Abstract
Colorectal cancer remains the most common cause of cancer-related deaths worldwide and it continues to lack an effective treatment. Here, we found that zinc finger E-box binding homeobox 2 (ZEB2) was overexpressed in several colorectal cancer cell lines and colorectal cancer specimens relative to adjacent non-cancerous tissues. Although ZEB2 has been reported to be associated with several tumors, its involvement in colorectal cancer progression remains unclear. In this study, we investigated the biological functions and molecular mechanisms of ZEB2 underlying colorectal carcinoma metastasis and angiogenesis. HCT116 colorectal cancer cells were treated with ZEB2 shRNA or recombinant ZEB2, and the expression of ZEB2 was assessed using reverse transcriptase polymerase chain reaction (RT-PCR) and immunoblotting, respectively. Ectopic expression of ZEB2 induced proliferation and epithelial-mesenchymal transition (EMT), and increased the metastatic capacity of HCT116 cells in vitro and in vivo. Furthermore, endothelial cell tube formation and angiogenesis in chick embryo chorioallantoic membrane (CAM) were accelerated by conditioned medium from ZEB2-overexpressing HCT116 cells. Further, overexpression of ZEB2 accelerated tumor growth and angiogenesis in xenotransplantation models. However, silencing endogenous ZEB2 caused an opposite outcome. Our results provide new evidence that ZEB2 promotes the progression of colon cancer, and thereby might represent a novel therapeutic target for colorectal carcinoma.
Keywords: ZEB2, metastasis, angiogenesis, colorectal cancer
Introduction
Despite advances in early diagnosis and comprehensive therapy, colorectal cancer remains the most frequent malignancies and one of the leading causes of cancer-related deaths worldwide [1]. The prognoses of patients with colorectal cancer are often poor due to metastasis, and the overall 5-year survival rate of the patients with distant metastatic colorectal cancer is only 12% [2]. Accumulating evidence has demonstrated that multiple genes and cellular pathways participate in the occurrence and metastasis of colorectal cancer. Therefore, elucidating the molecular mechanisms underlying colorectal cancer metastasis and identifying novel biomarker of migration and invasion are extremely essential for the development of more effective therapeutic strategy and improvement in the clinical outcome of patients with colorectal cancer [3]. The high throughput platforms for analysis of gene expression, such as microarrays, are increasingly valued as promising tools in medical oncology with great clinical applications. Many gene expression-profiling studies on colon cancer have been performed in the last decade using microarray technology and showed hundreds of differentially expressed genes involved in different pathways, biological processes, or molecular functions. We adopted an approach to gene expression dataset, which enabled the discovery and validation of differentially expressed genes in colorectal cancer. Among the differentially expressed genes associated with tumor metastasis, expression of zinc finger E-box binding homeobox 2 (ZEB2) gene was upregulated in colorectal cancer tissues as compared to normal tissues [4].
Members of the ZEB family have a similar structure consisting of two zinc finger clusters and a central repression region, including CtBP and Smad-interacting domains [5]. ZEB2, also known as SIP1, was originally identified as a transcription factor and a protein playing vital roles in the TGF-β signaling cascade. In addition, ZEB2 is a Smad-interacting, DNA-binding, multi-zinc finger transcription factor involved in multiple cellular functions [6]. Previous studies have documented that ZEB2 acts as a transcriptional repressor of E-cadherin and plays a role in epithelial-mesenchymal transition (EMT) in breast and liver cancers. EMT is a pathological process leading to specific morphological and phenotypic alterations in tumor cells during cancer metastasis. The occurrence of EMT is accompanied by various expression-specific epithelial and mesenchymal molecular markers [7]. During EMT, epithelial cell markers, such as E-cadherin, α-catenin, β-catenin, and γ-catenin, are downregulated, whereas the mesenchymal tissue markers, such as N-cadherin, vimentin, and fibronectin, are upregulated.
Except for its role in cancer cell metastasis and EMT, ZEB2 also plays an important role in tumor angiogenesis. Sustained angiogenesis plays an important role in the unrestrained growth of solid tumors and metastasis of cancer cells to distant organs [8]. Current clinical therapeutic trials have suggested that techniques targeting tumor angiogenesis are promising in the treatment of cancer metastasis and progression. In the last few years, anti-angiogenic therapy has emerged as a promising treatment strategy for tumors. However, while initial tumor shrinkage and survival benefits in terms of time to progression are observed, these effects are transient and tumor growth resumes. Consistently, treatment with angiogenesis-inhibiting anti-VEGF is linked to enhanced invasion and multifocal tumor recurrence, which is associated with aggravated cognitive decline. These findings urge for the identification of new targets to counteract the undesired side effects of anti-angiogenic therapy [9]. Experimental evidence in mouse models and human subjects have implicated that increased angiogenesis is associated with elevated ZEB2. ZEB2 overexpression increases VEGFA synthesis in breast cancer cells and increases formation of new blood vessels [10].
In this study, we investigated the level of ZEB2 expression in colorectal cancer tissues and reported its role in disruption of colorectal cancer cell metastasis and angiogenesis. By silencing and overexpressing ZEB2, we confirmed the role of ZEB2 in the cancer cell metastasis in vitro and in vivo. Furthermore, reduction in ZEB2 expression in HCT116 cells significantly impaired their angiogenesis in vivo. Together, our results provide new evidence that ZEB2 overexpression promotes colon carcinoma metastasis and might represent a novel therapeutic target for its treatment.
