (Invitrogen) to transfect oligonucleotides according to the manufacturer’s protocol. Cells were treated with cisplatin (0.4 μg/ml dissolved in PBS, Sigma) for 24 h as an apoptosis inducer.
Cell proliferation assay
Cell proliferation was assessed using an MTS (3-[4, 5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt) assay. Cells (3000) were plated in wells of a 96-well plate and MTS was added. Cells were incubated at 37°C for 4 h. Optical density was measured at 490 nm using a microplate reader (BioTek Instruments Inc.) to compare the treatment groups. Each group was repeated three times.
Flow cytometric analysis
Cells were counted in logarithmic growth phase and seeded in 6 plates. Cells were collected and washed twice with ice-cold PBS. A 70% ethanol solution was then added to fix the cells with an overnight incubation at 4°C. Cells were washed with 500 μL PBS and 10 μL RNase (10 g/L) at 37°C for 30 min. Cells were stained with 10 μL propidium iodide (PI, 1 g/L). Cell apoptosis and cell cycle progression were analyzed by flow cytometry.
Transwell cell invasion assay
Diluted extracellular matrix (ECM) glue (100 μL) was added to the Transwell chamber and incubated at 37°C for 60 min to ensure complete polymerization. Then, 600 μL RPMI-1640 medium containing 10% fetal bovine serum was added to the lower chamber and incubated for 60 min. Logarithmic PANC-1 cells were digested with 0.25% pancreatin and plated in the upper chambers at a density of 5×105 cells/well in 100 μL of cell suspension. After 48 h, the Transwell chamber was removed and a cotton swab was used to wipe the polycarbonate membrane ECM glue. The upper and lower chambers were rinsed with D-Hanks’ surface liquid rinse, cooled, and dried. Cells were stained with 0.1% crystal violet for 10 min. After removal of the excess crystal violet dye, plates were observed under a light microscope. Five fields (100× magnification) of each membrane were randomly selected for analysis. The number of cells on the surface of the polycarbonate membrane was counted. The invasion ability of the cells was expressed as the invasion cell number. The experiment was repeated three times.
Wound healing analysis
After transfection for 36 h, suspended cells were seeded in 6-well plates at a concentration of 5×105/well. The next day, a 20-μL gun head was used to plate vertical lines on the bottom of wells. Cells were then washed with PBS and incubated in serum-free medium. Photographs were obtained at 48 h to compare the migration ability of the three groups of cells.
Western blotting
The cells were centrifuged and total protein was extracted. Proteins were separated on a 15% acrylamide gel by electrophoresis and then transferred to a polyvinylidene fluoride (PVDF) membrane. Membranes were blocked in 5% skim milk for 1 h and then incubated with rabbit anti-human TNIP2, matrix metalloproteinase (MMP)-2, NF-κB, MMP-9, Bax, Bcl-2, cyclin D1, or p21 polyclonal antibodies (Abcam, 1:500) overnight at 4°C. After washing, membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (Abcam, 1:2000) at room temperature for 2 h. After washing, electrochemiluminescence (ECL) substrate was added, and images were assessed on x-ray film for data analysis and gray value detection. GAPDH was used as the internal reference.
Statistical analyses
All data were analyzed using SPSS 19.0 statistics software (IBM). Cell counts are expressed as the mean ± standard deviation (SD). Student’s t-tests were used to compare two groups. P<0.05 indicated a statistically significant difference.
Results
NF-κB and miR-1180 expression in pancreatic cancer tissue
IHC assays indicated that expression of NF-κB protein was significantly upregulated in pancreatic cancer tissue (Figure 1A) compared with the tissue adjacent to the carcinoma (Figure 1B). In addition, qRT-PCR assays demonstrated that miR-1180 expression was significantly increased in pancreatic cancer tissues (n=30) compared with the tissue adjacent to the carcinoma (P<0.05, Figure 1C). Next, we analyzed the relationship between NF-κB and miR-1180 in these samples and found that expression of NF-κB was positively correlated with miR-1180 expression (r=0.848, Figure 1D).
Figure 1.
