Abstract
Human malignant hepatocellular carcinoma (HCC) is a common tumor, which severely threatens human health and shortens longevity. The poor prognosis of HCC is primarily attributed to distant metastases. C-X-C motif chemokine 10 (CXCL10) regulates the control of several cellular and developmental processes including tumor cell proliferation, apoptosis, and cell metastasis. Previous studies have confirmed that CXCL10 functions as an oncogene in several cancers. However, the expression and biological functions of CXCL10 in HCC, especially with regard to metastasis, need further investigation. In this study, CXCL10 was found to be over expressed in invasive HCC cells and HCC clinical samples. While the over-expression of CXCL10 enhanced migration, invasion, and metastasis of HCC cells in vitro as well as in vivo, silencing of CXCL10 resulted in inhibition of HCC cell metastasis. Further, CXCL10 was found to accelerate epithelial-mesenchymal transition of HCC cells. The microarray analysis indicated that matrix metallopeptidase-2 (MMP-2) functions as a downstream factor of CXCL10. This study demonstrates that CXCL10 partakes in the metastasis of HCC by activating MMP-2 expression.
Keywords: CXCL10, hepatocellular carcinoma, EMT, MMP-2
Introduction
Hepatocellular carcinoma (HCC) is considered as the most prevalent cancer worldwide and the leading cause of cancer-related mortality among all cancers [1]. Several risk factors such as hepatitis B and C infections, xenobiotics, alcohol abuse, primary biliary cirrhosis, diabetes, non-alcoholic fatty liver disease, and genetic disorders have been identified in recent years [2]. Although the great advances in functional genomics of cancer provide an increasingly comprehensive understanding of HCC genesis and development, the molecular pathogenesis of HCC remains poorly understood [3]. Tumor resection, liver transplantation, and small molecule inhibitors of several tyrosine protein kinases are palliative treatments for HCC patients. HCC is clinically resistant to conventional chemotherapy treatments, which are rejected in any case by clinical practitioners as a treatment option for HCC because of its toxic side effects [4]. Therefore, early detection of HCC becomes important because effective treatment of small tumors is possible by surgical resection. The clinical heterogeneity of complex HCC patients combined with the lack of sensitive and early diagnostic biomarkers and treatment strategies have led to a high mortality rate in HCC patients. Meanwhile, the unfavorable prognosis of HCC is attributed to metastasis of HCC cells, frequent intrahepatic spread of hepatocellular carcinoma, and extrahepatic metastasis during the initial diagnosis of the disease [5]. Therefore, development of effective targeted therapies is necessary for treatment of HCC.
Tumor epithelial-mesenchymal transitions (EMT) are defined as specific phenotypic alterations and morphological changes in epithelial tumor cells, causing them to transform into mesenchymal cells during cancer metastasis [6]. In tumor EMT, tumor cells surrounding the epithelial cells and matrix lose their polarity and adhesive properties to become morphologically similar to fibroblasts, thereby enhancing the cells’ migratory and invasive abilities [7]. The occurrence of tumor EMT is accompanied by differential expression of epithelial and mesenchymal molecular tumor markers. In tumor EMT, epithelial molecular markers, including E-cadherin, α-catenin, γ-catenin, and β-catenin are down-regulated, whereas the expression of mesenchymal molecular markers, including N-cadherin, vimentin, and fibronectin are up-regulated [8]. In addition, EMT is also closely associated with tumor resistance to chemotherapy [9].
Chemokines are induced by inflammatory cytokines, growth factors, and pathogenic stimuli and secreted by different types of cells including tumor cells and tumor-infiltrating immune cells [10]. C-X-C motif chemokine 10 (CXCL10) is a small secretory immune modulator belonging to a larger CXC subfamily [11]. In line with the known functions of CXC cytokines, CXCL10 mediates inflammation, leukocyte trafficking, adaptive immunity, hematopoiesis, tumor metastasis, and angiogenesis [12]. CXCL10 signals through the G-protein-coupled (chemokine) receptors, which are divided into four subgroups (CC, CXC, CX3C, and C). Signaling occurs through the recruitment of CXCL10, with the downstream activation of multiple pathways involved in tumorigenicity, proliferation, apoptosis, angiogenesis, invasion, and metastasis of tumor cells [13]. In addition to tumor survival, metastasis, and angiogenesis, CXCL10 have recently been implicated in poor treatment response. A number of studies have established that radiation can exert considerable effect on CXCL10 expression, thus being indicative of the role of CXCL10 in resistance to chemo- and radiotherapy [14]. Therefore, CXCL10 appears to be an interesting molecular target, possessing characteristics of a natural immune modulator and considering that monoclonal antibodies against chemokine receptors have been used previously in experimental settings to inhibit growth and spread of malignant tumors. Chemokines such as CXCL12 and its ligands are often expressed in the inflammatory microenvironment of cancer cells in HCC [15]. However, the expression of CXCL10 and the role of CXCL10 in cancer-cell metastasis in HCC remains unknown.
In this study, we investigated the potential function of CXCL10 in the metastasis of HCC. We found that CXCL10 expression was up-regulated in the majority of HCC cell lines and tissues. Up-regulation of CXCL10 was significantly associated with poor prognosis in HCC patients. Moreover, in vitro and in vivo assays showed that CXCL10 knock-down significantly suppressed HCC cells metastasis, while, CXCL10 over-expression significantly enhanced the migration, invasion, and metastasis of HCC cells. Microarray analysis results suggested that CXCL10 performs its functions by regulating matrix metallopeptidase-2 (MMP-2). MMP-2 knockdown inhibited CXCL10-induced migration and invasion, and EMT of HCC cells. These results provide a clear understanding of the oncogenic functions of CXCL10 in liver cancers and the underlying mechanism by which CXCL10 inhibits HCC.
Materials and methods
Cell lines and cell culture
The human hepatocellular carcinoma cell lines (HepG2, MHCC97H, SMMC-7721, MHCC97L) and the non-tumorigenic human liver cells LO2 used as control were obtained from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (CBTCCCAS, Shanghai, China). The hepatocellular carcinoma cell lines were cultured as monolayers in RPMI 1640 culture media or Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Wisent, Quebec, Canada), penicillin (100 μg/mL), and streptomycin (100 μg/mL) and LO2 was cultured in RPMI 1640 culture media (Invitrogen, Carlsbad, CA) supplemented with 10% FBS. All cell lines were maintained in an incubator with 5% CO2/95% air atmosphere at 37°C.
Oncomine data analysis
To determine the expression pattern of CXCL10 in hepatocellular carcinoma, the Mas Liver [16] dataset in Oncomine database (www.oncomine.org) was used. The gene expression of CXCL10 was compared between hepatocellular carcinoma tissues with normal liver tissues according to the standard procedures as previously described.