Materials and methods
Colon cancer samples and cell lines
A total of 126 paired specimens (adjacent non-tumor tissues and surgically resected colon cancer) were collected from The First Affiliated Hospital of Sun Yat-sen University between January 2006 and March 2007. These tissue samples were obtained from 60 male and 66 female patients with a mean age of 57.5 years (range from 33 to 85 years). Clinic-pathological features of these patients are shown in Supplementary Table 1. All patients were not treated with any chemotherapy or radiotherapy before surgery. The last patient follow-ups were conducted at the end of October 2012. The patients who were lost to follow-up or death from causes other than colon cancer were regarded as censored data during the survival analysis. The samples used in this study were approved by the Committee for Ethical Review of Research at Sun Yat-sen University. The colon cancer cell lines (HT-29, SW480, LOVO and HCT116) was obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle medium (DMEM) (HyClone, USA) or RPMI-1640 (HyClone, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), penicillin (100 IU/ml), and streptomycin (0.1 mg/ml) in 5% CO2 at 37°C. The healthy human colonic epithelial HCoEpiC cell line was obtained from Sciencell (Sciencell Research Laboratories, Carlsbad, CA, USA) and cultured in RPMI-1640 (HyClone, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), penicillin (100 IU/ml), and streptomycin (0.1 mg/ml) in 5% CO2 at 37°C. HUVECs was obtained from CHI Scientific, Inc and cultured in M199 medium supplemented with 10% FBS, penicillin, and streptomycin in 5% CO2 at 37°C.
Establishment of stable expression and knockdown cell lines
Full-length human ZEB2 complementary DNA (cDNA) was amplified with PCR and cloned into the pcDNA3.1 (+) expression vector (Invitrogen, Catalog #V79020) before being transfected into HCT116 cells using Lipofectamine 2000 (Invitrogen, Catalog #11668027) according to the manufacturer’s instructions. Cells transfected with empty vector were used as controls. Lentiviruses containing shRNA targeting ZEB2 were purchased from Santa Cruz Biotechnology (Santa Cruz, Catalog #sc-38642-SH). Cells transfected with scrambled shRNA were used as controls (Santa Cruz, Catalog #sc-108060). The expression of ZEB2 was confirmed by qRT-PCR and Western blotting analysis [11].
MTT assay
HCT116 cells (4×103/well) were seeded with 200 µl of DMEM medium in a 96-well plate and grown up to 24, 48, 72 and 96 hours. At the end of the experiments, 100 µl 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide (MTT, 0.5 mg/ml, Sigma-AldrichCatalog #M2003) was added to each well. The cells were then cultured at 37°C for 4 h, and 200 µl DMSO was added into each well and mixed by shaking at room temperature for 15 min. After that, the absorption rate was measured at 490 nm using a spectrophotometer [12].
Wound healing and transwell invasion assay
HCT116 cells was seeded in six-well culture dishes and cultured at 37°C to form a confluent monolayer. After starvation in serum-free medium for 24 h, a wound was made by scratching the monolayer with a 100 µl pipette tip. Then, the wounded monolayer was washed to remove cell debris with PBS and incubated with fresh normal medium. After scratching, the area of cell-free scratch was photographed at 0 h and 24 h. The wound healing effect was assayed by accounting the number of migrated HCT116 cells [13]. Invasion of HCT116 cells was measured in Matrigel (BD, Catalog #356234) coated Transwell inserts containing polycarbonate filters with 8-μm pores as detailed previously. The inserts were coated with 50 μl of 1 mg/ml Matrigel matrix according to the manufacturer’s recommendations. 2×105 HCT116 cells in 200 μl of serum-free medium were plated in the upper chamber, whereas 600 μl of medium with 10% fatal bovine serum were added to lower well. After 6 h incubation, cells that migrated to the lower surface of the membrane were fixed and stained with 1% crystal violet. For each membrane from each group, five random fields were choose and counted cells invasion number [14].
Colony formation assay
Soft agar colony-formation assays were performed as previously described with minor modifications. HCT116 (1×104) cells in 1.5 ml of DMEM medium were mixed with 1 ml of 0.1% agarose in warmed growth medium containing vehicle (0.1% DMSO) and layered on 1% base agar in 60-mm cell culture dishes. DMEM medium was added every week for 21 days until large colonies were evident. Cells were stained with crystal violet for colony counting [15].
Tube formation assay
The tube formation assay was performed using 12-well plate coated with 100 µl Matrigel basement membrane matrix (BD, Catalog #356234) per well and polymerized at 37°C for 30 min. HUVECs suspended in condition medium from HCT116-shZEB2 or HCT116-ZEB2 cells were plated on the Matrigel at a density of 2×105 cells/well. After 24 h, The Matrigel-induced morphological changes were photographed and the extent of capillary tube formation was evaluated by measuring the total tube length per field [16].
Chick chorioallantoic membrane assay
Fertilized white leghorn chicken embryos were incubated for 3 days at 37°C and 70% humidity. A small hole was made over the air sac at the end of the egg, and a second hole was made directly over the embryonic CAM. After 10 d, supernatant from indicated HCT116 cells or control cells were dropped onto the CAM to form a plug. After 48 h, CAMs were fixed with PBS solution/3.7% paraformaldehyde for 10 min at room temperature, and images were taken with a Nikon digital color camera [17].
Western blotting
Total protein were extracted from HCT116 cells and determined by the Bradford method. 20 μg of lysis were separated by reducing SDS-PAGE and separated proteins were then electronically blotted onto polyvinylidenedifluoride (PVDF) membranes (Millipore, MA). The membranes were then blocked with 5% skim milk and incubated with anti-ZEB2 antibody (1:1000; Santa Cruz Biotechnology, Catalog #sc-271984), anti-MMP-2 (1:1000; Santa Cruz Biotechnology, Catalog #sc-13594), anti-MMP-9 (1:1000; Santa Cruz Biotechnology, Catalog #sc-21733), anti-VEGF-A (1:1000; Santa Cruz Biotechnology, Catalog #sc-152), anti-GAPDH antibody (1:1000; Santa Cruz Biotechnology, Catalog #sc-293335) or anti-β-tublin antibody (1:1000; Santa Cruz Biotechnology, Catalog #sc-73242). Then, blots were washed and probed with respective secondary peroxidase-conjugated antibodies, and the bands visualized by chemoluminescence (Millipore).