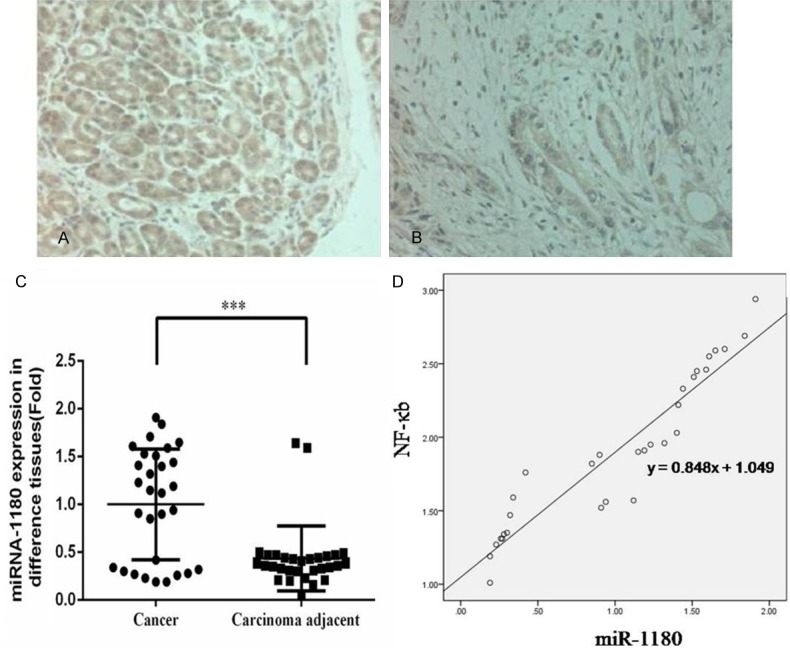
Clinical relevance analysis. A. The NF-κB protein expression in pancreatic cancer tissue by IHC (×200). B. The NF-κB protein expression in carcinoma adjacent tissue by IHC (×200). C. The miR-1180 gene expression in cancer and carcinoma adjacent tissues (Fold). ***: P<0.05, compared with NC group. D. The correlation between miR-1180 gene expression and NF-κB protein expression.
miR-1180 expression affects cell proliferation
Among the PANC-1 cells treated with NC, miR-1180 mimic, and miR-1180-in, differences in cell proliferation were noted. Cell proliferation of the miR-1180 cells was significantly increased compared to the NC cells (P<0.05). However, the cell proliferation rate of miR-1180-in cells was significantly decreased compared to that of the NC cells (P<0.05) and the miR-1180 cells (P<0.05, Figure 2).
Figure 2.
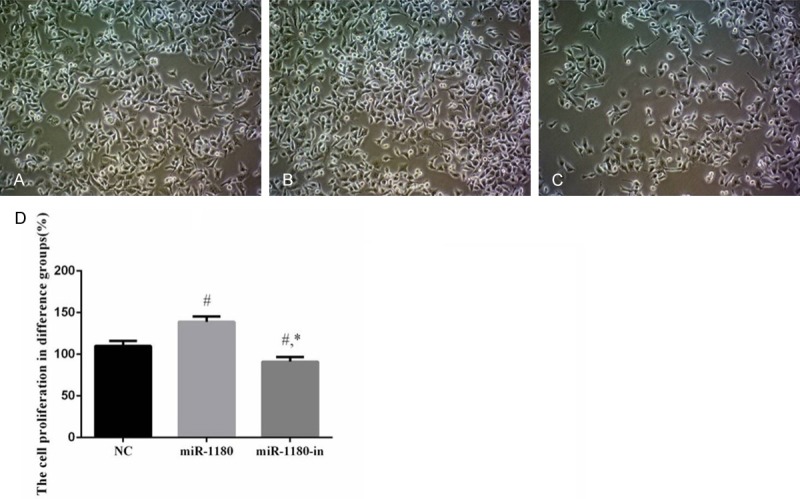
The cell proliferation of difference groups. A. The cell proliferation of NC group (×100). B. The cell proliferation of miR-1180 group (×100). C. The cell proliferation of miR-1180-in group (×100). D. Analysis the cell proliferation of difference groups. #: P<0.05, compared with NC group. *: P<0.05, compared with miR-1180 group.
miR-1180 expression affects the invasion ability of PANC-1 cells
The invasion ability of PANC-1 cells treated with the miR-1180 mimic was significantly higher than that of the NC cells (P<0.05). However, the invasion ability of PANC-1 cells treated with miR-1180-in was decreased compared to the NC and miR-1180 cells (P<0.05, Figure 3).