Plasmid constructs and transfection
Full-length human CXCL10 complementary DNA (cDNA) was amplified with PCR and cloned into the pcDNA3.1 (+) expression vector (Invitrogen) before being transfected into HepG2 and MHCC97H cells using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s instructions. HepG2 and MHCC97H cells transfected with empty vector was used as control. Lentiviruses containing shRNAs targeting CXCL10 were purchased from GeneChem (GeneChem, China). The shCXCL10 target sequence was 5’-TAGATTCCGGATTCAGACATC-3’. Cells transfected with scrambled shRNA was used as control [17].
RNA interference
Oligonucleotides for human MMP-2 siRNA kit were purchased from OriGene (Rockville, MD, USA). The kit contains three predesigned duplexes targeting a specific gene of interest, and we used a pool of three target siRNAs to ensure work efficiency. Cells were transfected with MMP-2 siRNA or non-specific siRNA using the opti-MEM plus X-tremeGENE siRNA transfection reagent (Roche, Mannheim, Germany) according to the instruction manual. After 24 h post-transfection, the cells were further performed western blot analyses and migration assay [18].
Wound-healing assay
Cells migration in vitro was examined by wound-healing assay. Briefly, HepG2 or MHCC97H cells were cultured to about 90% confluence in a 6-well plate. Then a wound was created by a sterile 100 μL micropipette tip. Cell debris was washed with phosphate buffer saline (PBS) three times, and then 1 mL serum-free culture medium was added. The migrated cells count was determined at 0 h and 48 h [19]. The wound healing experiment was performed in triplicate.
Cell invasion assay
Invasion of HepG2 or MHCC97H cells was measured in Matrigel (BD) coated Transwell inserts containing polycarbonate filters with 8 μm pores (Costar). The inserts were coated with 1 mg/ml Matrigel matrix (100 μl). 1×104 cells in 200 μl of serum-free culture medium were plated in the upper chamber, whereas 600 μl of culture medium containing 10% FBS were added to lower chamber. After 6 h incubation, HepG2 or MHCC97H cells that migrated to the lower surface of the membrane were fixed with 1% paraformaldehyde and stained with 1% crystal violet [20].
Western blotting
After cells transfection, cells were harvested and total protein was extracted with 1×SDS Sample Buffer [100 mM Tris-HCl, 4% SDS, 10% Glycine, 10 mM EDTA]. The protein concentration was determined with BCA Protein Assay Kit. Protein extracts were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were then blocked with 5% skim milk in TBST for 1.5 h, and then incubated overnight at 4°C in TBST with primary antibody, including rabbit anti-CXCL10 (1:1000, Sigma), anti-E-cadherin (1:1000, Cell Signaling Technology), anti-N-cadherin (1:1000, Cell Signaling Technology), anti-fibronection (1:1000, Cell Signaling Technology), anti-vimentin (1:1000, Cell Signaling Technology), anti-MMP-2 (1:1000, Cell Signaling Technology), anti-MMP-9 (1:1000, Cell Signaling Technology) and GAPDH (1:1000, Cell Signaling Technology). Following incubation with horseradish peroxidase-conjugated IgGs (1:10000, Bioworld Biotechnology) for 1.5 h, the membranes were detected using enhanced chemiluminescence (ECL) kit (PerkinElmer) and visualized with the ChemiDoc XRS system (Bio-Rad). GAPDH was used as a control to verify equal protein loading [21].
Quantitative RT-PCR
PCR reaction was conducted with 2 μL cDNA sample, 0.4 μL forward primer (10 μmol/L), 0.4 μL reverse primer (10 μmol/L), 11.2 μL RNase-free water, and 6 μL 2× EsayTaq PCR SuperMix (TransGen BIotech, Beijing, China). PCR reaction was performed using the following cycle parameters: 95°C for 5 minutes, (94°C for 30 seconds, 56°C for 30 seconds, 72°C for 45 seconds) for 30 cycles, 72°C for 7 minutes. RT-PCR products were separated on 2% agarose gels. After stained with ethidium bromide, gel images were photographed with ChemiImagerTM 4400. RT-PCR was performed at least 3 times for each sample. The sequences of the primer pairs are: CXCL10 forward: 5’-GTGGCATTCAAGGAGTACCTC-3’, CXCL10 reverse: 5’-TGATGGCCTTCGATTCTGGATT-3’, MMP-2 forward: 5’-GATACCCCTTTGACGGTAAGGA-3’, MMP-2 reverse: 5’-CCTTCTCCCAAGGTCCATAGC-3’, MMP-9 forward: 5’-GGGACGCAGACATCGTCATC-3’, MMP-9 reverse: 5’-TCGTCATCGTCGAAATGGGC-3’, E-cadherin forward: 5’-AAAGGCCCATTTCCTAAAAACCT-3’, E-cadherin reverse: 5’-TGCGTTCTCTATCCAGAGGCT-3’, N-cadherin forward: 5’-TCAGGCGTCTGTAGAGGCTT-3’, N-cadherin reverse: 5’-ATGCACATCCTTCGATAAGACTG-3’, Fibronection forward: 5’-CGGTGGCTGTCAGTCAAAG-3’, Fibronection reverse: 5’-AAACCTCGGCTTCCTCCATAA-3’, Vimentin forward: 5’-TCCACACGCACCTACAGTCT-3’, Vimentin reverse: 5’-CCGAGGACCGGGTCACATA-3’, GAPDH forward: 5’-ACAACTTTGGTATCGTGGAAGG-3’, GAPDH reverse: 5’-GCCATCACGCCACAGTTTC-3’. The GAPDH was used as internal control. Relative quantitation was analyzed by taking the difference ΔC(T) between the C(T) of GAPDH and C(T) of indicated genes and computing 2-ΔΔC(T) as described previously [22].
Gene expression profiling
The total RNA quantity from indicated cells were assayed by NanoDrop ND-1000 and Agilent 2100 Bioanalyzer. The GeneChip Human Genome HU U133 plus 2.0 arrays (Affymetrix) were used according to manufacturer’s protocol. The data was normalized and summarized using robust multiarray average (RMA) normalization algorithms in Affymetrix® Expression Console Software. Altered genes between CXCL10 knock-down cells and its control cells were indicated significantly by scatter plots and the genes up-regulated and down-regulated ≥ 5-fold. Clustering analysis was done using Gene Cluster v2.0 software, and the heatmap was visualized by Java TreeView v1.1.4 software. Gene set enrichment analysis was performed using the gene set enrichment and gene set relation mapping tool: ConceptGen [23].