Quantative real-time PCR
Total RNA was extracted from indicated HCT116 cells or colon cancer samples using TRIzol reagent (Invitrogen) and we synthesized cDNA was using SuperScript II Reverse Transcriptase (Invitrogen). Quantitative PCR was performed using an ABI Prism 7900HT Sequence detection system (Applied Biosystems, Foster City, CA, USA). Gene’s amplification was performed using the following primers: ZEB2: forward, 5’-CAAGAGGCGCAAACAAGCC-3’; and reverse, 5’-GGTTGGCAATACCGTCATCC-3’. MMP-2: forward, 5’-GGCTCATGCCTTCGCCCCAG-3’; and reverse, 5’-ACTCCCCATCGGCGTTCCCA-3’. MMP-9: forward, 5’-TGACAGCGACAAGAAGTG-3’; and reverse, 5’-CAGTGAAGCGGTACATAGG-3’. GAPDH: forward, 5’-CATCAAGAAGGTGGTGAAGCAG-3’; and reverse, 5’-CGTCAAAGGTGGAGGAGTGG-3’. Samples were run in triplicates, and the samples were normalized to the internal control gene (GAPDH) using the comparative Ct method (ΔΔCt).
In vivo tumor metastasis
Nude mice were purchased from the Shanghai Slac Laboratory Animal Co. Ltd and maintained in microisolator cages (The number of the using of laboratory animal is SCXK (Hu) 2012-0002). This study was approved by the Animal Ethical and Welfare Committee of Sun Yat-sen University (approval number IACUC-DB-16-0216, Guangzhou, China). For metastasis assays, cells were re-suspended in PBS at a concentration of 1×107 cells ml-1. Cell suspension (0.1 ml) was injected into tail veins of nude mice. All of the mice were killed by CO2 14 days after inoculation [18].
Xenograft models and immunohistochemistry detections
This study was approved by the Animal Ethical and Welfare Committee of Sun Yat-sen University (approval number IACUC-DB-16-0216, Guangzhou, China). 3×106 HCT116 cells were subcutaneously implanted into female, BALB/c nude mice to build colon cancer xenograft. Mice with appropriate size of tumors were divided randomly into two groups and tumor volume and mice body weight were measured every 3 days. Tumor volume was calculated as mm3 = 0.5× length (mm) width (mm)2. After sacrificing mice on day 25, formalin-fixed paraffin-embedded tumor sections were stained with specific antibodies including CD31 and Ki67. Ki67 positive cells were counted in five random high-power fields per section and were reported as a percentage of positive cells in each cellular compartment [19]. Mean integrated optical density (mean IOD) of blood vessels accords to the following formula: mean IOD = IOD/area of the tumor section [20]. All animal experiments were carried out in compliance with the Ethical Guidelines for the Sun Yat-sen University.
Statistical analysis
Data was described as the mean ± SD. Comparisons between different groups were undertaken using the Student’s two-tailed t-test or ANOVA test. The statistical significance of the differences between mean values was determined by P < 0.05. Statistical analysis was done with GraphPad Prism 5.
Results
ZEB2 expression was increased in human colon cancer samples and correlated with tumor prognosis
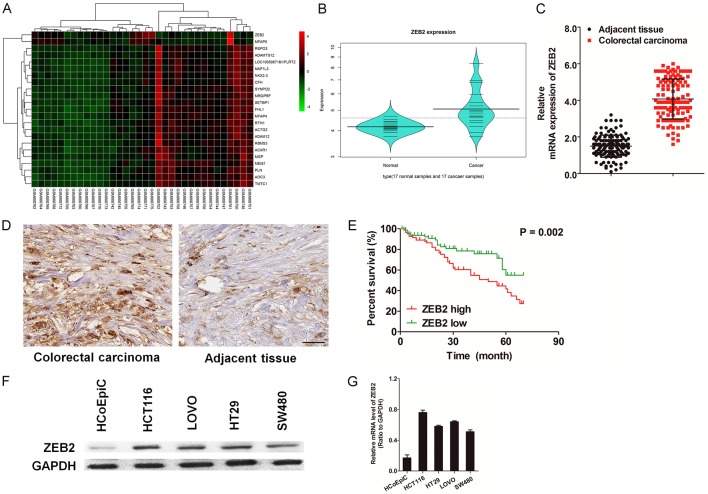
A total of 17 colorectal cancer samples and 17 normal samples were analyzed in GSE32323. The series from each chip was analyzed separately using limma software and finally the list of differentially expressed genes was created. Because of the heterogeneity of gene expression within and among cancer samples and datasets, we used a threshold (P-value ≤ 0.01, Fold change ≥ 2) for differential analysis and screened out the expression genes. As shown in Figure 1A and 1B, we observed that ZEB2 was one of the most upregulated genes in colon cancer tissues. Next, we performed real-time reverse transcriptase polymerase chain reaction (qRT-PCR) to assess ZEB2 expression in 126 samples of colorectal cancer and matched normal tissues. A similar result that ZEB2 was significantly overexpressed in several colorectal cancer samples as compared to corresponding normal tissues was obtained (Figure 1C). We further detected the expression levels of ZEB2 protein in paraffin-embedded colorectal cancer samples and non-cancerous samples using immunohistochemical (IHC) staining (Figure 1D), and observed that ZEB2 protein was overexpressed in colorectal cancer samples. Moreover, the Kaplan-Meier analysis (Figure 1E) demonstrated that the overall survival was significantly impaired in patients with a high level of ZEB2 protein as compared to patients with a low level of ZEB2 protein in the tumors (P = 0.002) (Supplementary Table 1). The association between cancer progression and ZEB2 overexpression was also confirmed in a panel of colon cancer cell lines (Figure 1F). ZEB2 was expressed at relatively high levels in the colon cancer cell lines (HCT116, SW480, LOVO, HT29), but was markedly reduced in healthy human colonic epithelial cell line (HCoEpiC). This reduction was partially due to a decrease in the ZEB2 mRNA levels as shown by qRT-PCR (Figure 1G).
Figure 1.
ZEB2 is significantly up-regulated in human colon cancer tissues and cells. A. Increased ZEB2 expression was shown in colorectal cancer by microarray data analysis of GSE32323 data set retrieved from the GEO database. B. Violin plot summarized ZEB2 expression in the colorectal cancer tissue versus normal tissue according to the heatmap. C. Expression of ZEB2 mRNA was increased in 126 cases of colorectal carcinoma tissues compared with normal tissues by real-time PCR. D. Immunohistochemical staining of ZEB2 in colorectal cancer tissues and matched normal tissues (Scale bar: 100 μM). E. Survival curve for 126 colorectal cancer patients according to ZEB2 protein expression status. High expression of ZEB2 protein was closely correlated with inferior overall survival (OS). F. Western blotting analysis of the levels of ZEB2 in various colon cancer cell lines. Tubulin was used as loading control. G. qRT-PCR analysis of ZEB2 mRNA levels in colonic epithelial cells HCoEpiC and various colon cancer cell lines. PCR values were normalized to the levels of GAPDH. Data are presented as the mean ± SD from three independent measurements.