Figure 3.
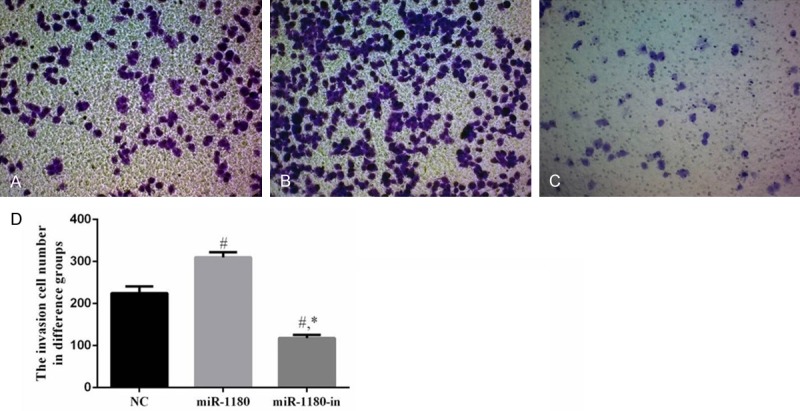
The invasion cell of difference groups. A. The invasion cell of NC group (×100). B. The invasion cell of miR-1180 group (×100). C. The invasion cell of miR-1180-in group (×100). D. The invasion cell number in difference groups. #: P<0.05, compared with NC group. *: P<0.05, compared with miR-1180 group.
miR-1180 expression impacts the migration ability of PANC-1 cells
Compared with the NC cells, the wound healing rates of PANC-1 cells treated with the miR-1180 mimic or miR-1180-in were significantly different (P<0.05). The wound healing ability of miR-1180-in cells was significantly lower than that of the miR-1180 cells (P<0.05, Figure 4).
Figure 4.
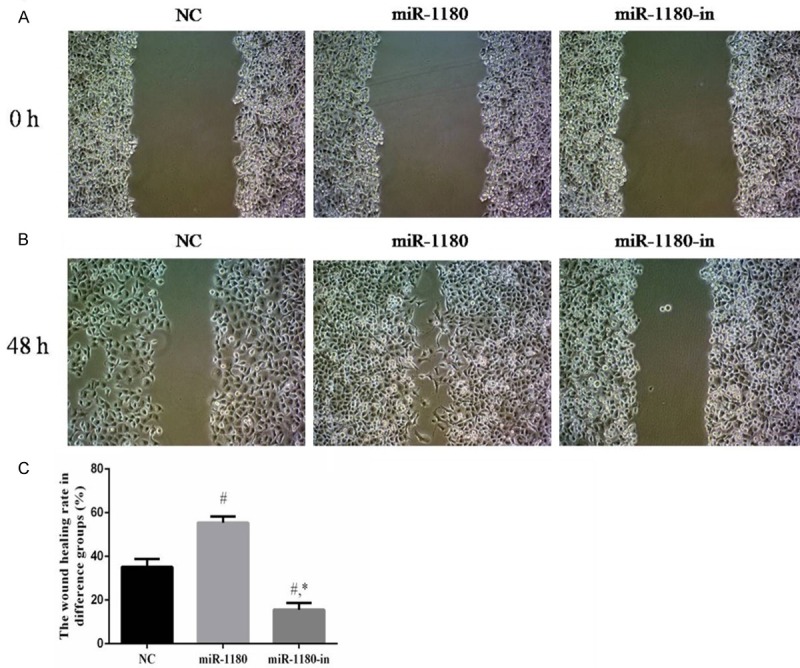
The wound healing testing of difference groups. A. The wound healing testing of difference groups at 0 h (×100). B. The wound healing testing of difference groups at 48 h (×100). C. The wound healing testing of difference groups (%). #: P<0.05, compared with NC group. *: P<0.05, compared with miR-1180 group.
miR-1180 expression affects cell apoptosis
Compared with the NC cells and the cells treated with the miR-1180 mimic, apoptosis of the miR-1180-in cells was significantly increased (P<0.05). The apoptosis rate of miR-1180 cells was significantly reduced due to the miR-1180 overexpression (P<0.05, Figure 5).