In vivo tumor metastasis
BALB/c nude mice were purchased from Shanghai Slac Laboratory Animal Co. Ltd and maintained in SPF conditions. All animals were used in accordance with institutional guidelines and the current experiments were approved by the Use Committee for Animal Care of the Affiliated Baiyun Hospital of Guizhou Medical University. For HCC cells metastasis assays, 1×107 HepG2 or MHCC97H cells transduced with control vector or CXCL10 plasmid were re-suspended in PBS and were injected into the tail vein of BALB/c nude mice. All the mice were killed by CO2 25 days after injection. The metastasis nodules in the lung tissues were stained with hematoxylin and eosin [24].
Statistical analysis
The data were described as mean ± SD. Differences in the results of two groups were evaluated using either Student’s two-tailed t-test or ANOVA test. The differences between mean values with P < 0.05 were considered statistically significant.
Results
CXCL10 is highly expressed in human hepatocellular carcinoma
Compared to the normal human liver cell line, LO2, the expression of CXCL10 was significantly higher in the tested HCC cells, particularly in invasive HCC cells, such as MHCC97H and SMMC-7721 (Figure 1A). The mRNA levels of CXCL10, when determined by qRT-PCR analysis, were found to be consistent with those obtained by western blot (Figure 1B). Next, we evaluated whether CXCL10 expression was up-regulated in the clinical specimens. By analyzing the published mRNA expression profiles obtained from 19 normal liver tissues and 38 hepatocellular carcinoma tissues, we found that CXCL10 expression was significantly up-regulated in HCC tissues compared to the normal tissues (Figure 1C). To investigate the association between CXCL10 levels and the survival of HCC patients, the biomarker was assessed using the online biomarker validation tool, SurvExpress, in 47 HCC and non-tumor liver samples (Tsuchiya Rusyn Liver GSE17856) [25]. As shown in Figure 1D, the survival curves indicated that HCC patients with higher CXCL10 levels had poor survival rates than those patients with lower CXCL10 expression. Together with the systemic analysis results, these findings suggested a negative correlation between the over-expression of CXCL10 and longer survival rate in HCC patients.
Figure 1.
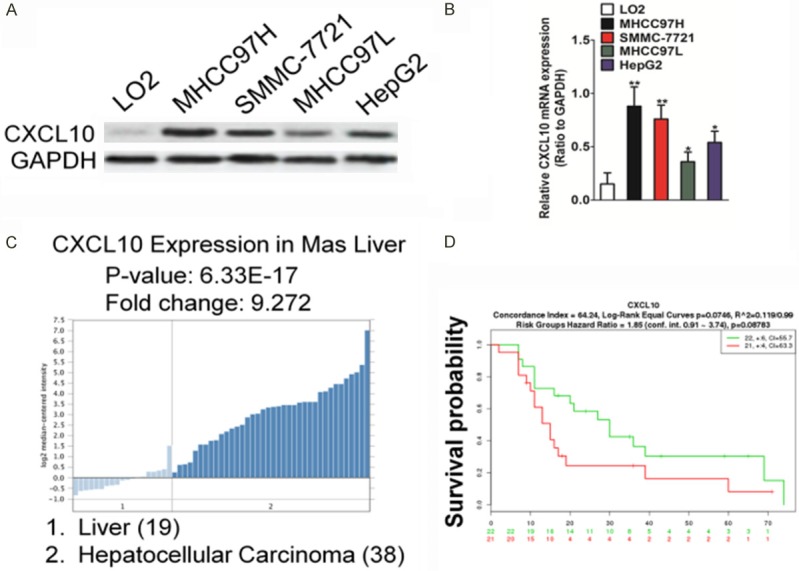
CXCL10 is highly expressed in HCC. A. CXCL10 expression in LO2 and several HCC cell lines as determined by western blot analysis (left). Quantitative analysis of CXCL10 protein level in HCC cell lines are shown (right). B. Total RNA was extracted from LO2 and all HCC cells. CXCL10 mRNA level was determined by quantitative real-time PCR (qRT-PCR) and normalized to the mRNA level of GAPDH. The fold changes of mRNA expression of CXCL10 gene in HCC cells were compared as a ratio to that of the LO2 cells. Data are shown as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with the LO2 cells. C. Box plots derived from gene expression data in Oncomine comparing expression of CXCL10 gene in normal liver (left plot) and liver cancer (right plot). D. Kaplan-Meier survival curve (SurvExpress, Tsuchiya Rusyn Liver GSE17856) shows the survival of HCC patients with high or low CXCL10 expression. High and low expression levels are represented in red and green, respectively.
Down-regulation of CXCL10 suppresses migration and invasion of HCC cells
To delineate the underlying role of the effect of SOSTDC1 on HCC cells metastasis, the silencing of CXCL10 expression in a highly metastatic HCC cell line MHCC97H was established and designated as MHCC97H-shCXCL10. The mRNA and protein expression levels of CXCL10 were verified in MHCC97H-shCXCL10 cells by western blotting and qRT-PCR. (Figure 2A and 2B). Cancer-cell migration and invasion are considered critical for tumor metastasis. Wound healing analysis and Transwell Matrigel assay were performed to evaluate the effects of CXCL10 on migration and invasion in the MHCC97H-shCXCL10 and control cells. As shown in Figure 2C, the wound closure ratio was markedly reduced to 61% in CXCL10-shRNA transfected MHCC97H cells, compared to the rates in control cells. Likewise, silencing of CXCL10 expression significantly reduced the invasion by MHCC97H cells through the Matrigel-coated membrane in the Transwell chamber compared with that of control cells (Figure 2D). To further examine the function of CXCL10 in MHCC97H metastasis in vivo, MHCC97H-shCXCL10 and the corresponding control cells were injected into nude mice via the tail vein. The number of mice with distant metastasis was found to have significantly decreased after injecting with MHCC97H-shCXCL10 cells (Figure 2E). As expected, the mean incidences of metastatic foci in the liver of each mouse after injecting with the MHCC97H-shCXCL10 cell group and with the control group were 80% and 20%, respectively (Figure 2F). All these results suggested that shCXCL10 could efficiently inhibit cell migration and invasion of HCC cells in vitro and inhibit HCC cell metastasis in vivo.
Figure 2.