Down-regulation of ZEB2 inhibits colorectal cancer cell growth and metastasis
To ascertain the precise biological role of ZEB2 upregulation in colorectal carcinoma, we silenced ZEB2 expression in colon cancer HCT116 cells with an shRNA-incorporated plasmid targeting a specific site of human ZEB2 mRNA (Supplementary Figure 1A and 1B). We found that downregulation of ZEB2 in HCT116 cells markedly decreased the growth rate (Figure 2A) and abrogated the anchorage-independent growth ability (Figure 2B). To investigate if functional loss of ZEB2 affected cell migration and invasion, transfected cells were then subjected to wound healing and Transwell invasion assays. Disruption of ZEB2 expression significantly reduced HCT116 cell migration, and wound area recovery by the migration of HCT116-shZEB2 cells was not distinct from that by migration of scrambled shRNA-transfected cells (Figure 2C). Consistently, less HCT116-shZEB2 cells migrated across the membrane of the Transwell chamber than the wild type cells did (Figure 2D). Matrix metalloproteinases (MMP), which are capable of degrading various structural components of the extracellular matrix (ECM), play a critical role in tumor invasion and metastasis. The effect of ZEB2 downregulation on the expression of MMP-2/9 was assessed by qPCR. As expected, MMP-2/9 expression was significantly downregulated at the mRNA level after ZEB2 downregulation (Supplementary Figure 1C). Consistent with this result, the activity of MMP-2/9 was also markedly decreased with the downregulationof ZEB2 (Supplementary Figure 1D). As invasive potential and aggressiveness of colorectal cancer cells were increased, we next investigated the role of ZEB2 in EMT of HCT116 cells. Immunoblot analysis revealed that HCT116-shZEB2 cells showed low expression levels of mesenchymal markers N-cadherin, vimentin, and Twist, and increased expression level of the epithelial marker E-cadherin as compared with those inthe control cells (Figure 2E). Collectively, these results suggest that ZEB2 promotes growth and migration of colorectal cancer cells in vitro.
Figure 2.
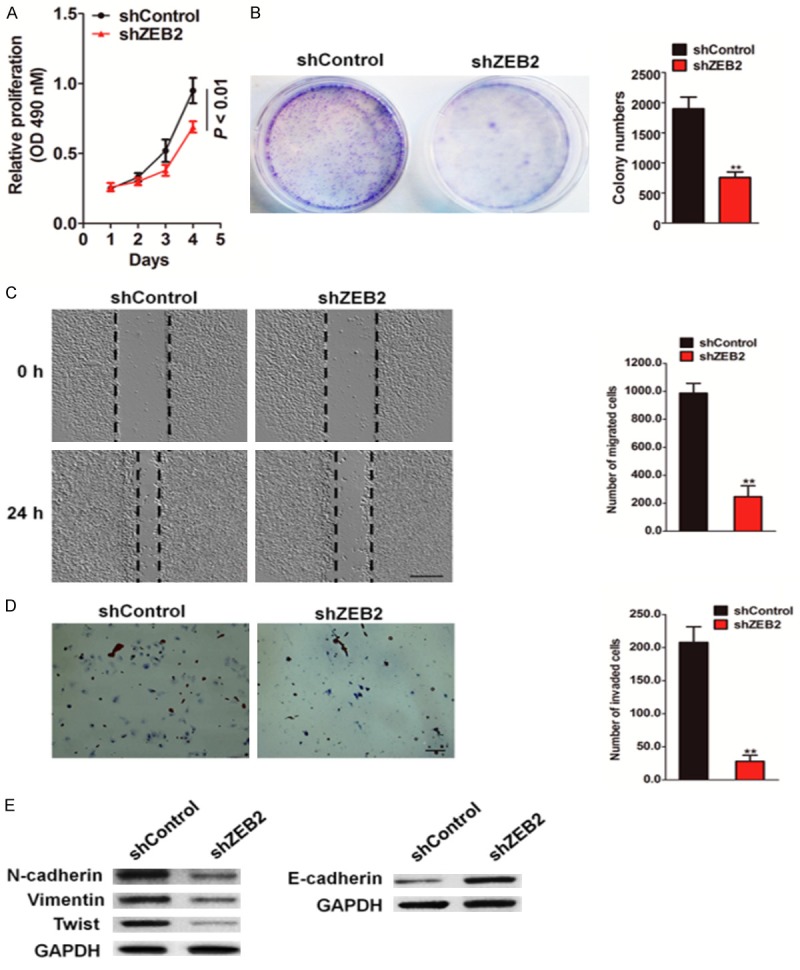
Knock-down of ZEB2 repressed the metastasis capacity of HCT116 cells. A. MTT assay analysis of cell proliferation in HCT116-shZEB2 and control cells at 24, 48, 72 and 96 hours, respectively (*P < 0.01). B. Representative micro-graphs of the colonies formed by indicated HCT116 cells (left panel). Histograms reported the mean ± SD of the colonies formed by indicated cells. C. ZEB2-shRNA and control-shRNA transfected HCT116 cells were wounded with pipette and wound closure percentage was quantified 24 h after scratch relative to that at 0 h. *P < 0.05 compared to the control cells. Scale bar represents 100 μm. D. HCT116 cells transfected with ZEB2 shRNA or control shRNA were seeded in the upper chamber. After 24 h, the cells invaded through the membrane were stained and counted in five random microscopic fields (Scale bar: 50 μM). Representative a histogram of the quantification of invasion number was shown. E. Expression levels of EMT related proteins were investigated by immunoblotting. GAPDH was used as a loading control. *P < 0.05, **P < 0.01 compared between control-shRNA transfected HCT116 cells and ZEB2-shRNA cells; Data were presented as the mean ± SD from three independent measurements.