Figure 5.
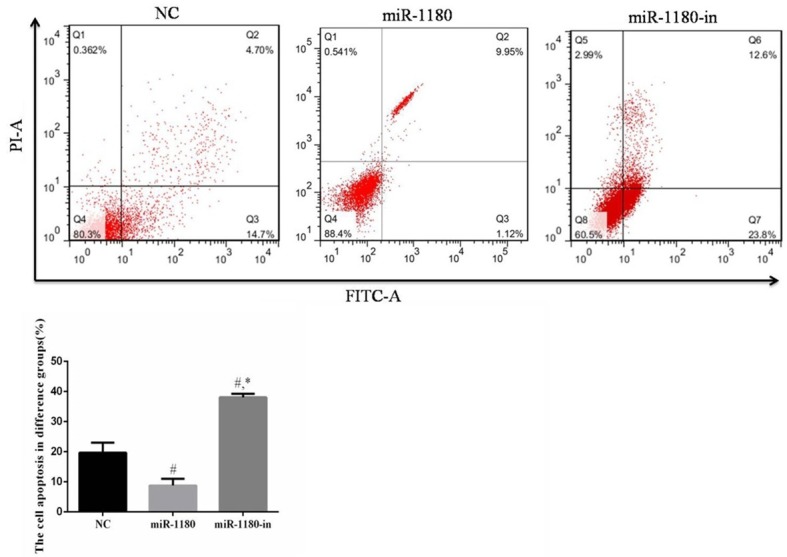
The cell apoptosis of difference groups. #: P<0.05, compared with NC group. *: P<0.05, compared with miR-1180 group.
miR-1180 expression affects cell cycle progression
Compared with the NC cells, the number of cells in the G1 phase of the miR-1180 group was significantly lower (P<0.05). However, the number of miR-1180-in cells in the G1 phase was significantly higher than that of the NC or miR-1180 cells (P<0.05, Figure 6).
Figure 6.
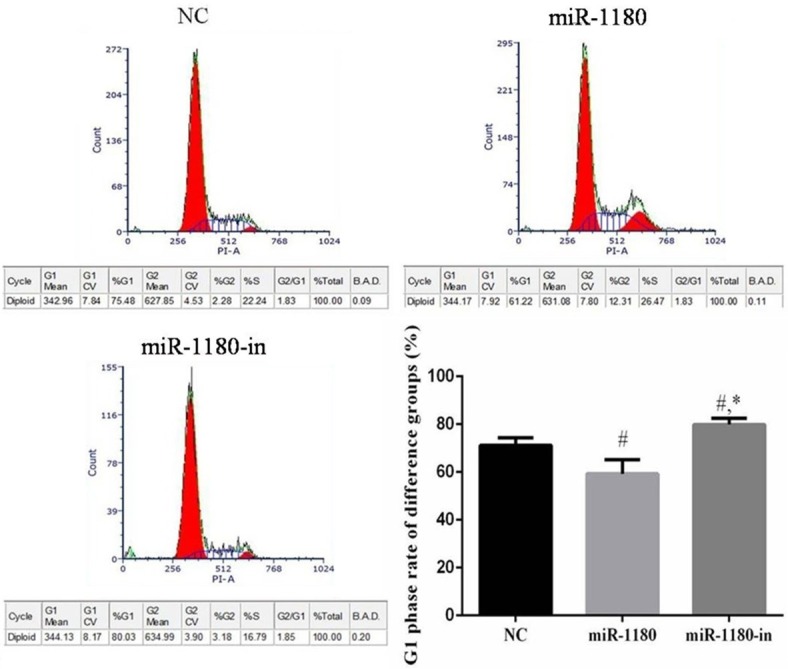
The cell cycle of difference groups. #: P<0.05, compared with G1 phase of NC group. *: P<0.05, compared with G1 phase of miR-1180 group.