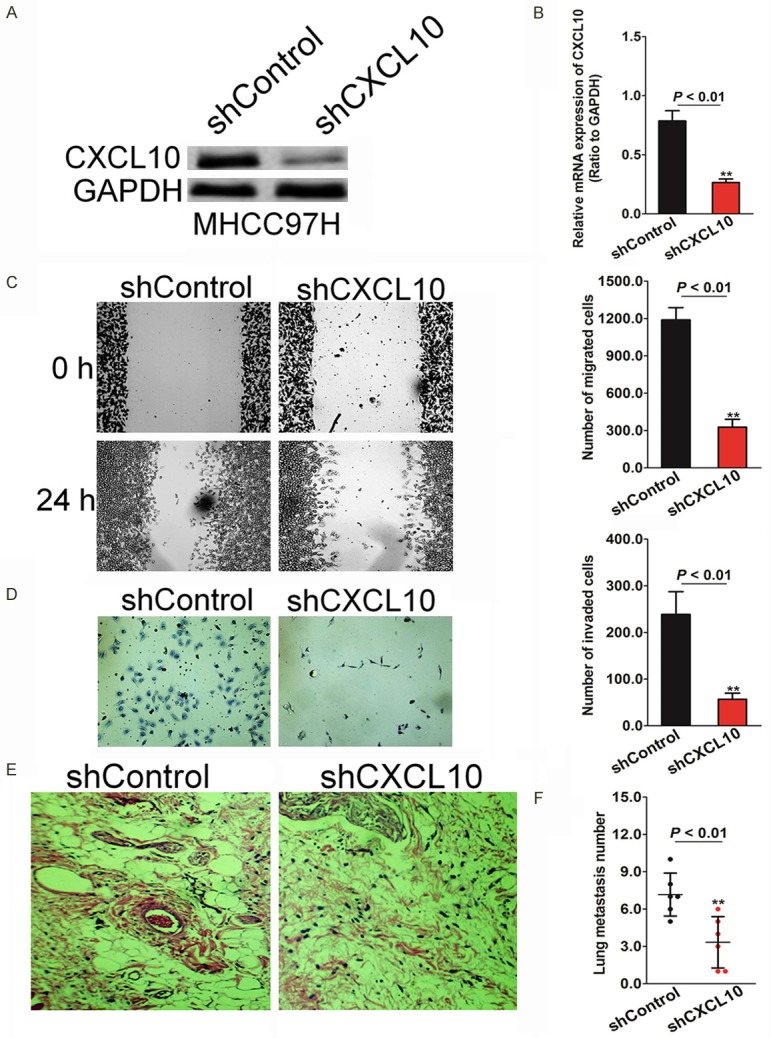
Silencing CXCL10 decreases the migration and invasion of MHCC97H cells. A. Control shRNA or shRNA against CXCL10 were transfected into MHCC97H cells. After transfection, both cell types were subjected to western blot analysis for measuring the CXCL10 levels in them. B. MHCC97H cells were incubated with control shRNA or shRNA against CXCL10 followed by analysis of the mRNA level of CXCL10 in the established cell lines by qRT-PCR. C. CXCL10- and control-shRNA were transfected into MHCC97H cells wounded with a pipette, and the wound closure percentage was quantified at 48 h after scratch relative to that at 0 h. D. MHCC97H cells with silenced CXCL10 exhibit less invasiveness compared with that of control cells in Transwell Matrigel assay. E. Silencing CXCL10 significantly decreases the number of mice with distant metastasis. Representative images of H&E stained sections derived from lung metastatic nodules. Metastatic lesions in the lungs of mice injected with MHCC97H-shCXCL10 cells or control cells are shown at 6 weeks after implantation. F. Fewer metastatic foci in the liver are observed in mice injected with CXCL10-silenced MHCC97H cells. Each bar represents the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01.
Over-expression of CXCL10 promotes metastasis of HCC cells
To investigate the biological role of up-regulation of CXCL10 expression in HCC metastasis, HepG2 cell line that stably expressed CXCL10 was established and designated as HepG2-CXCL10. Both mRNA (Figure 3A) and protein levels (Figure 3B) of CXCL10 were found to increase in HepG2 cells compared to the cells transfected with the control vector. We found that ectopic expression of CXCL10 in HepG2 cells markedly increased the migration rate as shown in the wound healing assay (Figure 3C). Matrigel assay results were also evaluated to determine the invasive potential of HepG2 cells in the event of CXCL10 over-expression. Consistent with the migration assay results, invasion by HepG2 cells significantly increased in cells with high CXCL10 expression (Figure 3D). To further investigate the role of CXCL10 in HepG2 cell metastasis in vivo, HepG2-CXCL10 cells and the corresponding control cells were injected into immunocompromised mice via the tail vein. As shown in Figure 3E, up-regulation of CXCL10 expression increased the number of mice with incidence of metastatic foci in the liver, the incidence observed being 30% and 76% in mice injected with the HepG2-CXCL10 cell and control groups, respectively (Figure 3F). Collectively, these results suggest that CXCL10 up-regulation promotes aggressive metastasis of HCC cells in vitro and in vivo.
Figure 3.
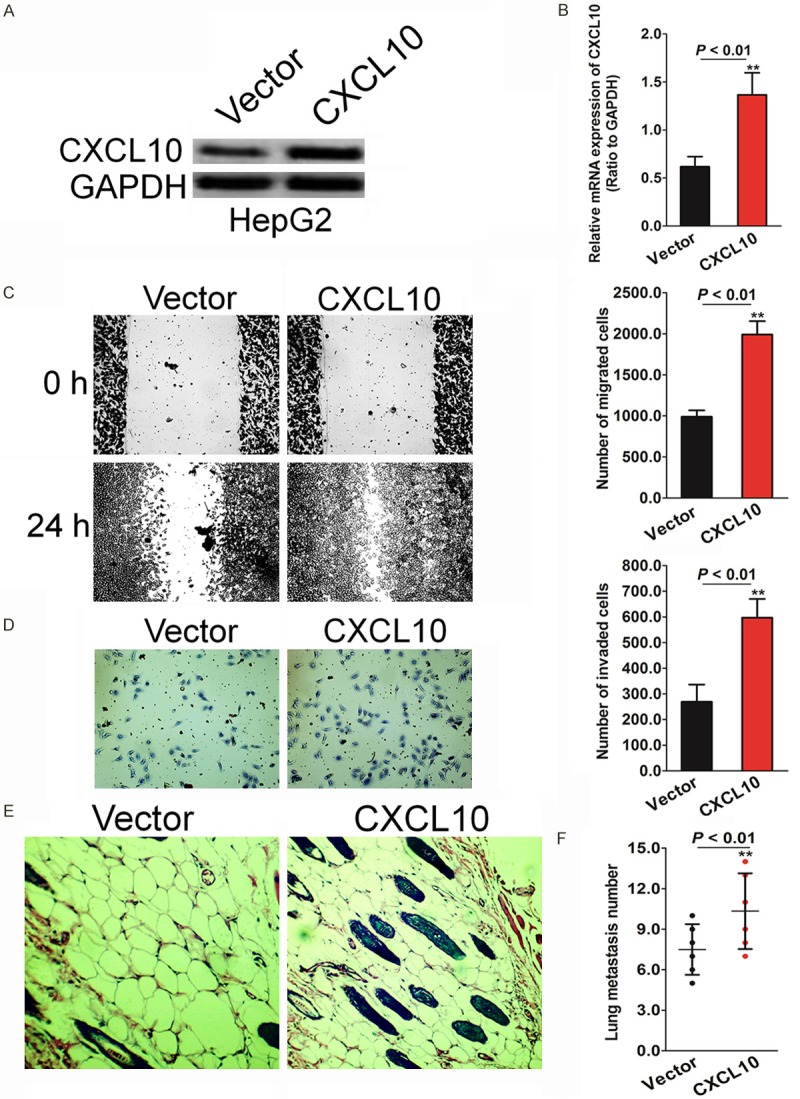
CXCL10 over-expression increases HepG2 cells migration and invasion. A. Control vector or CXCL10 were transfected into HepG2 cells for 24 hours. After transfection, the established cells were subjected to western blot analysis for measuring the CXCL10 level. B. qRT-PCR analysis was performed to evaluate the CXCL10 mRNA level in the established HepG2 cell lines. C. Wound healing assay was conducted to evaluate the HepG2 cell motility after transfection with CXCL10 or control vector. Each bar represents the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with cells transfected with control vector. D. HepG2 cells with high levels of CXCL10 expression exhibit stronger invasive abilities in Transwell Matrigel assays. E. CXCL10 over-expression significantly increased the number of mice with distant metastasis. F. High numbers of metastatic foci in liver were counted in each mouse injected with HepG2 over-expressing CXCL10. Each bar represents the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with mice injected into control HepG2 cells.