Confirmation of the role of ZEB2 in colon cancer cell growth and metastasis by gene overexpression
To further confirm the role of ZEB2 in colon carcinoma growth and metastasis, ZEB2 was cloned into pcDNA vector and transfected into HCT116 cells. The transfection efficiency was confirmed by western blotting using ZEB2 antibody, and qRT-PCR (Supplementary Figure 2A and 2B). MTT assays were performed on HCT116-ZEB2 and control cells. The results showed an increase in the proliferation rate of HCT116-ZEB2 cells compared to that of the control cells at 24, 48, 72, and 96 h (Figure 3A). Ectopic expression of ZEB2 in HCT116 cells also markedly augmented the anchorage-independent growth ability (Figure 3B). The migration of ZEB2-overexpressed cells was then examined by wound healing and Transwell assays. As shown in Figure 3C, the wound gaps of cells, which were stably expressing ZEB2 gene, healed faster than that of the control cells did. In accordance with this result, more ZEB2-overexpressed HCT116 cells migrated across the membrane (Figure 3D). To determine if ZEB2 overexpression affected the transcription level of MMP-2/9, the effect of ZEB2 overexpression on the expression of MMP-9 was assessed by qPCR. As expected, the mRNA expression of MMP-9 was significantly upregulated after ZEB2 overexpression (Supplementary Figure 2C). Consistent with the qPCR result, the activity of MMP-2/9 was also markedly increased with ZEB2 overexpression (Supplementary Figure 2D). Next, the expression of EMT markers following ZEB2 overexpression was examined. The results of western blot analysis revealed that expression of the epithelial biomarker, E-cadherin, decreased, whereas that of the mesenchymal markers increased in HCT116-ZEB2 cells (Figure 3E). Collectively, these results suggest that ZEB2 promotes migration of melanoma cells.
Figure 3.
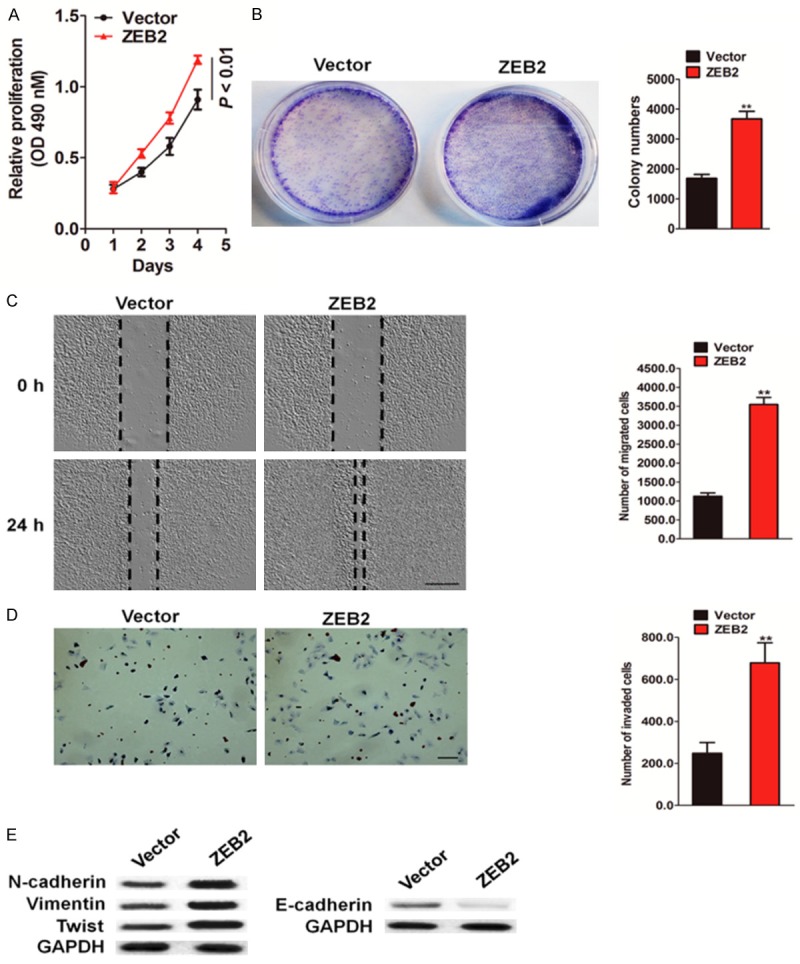
ZEB2 overexpression increases HCT116 cells growth and metastasis. A. MTT assay analysis of cell proliferation in HCT116-ZEB2 and control cells (*P < 0.01). B. Tumor sphere forming ability of HCT116 cells following ZEB2 over-expression. The number of colonies was quantified and shown in the graph right. C. Wound-healing migration assay wad performed with HCT116 cells that transfected with ZEB2 or control vector. Scale bar represents 100 μm. D. Representative images showed that HCT116 cells with high ZEB2 expression exhibit stronger invasive abilities in Matrigel coated Transwell assays. Scale bar represents 50 μm. E. Immunoblots showed the effect of ZEB2 knock-in on N-cadherin, vimentin, Twist and E-cadherin protein in HCT116 cells. *P < 0.05, **P < 0.01 compared between control cells (Vector) and HCT116-ZEB2 cells; Data were presented as the mean ± SD from three independent measurements.