Comparison of relative protein expression in the different cell groups
The protein expression of NF-κB, MMP-2, MMP-9, Bcl-2, and cyclin D1 was significantly downregulated and the expression of TNIP2, Bax, and p21 proteins were significantly upregulated in cells treated with miR-1180-in compared with expression levels in the NC cells or the cells treated with the miR-1180 mimic (P<0.05, Figure 7). Furthermore, these relative protein expression trends were different in the miR-1180 group compared with those of miR-1180 group. The relative expression of these proteins in the miR-1180 cells was significantly different compared with that in the NC cells (P<0.05, Figure 7).
Figure 7.
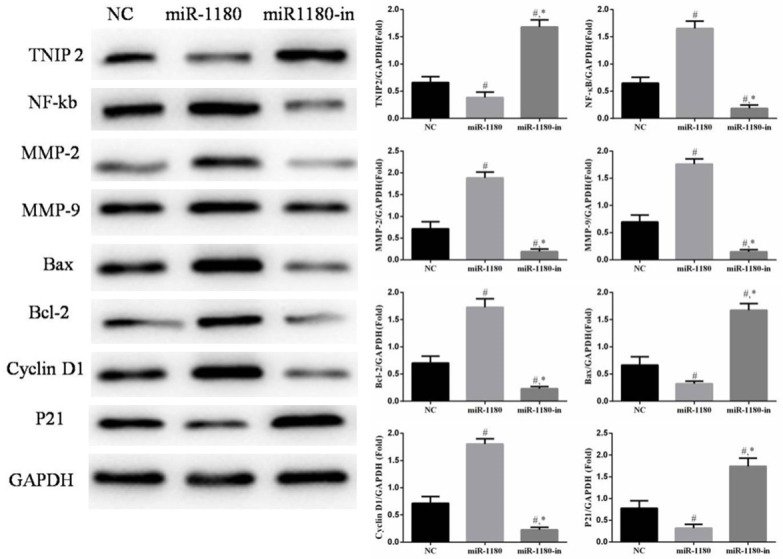
The relative proteins of difference groups. #: P<0.05, compared with NC group. *: P<0.05, compared with miR-1180 group.
Discussion
Previous studies showed that miR-1180 expression is significantly upregulated in cancer tissues and cell lines [8-10]; however, the expression of this miRNA has not been described in pancreatic cancer. Here, we demonstrated that miR-1180 and NF-κB expression were significantly increased in pancreatic cancer tissues and uncovered a positive correlation between their expression (r=0.848).
Inhibition of miR-1180 increased cisplatin-induced PANC apoptosis
Previously, miR-1180 was found to directly target TNIP 2 [8]. In the present study, we found that TNIP2 protein expression was significantly downregulated in cells treated with miR-1180-in. TNIP2 functions as a NF-κB signaling inhibitor. We found that the NF-κB pathway was stimulated by miR-1180 overexpression. Thus, this pathway is important in suppression of cell activation (cell proliferation, invasion, and migration) via regulation of NF-κB and its downstream signaling pathway in PANC-1 cells.
MMP-2 and MMP-9 are two important factors in NF-κB downstream signaling [11-13]. The invasion of tumors is regulated by these MMPs, which are zinc ion-dependent endonucleases that regulate the biological behavior of cells. The MMP family is comprised of 20 different enzymes that are classified as different subtypes according to their substrate specificities and sequence characteristics [14]. MMP-2 and MMP-9 can degrade the main component of type IV collagen in the basement membrane and ECM [15]. MMP-2 and MMP-9 are closely related to tumor invasion and metastasis [16,17]. Indeed, Farina et al. [18] reported that the expression of MMP-9 is regulated by NF-κB at the transcriptional level. Bond et al. [19] found that activation of NF-κB is essential for the upregulation of MMP-9 expression. Philip et al. [20] reported an increase in MMP-2 activity in the mouse melanoma cell line B16F10 and a concomitant activation of NF-κB. We found that inhibition of miR-1180 can effectively reduce the invasion and migration of PANC-1 cells. The mechanism underlying this effect may be related to inhibition of the activities of NF-κB, MMP-2, and MMP-9.