CXCL10 regulates the transition between epithelial and mesenchymal phenotypes in HCC cells
EMT is a key step for tumor progression and plays an important role in migration and invasiveness of cancer cells. EMT progression is inherently marked by a decrease in tumor cell-cell adherence via the transcriptional repression of cadherins and the functional loss of E-cadherin. Mesenchymal-epithelial transition typically increases the functional loss of N-cadherin while the equivalent epithelial filament protein E-cadherin is increased. In this study, the expression levels of the EMT protein markers were evaluated to explore the association between CXCL10 and EMT. In the CXCL10-silenced MHCC97H cells, the epithelial cell marker (E-cadherin) was up-regulated, while the mesenchymal cell markers (N-cadherin, fibronectin, and vimentin) were down-regulated, as determined by western blot analysis (Figure 4A) and qRT-PCR (Figure 4B) assay. Furthermore, CXCL10 over-expression significantly increased levels of the mesenchymal cell markers (N-cadherin, fibronectin, and vimentin) and decreased the epithelial cell marker (E-cadherin), as determined by western blot analysis in CXCL10 over-expressed HepG2 cells (Figure 4C). The mRNA of the EMT markers exhibited a trend consistent with the above-mentioned immunoblotting results (Figure 4D). This suggested that CXCL10 plays important roles in EMT.
Figure 4.
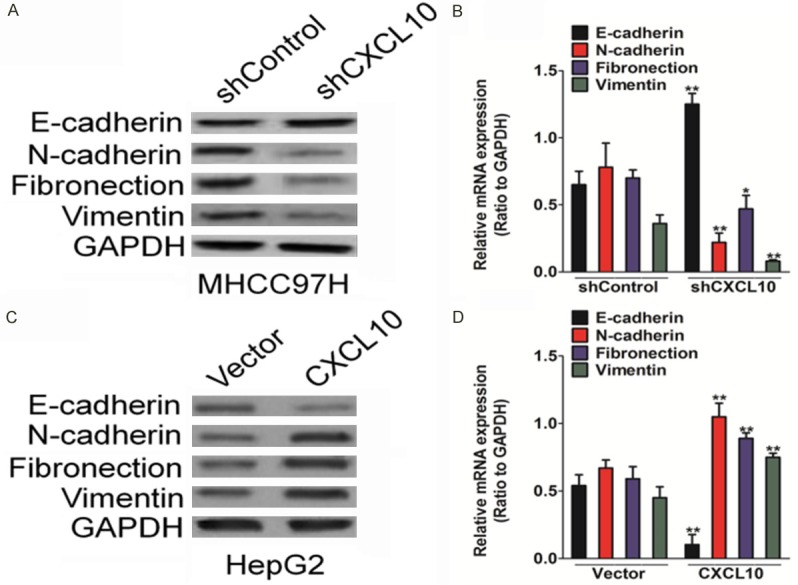
CXCL10 regulates the EMT in HCC cells. A. Silencing CXCL10 in MHCC97H increased up-regulation of E-cadherin and down-regulation of N-cadherin, fibronectin, and vimentin. MHCC97H cells, seeded in 6-well plates, were transfected with shCXCL10 or control shRNA. After 24 h since transfection, cells were subjected to western blot analysis for measuring protein levels. B. MHCC97H cells, seeded in 6-well plates, were transfected with shCXCL10 or control shRNA. After 24 h since transfections, qRT-PCR analysis was performed to assess epithelial and mesenchymal cell marker levels. C. Western blot analysis was performed to determine the epithelial and mesenchymal cell marker expression levels in HepG2 cells transfected with CXCL10 plasmid or control vector. D. HepG2 cells were seeded in 6-well plates and then transfected with CXCL10 or control vector. qRT-PCR analysis showed that CXCL10 over-expression led to the down-regulation of epithelial cell marker (E-cadherin) and up-regulation of mesenchymal cell marker (N-cadherin, fibronectin, and vimentin) mRNA levels.