ZEB2 regulates colorectal cancer cell induced angiogenesis
Angiogenesis supplies nutrients for tumor growth and provides the principal route to tumor metastasis. To further investigate the role of ZEB2 in tumor angiogenesis, the culture supernatants derived from ZEB2-depleted HCT116 cells and control cells were collected. The human umbilical vein endothelial cells (HUVECs) were treated with the culture supernatants for assessing tube formation and microvessel formation assays in chick embryo chorioallantoic membrane (CAM). In tube formation assay, the cumulative number of tubular structures formed by HUVECs treated with the culture supernatants were significantly decreased (Figure 4A). After incubation with the culture medium from shRNA-transfected HCT116 cells, microvessel sprouting in CAM was significantly inhibited (Figure 4B). VEGF-A is one of the most well-known potent pro-angiogenic peptides, and modulation of this peptide will likely have a significant consequence on angiogenesis. On treatment of ZEB2 shRNA transfected-HCT116 cells in vitro, ZEB2 downregulation resulted in lower levels of VEGF-A protein expression (Figure 4C) and secretion in conditioned medium (Figure 4D) than that in the control cells. Meanwhile, the ability of the conditioned medium from ZEB2-overexpressed HCT116 cells to induce HUVEC tube formation (Figure 4E) and CAM neovascularization (Figure 4F) was significantly increased. In addition, expression and secretion levels of VEGF-A protein in HCT116-ZEB2 cells were measured by western blotting and enzyme-linked immuno sorbent assay (ELISA). Notably, ZEB2 upregulated VEGF-A protein expression (Figure 4G) and secretion in the medium (Figure 4H). Collectively, these results illustrated that ZEB2 downregulation inhibits HCT116-induced tumor angiogenesis in vitro.
Figure 4.
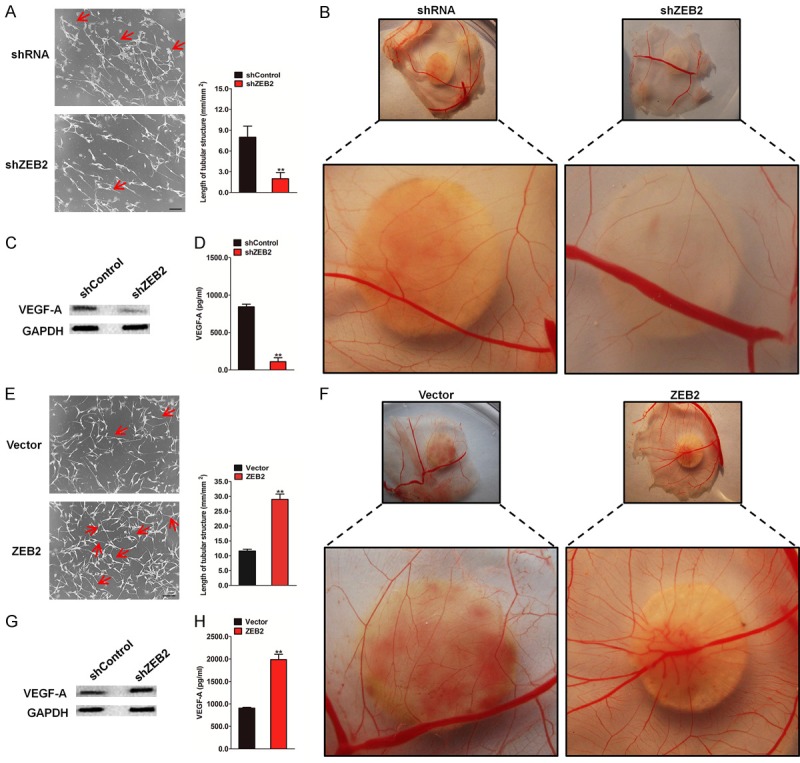
Effect of ZEB2 silencing or over-expression on tumor angiogenesis. A. Tubee formation was analyzed after HUVECs were cultured with conditioned medium from HCT116 cells transfected with shZEB2 or control. The quantification of the tube length was done by Image Pro Plus software and was presented in the histogram right. Scale bar represents 50 μm. B. Representative images of the CAM blood vessels stimulated with conditioned medium from ZEB2-depleted HCT116 cells or control cells. C. Western blotting with anti-VEGF-A antibody of cell lysates from the indicated HCT116 cells. D. Levels of VEGF-A in the medium conditioned from the indicated cells and harvested at 24 h. Each bar represents the mean ± SD of three independent experiments. **P < 0.01. E. Representative images (left panel) and quantification (right panel) of HUVECs cultured on matrigel-coated plates with conditioned medium from ZEB2-transfected HCT116 cells or control cells. Scale bar represents 50 μm. F. Representative images of the CAM blood vessels stimulated with conditioned medium from indicated HCT116 cells. G. Western blotting analysis of ZEB2 levels in HCT116 control and HCT116-ZEB2 cells. H. Levels of VEGF-A in the medium conditioned from the indicated cells and harvested at 24 h. Each bar represents the mean ± SD of three independent experiments. **P < 0.01.
ZEB2 contributes to the growth and metastasis of HCT116 cells in vivo
To further investigate the role of ZEB2 in the metastasis of HCT116 cells in vivo, an experimental metastasis assay was performed. HCT116 cells transfected with ZEB2 shRNA or control shRNA were injected into the lateral tail vein of nude mice. After 2 weeks post inoculation, animals were sacrificed and all the major organs were examined for the presence of tumor metastasis. Tumor metastasis was mainly observed in the lungs as previously reported. The results revealed that administration of HCT116 control cells resulted in the formation of numerous lung colonies, whereas silencing of ZEB2 significantly suppressed pulmonary metastasis and generated only a few lung colonies (Figure 5A). In contrast, more metastatic tumors were detected in mice injected HCT116-ZEB2 cells compared to those injected with control cells (Figure 6A). These results implied that ZEB2 silencing indeed perturbed the metastasis of HCT116 cells not only in vitro, but also in vivo. Considering our findings that ZEB2 depletion causes the loss of proliferation and tumor sphere forming ability of HCT116 cells, the in vivo relevance of ZEB2 in colon cancer was addressed. Tumor growth after implantation of ZEB2-depleted or control cells into mice was monitored over 25-day duration. Over this period, ZEB2 inhibition significantly impaired the growth of xenografts (Figure 5B). Conversely, tumors formed by ZEB2-overexpressing HCT116 cells were largerthan those formed by control cells (Figure 6B). IHC analysis revealed that ZEB2-silenced tumors displayed lower Ki67 proliferation index and microvascular density (MVD) (Figure 5C), whereas ZEB2-overexpressing tumors showed increased percentage of Ki67-positive cells and higher MVD (Figure 6C). Taken together, our finding indicates that ZEB2 contributes to colon growth and angiogenesis in vivo.
Figure 5.