Bcl-2, Bax, p21, and cyclin D1 are additional players in the NF-κB signaling pathway [21-23]. Bcl-2 abrogates apoptosis induced by free oxygen radicals, inhibits the transmembrane transport of Ca2+, and inhibits cell apoptosis induced by Ca2+ [24-26]. Cyclin D1 is a key positive regulator of the G1/S phase, as this protein forms a complex with CDK4 to promote cell proliferation. In vitro studies showed that downregulation of cyclin D1 expression effectively inhibits the development of tumors [27,28]. In addition, p21 is an inhibitor of a wide range of cyclin-dependent kinases (CDKs) [29,30] and combinations of the corresponding cyclins/CDKs. Thus, CDK inactivation by overexpression of p21 halts the progression of cells in the G1 phase, leading to cell apoptosis. The results of this study confirmed that miR-1180 effectively inhibits the increase of the apoptosis factor Bax and p21 expression and inhibits the expression of Bcl-2 and the cancer-promoting factor cyclin D1, stranding many PANC-1 cells in G1 phase, thus resulting in increased apoptosis.
In conclusion, our findings suggest that suppressing miR-1180 expression may exert anti-cancer effects on pancreatic cancer cells via regulation of the TNIP2/NF-κB signaling pathway and its downstream proteins, including MMP-2, MMP-9, Bax, Bcl-2, p21, and cyclin D1.
Disclosure of conflict of interest
None.
References
- 1.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Okada H, Kohanbash G, Lotze MT. MicroRNAs in immune regulation-opportunities for cancer immunotherapy. Int J Biochem Cell Biol. 2010;42:1256–1261. doi: 10.1016/j.biocel.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llave C, Xie Z, Kaschau KD, Carrington JC. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.1076311. [DOI] [PubMed] [Google Scholar]
- 5.Nomura A, Majumder K, Giri B, Carrington JC. Inhibition of NF-kappa B pathway leads to deregulation of epithelial-mesenchymal transition and neural invasion in pancreatic cancer. Lab Invest. 2016;96:1268–1276. doi: 10.1038/labinvest.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farhadi E, Zaker F, Safa M, Rezvani MR. miR-101 sensitizes K562 cell line to imatinib through Jak 2 downregulation and inhibition of NF-κB target. Tumour Biol. 2016;37:14117–14128. doi: 10.1007/s13277-016-5205-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhong Z, Cao Y, Yang S, Zhang S. Overexpression of RRM2 in gastric cancer cell promotes their invasiveness via AKT/NF-κB signaling pathway. Pharmazie. 2016;71:280–284. [PubMed] [Google Scholar]
- 8.Tan G, Wu L, Tan J, Zhang B, Tai WC, Xiong S, Chen W, Yang J, Li H. MiR-1180 promotes apoptotic resistance to human hepatocellular carcinoma via activation of NF-κB signaling pathway. Sci Rep. 2016;6:22328. doi: 10.1038/srep22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Zhu HQ, Ma CQ, Li HG, Liu FF, Chang H, Lu J. MiR-1180 promoted the proliferation of hepatocellular carcinoma cells by repressing TNIP2 expression. Biomed Pharmacother. 2016;79:315–320. doi: 10.1016/j.biopha.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Ge Q, Wang C, Chen Z, Li F, Hu J, Ye Z. The suppressive effects of miR-1180-5p on the proliferation and tumorigenicity of bladder cancer cells. Histol Histopathol. 2017;32:77–86. doi: 10.14670/HH-11-772. [DOI] [PubMed] [Google Scholar]
- 11.Poudel B, Lee YM, Kim DK. DDR2 inhibition reduces migration and invasion of murine metastatic melanoma cells by suppressing MMP2/9 expression through ERK/NF-κB pathway. Acta Biochim Biophys Sin. 2015;47:292–298. doi: 10.1093/abbs/gmv005. [DOI] [PubMed] [Google Scholar]
- 12.Yang CJ, Liu YP, Dai HY, Shiue YL, Tsai CJ, Huang MS, Yeh YT. Nuclear HDAC6 inhibits invasion by suppressing NF-κB/MMP2 and is inversely correlated with metastasis of nonsmall cell lung cancer. Oncotarget. 2015;6:30263–30276. doi: 10.18632/oncotarget.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao C, Ma J, Zhang Y, Chu Y, Li J, Kuai R, Wang S, Peng H. Perfluorooctanoic acid enhanceds colorectal cancer DLD-1 cells invasiveness through activating NF-κB mediated matrix metalloproteinase-2/-9 expression. Int J Clin Exp Pathol. 2015;8:10512–10522. [PMC free article] [PubMed] [Google Scholar]