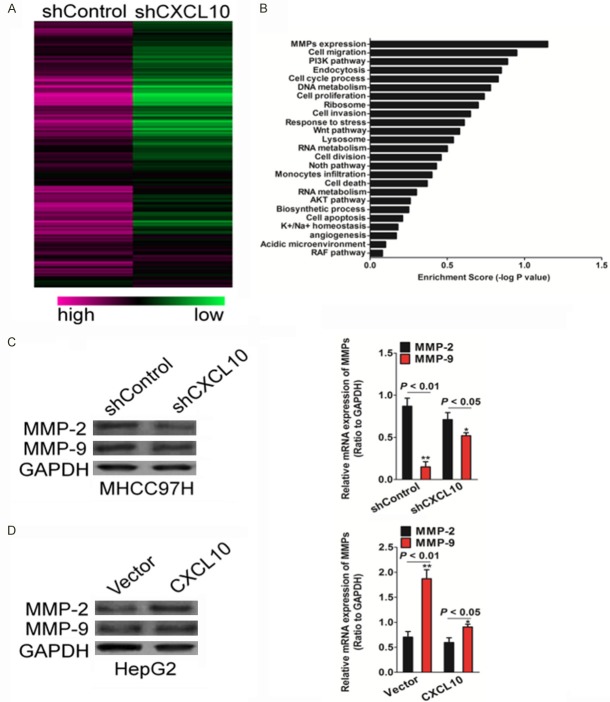
CXCL10 regulates MMP-2 expression
To elucidate the mechanisms by which CXCL10 is engaged in the metastasis of HCC cells, microarray assay was performed using the MHCC97H-shCXCL10 cell line, and appropriate control cells with an empty vector. Microarray results revealed a list of genes that were differentially expressed subsequent to the silencing of CXCL10 (Figure 5A). Gene set enrichment analysis indicates that matrix metalloproteinases (MMPs) were significantly enriched in CXCL10 knockdown cells (Figure 5B). This result supports the hypothesis that CXCL10 regulates EMT and that the HCC cell invasion and metastasis might be mediated by MMPs. To further verify the relationship between CXCL10 and MMPs, MMP-2 and MMP-9 expression in the above mentioned HCC cell lines was evaluated by western blot analysis and qRT-PCR assay. In cell lines with CXCL10 silencing, the MMP expression, especially that of MMP-2, decreased significantly as compared to that in the control vector cells (Figure 5C), while CXCL10 over-expression could increase the MMP-2 protein and mRNA expression levels (Figure 5D).
Figure 5.
CXCL10 regulates MMPs expression. A. Clustering of genes differentially expressed after CXCL10 silencing. B. Enrichment scores of differential gene expression in the CXCL10-silencing cell line. MMP expression was affected by CXCL10. C. MHCC97H cells were seeded in 6-well plates and transfected with shCXCL10 or control shRNA. After 24 h since transfection, total protein samples were subjected to western blot analysis for measuring MMP-2 and MMP-9. D. The MMP-2 and MMP-9 expression levels in the CXCL10 silencing cell line were assayed by qRT-PCR analysis. The data were presented as mean ± SD. For indicated comparisons, *P < 0.05 and **P < 0.01 compared to control MHCC97H cells. E. Control vector or CXCL10 were transfected into HepG2 cells. After 24 h since transfection, MMP-2 and MMP-9 expression in CXCL10 overexpression cell line was detected by western blot analysis. F. The mRNA levels of MMP-2 and MMP-9 in indicated HepG2 cells was measured by qRT-PCR. The data are presented as mean ± SD. For indicated comparisons, *P < 0.05 and **P < 0.01 compared to control HepG2 cells.
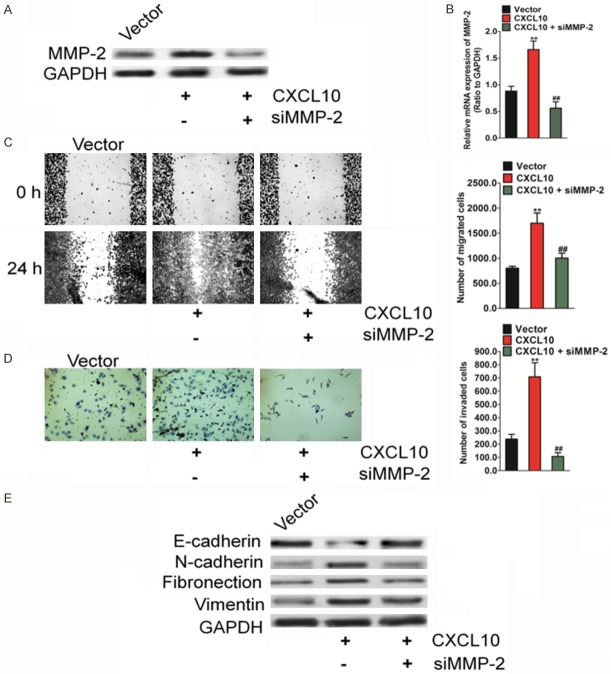
MMP-2 mediates CXCL10-induced migration, invasion, and EMT in HCC cells
To evaluate whether the migration and invasion of CXCL10-induced HCC cells were mediated by MMP-2, siRNA was used to silence the MMP-2 expression in HepG2-CXCL10 cells. The presence of HepG2 cells with MMP-2 silencing was verified by western blot (Figure 6A) and qRT-PCR analyses (Figure 6B). The knock-down of MMP-2 decreased the migration capacity of HepG2-CXCL10 cells, as shown by the wound healing assay (Figure 6C). Consequent to the silencing of MMP-2 by siRNA in HepG2-CXCL10 cells, HepG2 cell invasion was also inhibited (Figure 6D). Levels of the epithelial marker, E-cadherin, increased, whereas those of mesenchymal markers, including N-cadherin, fibronectin, and vimentin, decreased (Figure 6E). Overall, these results show that MMP-2 mediates CXCL10-induced EMT, migration, and invasion in HCC cells.
Figure 6.
MMP-2 is involved in CXCL10-regulated HCC cell metastasis. A. Western blot results show that the expression of MMP-2 was elevated in cells transfected with CXCL10. GAPDH was used as a loading control. B. qRT-PCR analysis of MMP-2 level in the established HepG2 cells. PCR values were normalized to the levels of GAPDH. Data are presented as the mean ± SD from three independent measurements. C. In the presence of siRNA targeting MMP-2, confluent cell monolayers were wounded to evaluate HepG2 cell invasiveness after transfection with CXCL10 pcDNA3.1. The wound closure was monitored at 0 hour and 24 hours. D. In the presence of siRNA targeting MP-2, Transwell assay was conducted to evaluate HepG2 cell invasiveness after transfection. Silencing MMP-2 in HepG2 cells results in decreased cell invasion induced by CXCL10 over-expressiong. E. Silencing MMP-2 reverses the CXCL10-induced changes in the EMT marker in HepG2 cells. Column data are presented as mean ± SD of three independent experiments. For indicated comparisons, *P < 0.05, **P < 0.01, compared to control cells.
Discussion
HCC remains a major clinical challenge because of its aggressive metastasis and the minimal effective strategies available against metastasis [26]. Our study reported that CXCL10 plays a functional role in HCC metastasis and EMT [27]. Silencing of CXCL10 in HCC cells inhibits EMT, cell migration, and invasion in vitro and decreases the metastatic capacities in vivo. By contrast, CXCL10 over-expression reverses these events in the otherwise poorly aggressive and invasive HCC cells. Microarray data showed that CXCL10 regulates matrix metalloproteinase-2 (MMP-2) expression in HCC cells. Effects of silencing MMP-2 in CXCL10 over-expressed HCC cells are similar to those caused by CXCL10 knock-down. In conclusion, our results reveal a new target for intervention in HCC metastasis and may increase the future treatment options of hepatocellular carcinoma.