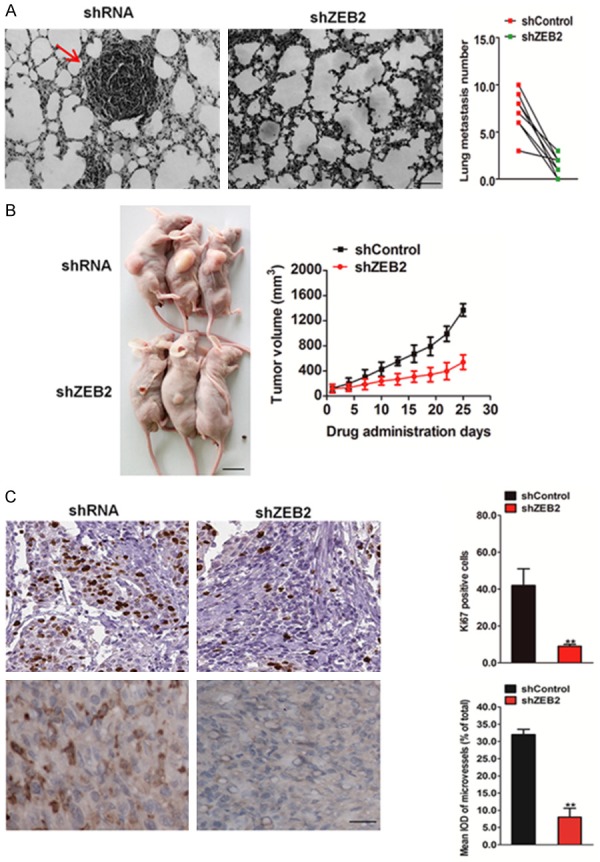
The effects of ZEB2 on growth and metastasis of HCT116 cells in vivo. A. Representative images of histologic inspection of a mouse lung for the presence of microscopic lesions twelve weeks after tail vein injection with HCT116 cells (left). Quantification of lung microscopic nodules in the lungs of each group was shown (right). Scale bar represents 100 μm. B. As a subcutaneous tumor model, BALB/c nude mice were injected subcutaneously with HCT116 cells and followed by the determination of the tumor volume. Scale bar represents 1 cm. C. Mice bearing HCT116 tumor xenograft were sacrificed at the end of the experiment, and tumor tissues were removed for future immunohistochemistry analysis with anti-Ki67 and anti-CD31 antibodies. The data were presented as the mean ± SD. *P < 0.05, **P < 0.01 vs. sh Control group.
Figure 6.
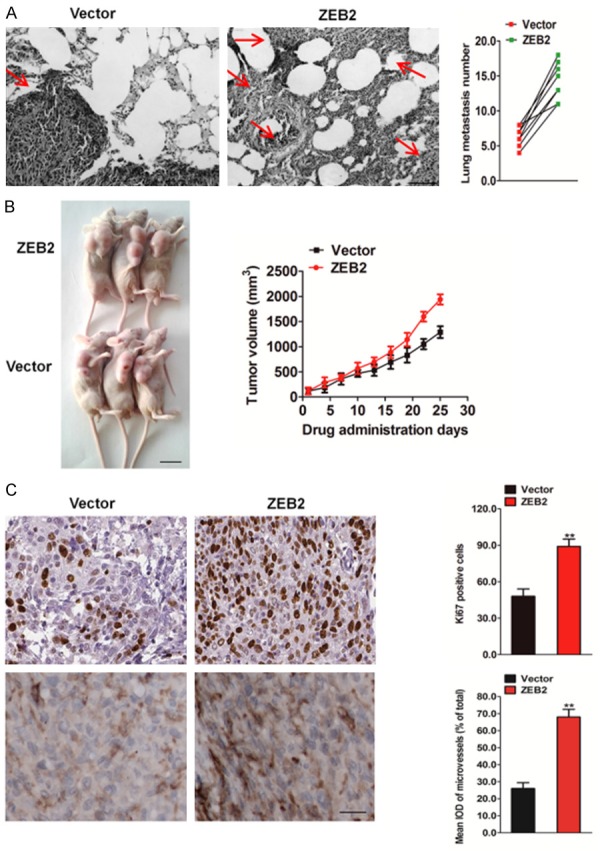
Over-expression of ZEB2 contributes to HCT116 cells growth and metastasis in vivo. A. Metastatic lesions in the lungs of the mice which injected with HCT116-ZEB2 cells or control cells via tail vein (left). The total numbers of lung metastatic lesions in the ZEB2 transfection groups were much higher than those in controls (right). Scale bar represents 100 μm. B. The tumor growth was significantly promoted in mice subcutaneously inoculation with HCT116-ZEB2 cells versus control. Representative images of tumor-bearing mice (left) and tumor volumes were measured on the indicated days (right). Scale bar represents 1 cm. C. Mice bearing HCT116 tumor xenograft were sacrificed at the end of the experiment, and tumor tissues were removed for immunohistochemistry analysis with anti-Ki67 and anti-CD31 antibodies. Immunohistochemistry staining demonstrated the expression of Ki67 and CD31 positive cells in the indicated tissues. The data were presented as the mean ± SD. *P < 0.05, **P < 0.01 vs. vector group.
Discussion
To advance the human colorectal cancer treatment, it is necessary to identify candidate genes inducing tumor metastasis and to functionally validate their biological significance as well as the molecular mechanisms underlying metastasis [21]. In the present study, we found that ZEB2 expression was higher in colon cancer tissues than in non-tumor tissues, and it positively correlated with infiltration depth, lymph node and distant metastases, and advanced tumor-node-metastasis (TNM) stages of colon cancer. This finding is consistent with ZEB2 expression in colorectal cancer cell lines, which is higher than that in a normal cell line. Functional studies identified that ZEB2 is necessary for colon cell proliferation and colony formation, as well as migration, invasion, and tumor cell-induced angiogenesis. Thus, our study provides the mechanistic basis for targeting ZEB2 in patients with metastatic colon cancer.