- 14.Sternlicht MD, Werb Z. How matrix metallo proteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran S, Murray GI. Matrix metalloproteinases in tumor invasion and metastasis. J Pathol. 1999;189:300–308. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Chang YZ, Yang MW, Wang GJ. A new spectrometry electrospray tip obtained via precise mechanical micromachining. Anal Bioanal Chem. 2005;383:76–82. doi: 10.1007/s00216-005-3376-0. [DOI] [PubMed] [Google Scholar]
- 18.Farina AR, Tacconelli A, Vacca A, Maroder M, Guling A, Mackay AR. Transcriptional upregulation of matrix metalloproteinase-9 expression during spontancous epithelial to neuroblast phenotype conversion by SK-N-Shneuroblastoma cell, involved in enhanced invasivity, dependes upon GT-box and nuclear factor-kappa B elements. Cell Growth Differ. 1999;10:363–367. [PubMed] [Google Scholar]
- 19.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcrition factor NF-κB. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 20.Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor-kappa B-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001;276:44926–44935. doi: 10.1074/jbc.M103334200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Qin F, Yang L, Xian J, Zou Q, Jin H, Wang L, Zhang L. co-operation induces malignant conversion via p53 loss, elevated NF-κB and tenascinc associated rigidity, but p21 inhibits ROCK2/NF-κB-mediated progression. Oncogene. 2016 doi: 10.1038/onc.2016.402. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Zhou Y, Zheng X, Wu X, Liang Y, Wang S, Zhang Y. Sphk1 promotes breast epithelial cell proliferation via NF-κB-p65-mediated cyclin D1 expression. Oncotarget. 2016;7:80579–80585. doi: 10.18632/oncotarget.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hockenbery DM, Zutter MK, Hickey W, Nahm M, Korsmeyer SJ. Bcl-2 protein is topo-graphically restricted is tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991;88:6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by bcl-2: release of cytochromec from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 25.Pezzella F, Tse AG, Cordell JL, Pulford KA, Gatter KC, Mason DY. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990;137:225–232. [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang TS, Han HS, Hong YC, Lee HJ, Paik NS. Prognostic value of combined analysis of Cyclin D1 and estrogen receptor status in breast cancer patients. Pathol Int. 2003;53:74–80. doi: 10.1046/j.1440-1827.2003.01441.x. [DOI] [PubMed] [Google Scholar]
- 27.Koroxenidou L, Chison LC, Porsch Hallstrom I. Long-term 17alpha-ethinyl estradiol treatment decreases cyclin E and cdk2 expression, reduces cdk2 kinase activity and inhibits S phase entry in regenerating rat liver. J Hepatol. 2005;43:478–484. doi: 10.1016/j.jhep.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Liu Z, Sturgis EM, Shi Q, Chamberiain RM, Spitz MR, Wei Q. Genetic polymorphisms of p21 are associated with risk of squamous cell carcinoma of the head and neck. Carcinogenesis. 2005;26:1596–1602. doi: 10.1093/carcin/bgi105. [DOI] [PubMed] [Google Scholar]
- 29.Huang WS, Kou YH, Kou HC, Hsieh MC, Huang CY, Lee KC, Lee KF, Shen CH, Tung SY, Teng CC. CIL-102-induced cell cycle arrest and apoptosis in colorectal cancer cells via upregulation of p21 and GADD45. PLoS One. 2017;12:e0168989. doi: 10.1371/journal.pone.0168989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang WS, Kou YH, Kou HC, Hsieh MC, Huang CY, Lee KC, Lee KF, Shen CH, Tung SY, Teng CC. CIL-102-induced Cell Cycle Arrest and Apoptosis in Colorectal Cancer Cells via Upregulation of p21 and GADD45. PLoS One. 2017;12:e0168989. doi: 10.1371/journal.pone.0168989. [DOI] [PMC free article] [PubMed] [Google Scholar]