Malignant HCC has one of the highest risks of cancer deaths owing to its highly metastatic potential [28]. In recent years, the incidence and mortality rate of HCC continues to climb and there are dedicated efforts to identify newer therapeutic strategies. In most cases, for patients with HCC, surgical resection remains the curative treatment choice [29]. Currently, however, there is no effective therapeutic option for metastatic HCC owing to the complicated molecular mechanisms underlying metastasis in HCC cells. Therefore, an understanding of these novel cellular and molecular mechanisms is crucial for the exploitation of novel therapeutic approaches. The expression disorder of genes belonging to the chemokines family in tumor has been reported and investigators have proposed that CXC chemokines and their receptors may be involved in tumor development and metastasis [30]. CXCL10 is highly expressed in a wide range of human diseases and in cancer, CXCL10 is secreted from a variety of cells, such as epithelial cells, activated neutrophils, endothelial cells, eosinophils, monocytes, and stromal cells [31]. Interactions between chemokines and their receptors were proposed to be of importance in the initiation and metastasis of cancer. In breast cancer cells, RAS induces CXCL10 over-expression by way of both RAF and PI3 kinase (PI3K) signaling pathways. CXCL10 binds to its receptor CXCR3 and down-regulates CXCR3, further promoting cell growth in breast cancer [32]. CXCL10 has also been verified as an autocrine invasion factor in nasal natural killer/T-cell lymphoma, which promotes metastasis of colon-cancer cells and tumorigenesis in human glioma and basal cell carcinoma [33]. Our results suggest that the CXCL10 expression in HCC cells was higher than that in normal liver cells. CXCL10 expression in HCC tumor tissue has also been shown to be higher than that in normal liver tissue, which suggests that CXCL10 may function as an oncogene in HCC. CXCL10 has been found to regulate the tumor growth factor in tumor and the metastatic behavior of tumor cells in diseases such as lung cancer, breast cancer, and bladder cancer. In the present study, silencing CXCL10 significantly reduces the migration and invasion of HCC cells in vitro. The process also weakens the metastatic ability of HCC cells in vivo. CXCL10 over-expression confers the opposite action on the HCC cells.
EMT significantly regulates tumor progression and confers fundamental abilities to cancer cells that are essential for tumor metastasis [34]. EMT-driven cancer cells metastasize by generating cancer stem cells that are capable of colonizing other tissues to form secondary tumor lesions. The occurrence of EMT is accompanied by altered expression levels of several molecular markers, such as E-cadherin, N-cadherin, fibronectin, and vimentin. Our results also indicate that CXCL10 down-expression in highly metastatic HCC cells inhibits EMT, and over-expression of CXCL10 in HCC cells with poor metastatic potential promotes EMT. In addition, CXCL10 has been proven to induce EMT by repressing expression of E-cadherin and increasing that of mesenchymal cell markers (N-cadherin, fibronectin, and vimentin). Microarray analysis was conducted to investigate the mechanism of CXCL10 in regulating the metastasis of HCC cells. MMPs were identified as effective mediators of CXCL10-induced metastasis. MMPs are major proteolytic enzymes that are involved in tumor cell migration and metastasis. Silencing MMP-2 in CXCL10 over-expressing HCC cells resulted in a phenomenon similar to that caused by CXCL10 knock-down. Thus, the present study identified a novel function of CXCL10 in HCC cell metastasis by regulating EMT. In conclusion, CXCL10 up-regulation is correlated with HCC metastasis and CXCL10-mediated induction of EMT is MMP-2 dependent.
Disclosure of conflict of interest
None.
References
- 1.Wang C, Yao B, Xu M, Zheng X. RIP1 upregulation promoted tumor progression by activating AKT/Bcl-2/BAX signaling and predicted poor postsurgical prognosis in HCC. Tumour Biol. 2016;37:15305–15313. doi: 10.1007/s13277-016-5342-1. [DOI] [PubMed] [Google Scholar]
- 2.Ye J, Yao Y, Song Q, Li S, Hu Z, Yu Y, Hu C, Da X, Li H, Chen Q, Wang QK. Up-regulation of miR-95-3p in hepatocellular carcinoma promotes tumorigenesis by targeting p21 expression. Sci Rep. 2016;6:34034. doi: 10.1038/srep34034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Li J, Yu Z, Li J, Sun R, Kan Q. MiR-935 promotes liver cancer cell proliferation and migration by targeting SOX7. Oncol Res. 2016;25:427–435. doi: 10.3727/096504016X14747300207374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ZG, Zheng H, Gao W, Han J, Cao JZ, Yang Y, Li S, Gao R, Liu H, Pan ZY, Fu SY, Gu FM, Xing H, Ni JS, Yan HL, Ren H, Zhou WP. eIF5B increases ASAP1 expression to promote HCC proliferation and invasion. Oncotarget. 2016;7:62327–62339. doi: 10.18632/oncotarget.11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Yu Y, Sun S, Wang Z, Liu P, Liu S, Jiang J. Bradykinin promotes migration and invasion of hepatocellular carcinoma cells through TRPM7 and MMP2. Exp Cell Res. 2016;349:68–76. doi: 10.1016/j.yexcr.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Yan W, Chen J, Chen Z, Chen H. Deregulated miR-296/S100A4 axis promotes tumor invasion by inducing epithelial-mesenchymal transition in human ovarian cancer. Am J Cancer Res. 2016;6:260–269. [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Q, Ning F, Fang R, Wang HS, Zhang G, Quan MY, Cai SH, Du J. Endogenous nodal promotes melanoma undergoing epithelialmesenchymal transition via snail and slug in vitro and in vivo. Am J Cancer Res. 2015;5:2098–2112. [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7:2141–2158. [PMC free article] [PubMed] [Google Scholar]
- 9.Lu W, Xu Z, Zhang M, Zuo Y. MiR-19a promotes epithelial-mesenchymal transition through PI3K/AKT pathway in gastric cancer. Int J Clin Exp Pathol. 2014;7:7286–7296. [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Z, Li W, Zhang H, Wu W, Peng Y, Zeng Y, Wan Y, Wang J, Ouyang N. CCL18/PITPNM3 enhances migration, invasion, and EMT through the NF-kappaB signaling pathway in hepatocellular carcinoma. Tumour Biol. 2016;37:3461–3468. doi: 10.1007/s13277-015-4172-x. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Tang Y, Yu H, Yin Q, Li M, Shi L, Zhang W, Li D, Li L. CCL18 from tumor-cells promotes epithelial ovarian cancer metastasis via mTOR signaling pathway. Mol Carcinog. 2016;55:1688–1699. doi: 10.1002/mc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Wu J, Wang T, Zhang X, Liu D. CXCL10/CXCR3 axis promotes the invasion of gastric cancer via PI3K/AKT pathway-dependent MMPs production. Biomed Pharmacother. 2016;82:479–488. doi: 10.1016/j.biopha.2016.04.069. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang Y, Liu K, Hao M, Zheng R, Zhang C, Wu Y, Zhang X, Li N, Zheng J, Chen D. Radiofrequency ablation-increased CXCL10 is associated with earlier recurrence of hepatocellular carcinoma by promoting stemness. Tumour Biol. 2016;37:3697–3704. doi: 10.1007/s13277-015-4035-5. [DOI] [PubMed] [Google Scholar]
- 14.Bai M, Chen X, Ba YI. CXCL10/CXCR3 overexpression as a biomarker of poor prognosis in patients with stage II colorectal cancer. Mol Clin Oncol. 2016;4:23–30. doi: 10.3892/mco.2015.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Pan Z, Li A, Fu S, Lei Y, Sun H, Wu M, Zhou W. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol. 2008;5:373–378. doi: 10.1038/cmi.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mas VR, Maluf DG, Archer KJ, Yanek K, Kong X, Kulik L, Freise CE, Olthoff KM, Ghobrial RM, McIver P, Fisher R. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol Med. 2009;15:85–94. doi: 10.2119/molmed.2008.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Wu S, Yang Y, Cai J, Zhu X, Wu J, Li M, Guan H. SOSTDC1 is down-regulated in nonsmall cell lung cancer and contributes to cancer cell proliferation. Cell Biosci. 2016;6:24. doi: 10.1186/s13578-016-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Lan H, Li J, Su Y, Xu L. Muc1 promotes migration and lung metastasis of melanoma cells. Am J Cancer Res. 2015;5:2590–2604. [PMC free article] [PubMed] [Google Scholar]
- 19.Qin B, Cheng K. Silencing of the IKKepsilon gene by siRNA inhibits invasiveness and growth of breast cancer cells. Breast Cancer Res. 2010;12:R74. doi: 10.1186/bcr2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang J, Li L, Guo L, Su Y, Wang Y, Xu Y, Wang X, Meng S, Lei L, Xu L, Shao G. RNF8 promotes epithelial-mesenchymal transition of breast cancer cells. J Exp Clin Cancer Res. 2016;35:88. doi: 10.1186/s13046-016-0363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang G, Tao L, Shen S, Chen L. Hypoxia induced CCL28 promotes angiogenesis in lung adenocarcinoma by targeting CCR3 on endothelial cells. Sci Rep. 2016;6:27152. doi: 10.1038/srep27152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YQ, Xiao CX, Lin BY, Shi Y, Liu YP, Liu JJ, Guleng B, Ren JL. Silencing of Pokemon enhances caspase-dependent apoptosis via fas- and mitochondria-mediated pathways in hepatocellular carcinoma cells. PLoS One. 2013;8:e68981. doi: 10.1371/journal.pone.0068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv X, Li L, Lv L, Qu X, Jin S, Li K, Deng X, Cheng L, He H, Dong L. HOXD9 promotes epithelial-mesenchymal transition and cancer metastasis by ZEB1 regulation in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:133. doi: 10.1186/s13046-015-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, Wei JW, Zhou HJ, Ren N, Ye QH, Dong QZ, Qin LX. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58:158–170. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya M, Parker JS, Kono H, Matsuda M, Fujii H, Rusyn I. Gene expression in nontumoral liver tissue and recurrence-free survival in hepatitis C virus-positive hepatocellular carcinoma. Mol Cancer. 2010;9:74. doi: 10.1186/1476-4598-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan Y, Mou L, Cheng K, Wang C, Deng X, Chen J, Fan Z, Ni Y. Hepatocellular carcinoma stem cell-like cells are enriched following lowdose 5-fluorouracil chemotherapy. Oncol Lett. 2016;12:2511–2516. doi: 10.3892/ol.2016.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Liu F, Ao P, Li X, Zheng H, Wu D, Zhang N, She J, Yuan J, Wu X. Correlation of PDK1 expression with clinicopathologic features and prognosis of hepatocellular carcinoma. Onco Targets Ther. 2016;9:5597–5602. doi: 10.2147/OTT.S110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu M, Liu Q, Jia Y, Tu K, Yao Y, Liu Q, Guo C. BCAT1 promotes tumor cell migration and invasion in hepatocellular carcinoma. Oncol Lett. 2016;12:2648–2656. doi: 10.3892/ol.2016.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo HY, Zhong JH. Comment on stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma. J Surg Oncol. 2016;113:715. doi: 10.1002/jso.24192. [DOI] [PubMed] [Google Scholar]
- 30.Shah AD, Bouchard MJ, Shieh AC. Interstitial fluid flow increases hepatocellular carcinoma cell invasion through CXCR4/CXCL12 and MEK/ERK signaling. PLoS One. 2015;10:e0142337. doi: 10.1371/journal.pone.0142337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CX, Ling CC, Shao Y, Xu A, Li XC, Ng KT, Liu XB, Ma YY, Qi X, Liu H, Liu J, Yeung OW, Yang XX, Liu QS, Lam YF, Zhai Y, Lo CM, Man K. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J Hepatol. 2016;65:944–952. doi: 10.1016/j.jhep.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Ejaeidi AA, Craft BS, Puneky LV, Lewis RE, Cruse JM. Hormone receptor-independent CXCL10 production is associated with the regulation of cellular factors linked to breast cancer progression and metastasis. Exp Mol Pathol. 2015;99:163–172. doi: 10.1016/j.yexmp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Shin SY, Hyun J, Lim Y, Lee YH. 3’-Chloro-5,7-dimethoxyisoflavone inhibits TNFalpha-induced CXCL10 gene transcription by suppressing the NF-kappaB pathway in HCT116 human colon cancer cells. Int Immunopharmacol. 2011;11:2104–2111. doi: 10.1016/j.intimp.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Shi W, Tang Y, Liu Y, He K, Hu Y, Li J, Yang Y, Song J. Prefoldin 1 promotes EMT and lung cancer progression by suppressing cyclin A expression. Oncogene. 2017;36:885–898. doi: 10.1038/onc.2016.257. [DOI] [PMC free article] [PubMed] [Google Scholar]