ZEB2 has been predicted to play a role in cell adhesion and migration due to its leucine-rich repeats and tendency to bind to ECM proteins [22]. A previous study showed that ZEB2 promoted proliferation, migration, and invasion of glioma cells, and downregulated E-cadherin expression [23]. However, ZEB2 has been reported to act as a tumor suppressor in hepatocellular and endometrial carcinomas. The discrepancy in ZEB2 function in certain cancers may be due to its tissue specificity. Although ZEB2 is implicated in various malignant carcinomas, the biological functions of ZEB2 in colon cancer have not been fully elucidated [24]. The role of the ZEB2 in human colon cancer is so far poorly known, and our study demonstrates that ZEB2 functions as a potential promoter of human colon cancer growth and metastasis. To understand the role of ZEB2 in colon cancer, we knocked down ZEB2 expression in colon cancer cell line, HCT116. We further explored the role of ZEB2 in the growth of colon cancer cells in vitro. Depletion of ZEB2 was associated with impaired proliferation and tumor cell clone formation. Consistent with the results of two other previous studies on human cancers, the results of our study showed that downregulation of ZEB2 significantly inhibited colon cancer cell growth in nude mouse xenografts. ZEB2 positively regulated HCT116 cell proliferation and colony formation ability, which suggests that the downregulation of ZEB2 inhibits colorectal carcinoma progression. Meanwhile, our experiments demonstrated that knockdown of ZEB2 by shRNA significantly attenuated the migratory and invasive potency of colon cancer cells. Moreover, depletion of ZEB2 caused upregulation of E-cadherin epithelial marker and downregulation of N-cadherin, Twist, and Vimentin mesenchymal markers. In contrast to ZEB2 downregulation, ZEB2 overexpression in HCT116 cells accelerated proliferative potential and colony formation in vitro. At the molecular level, MMP-2 and MMP-9 are associated with cell migration influencing cancer development and progression [25]. We found that knockdown of ZEB2 expression suppressed MMP-2 and MMP-9 expression, indicating the biological significance of ZEB2 in colon cancer progression. In contrast, ZEB2 overexpression was able to upregulate MMP-2/9 expression and activity in HCT116 cancer cells, which is partially consistent with the results of another previous study, which showed that knockdown of ZEB2 reduces gastric cancer cell metastasis and MMP-2/9 downregulation [26].
Angiogenesis is critical for growth and metastasis of cancer cells to distant organs, and many studies have demonstrated that angiogenesis inhibitor provides significant therapeutic advantages. Cytokines derived from the tumor microenvironment could facilitate the activities of endothelial cells and promote angiogenesis. Here, we used the conditional medium from HCT116 cells to determine the modulation of endothelial cell tube formation, which is a critical indicator of angiogenic ability, and induction of vascular formation in chick embryo CAM by ZEB2 expression. We found that endothelial cells cultured in conditional medium from knockdown HCT116 cells exhibited a decreased tube formation capacity and the number of capillary blood vessels in CAM was also markedly reduced. In contrast, overexpression of ZEB2 in HCT116 cells promotes the endothelial cell tube formation and angiogenesis in CAM. These findings, together with previous studies, showed that ZEB2 promotes angiogenesis, which suggests that it might contribute to tumor growth by regulating crucial molecules involved in tumor vascularization. The fact that angiogenesis is regulated by ZEB2 urged us to evaluate the role of ZEB2 with respect to VEGF-A, a well characterized molecule in tumor angiogenesis, secretion, and expression. We showed that ZEB2 positively influenced VEGF secretion and expression in HCT116 cancer cells.
The biological effects of ZEB2 overexpression on colon cancer progression were examined using a tumor xenograft model. Tumors formed by ZEB2-overexpressing HCT116 cells were larger in size than tumors formed by the control cells. IHC analysis revealed that ZEB2-overexpressing tumors showed increased percentage of Ki67-positive cells and higher MVD, whereas tumors formed by ZEB2-silenced HCT116 cells were smaller and ZEB2 silenced tumors displayed lower Ki67 proliferation index and MVD. Taken together, our findings indicate that ZEB2 contributes to colon cancer growth and angiogenesis in vivo. In our metastatic model system, ZEB2 was identified as the promoter gene in HCT116 cell metastasis in vivo. Lung metastasis was apparent, and contrastingly a few metastatic tumors were detected in mice administered with HCT116-ZEB2 cells. Briefly, our results indicate that decreased ZEB2 expression inhibits HCT116 cell metastasis in vivo. In this study, we provided evidence for the first time that ZEB2 expression is closely associated with the clinico-pathological parameters of colon cancer, and ZEB2 promotes colon cancer cell growth, metastasis, and angiogenesis. These findings would facilitate the development of new drugs targetingZEB2 for anti-cancer therapy.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Hubbard J. Management of colorectal cancer in older adults. Clin Geriatr Med. 2016;32:97–111. doi: 10.1016/j.cger.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Vulcan A, Brändstedt J, Manjer J, Jirström K, Ohlsson B, Ericson U. Fibre intake and incident colorectal cancer depending on fibre source, sex, tumour location and tumour, node, metastasis stage. Br J Nutr. 2015;114:959–969. doi: 10.1017/S0007114515002743. [DOI] [PubMed] [Google Scholar]
- 3.Tian Y, Xu T, Huang J, Zhang L, Xu S, Xiong B, Wang Y, Tang H. Tissue metabonomic phenotyping for diagnosis and prognosis of human colorectal cancer. Sci Rep. 2016;6:20790. doi: 10.1038/srep20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahlert C, Lahes S, Radhakrishnan P, Dutta S, Mogler C, Herpel E, Brand K, Steinert G, Schneider M, Mollenhauer M, Reissfelder C, Klupp F, Fritzmann J, Wunder C, Benner A, Kloor M, Huth C, Contin P, Ulrich A, Koch M, Weitz J. Overexpression of ZEB2 at the invasion front of colorectal cancer is an independent prognostic marker and regulates tumor invasion in vitro. Clin Cancer Res. 2011;17:7654–7663. doi: 10.1158/1078-0432.CCR-10-2816. [DOI] [PubMed] [Google Scholar]
- 5.Nam E, Lee Y, Park Y, Lee J, Kim S. ZEB2 upregulates integrin α5 expression through cooperation with Sp1 to induce invasion during epithelial-mesenchymal transition of human cancer cells. Carcinogenesis. 2012;33:563–571. doi: 10.1093/carcin/bgs005. [DOI] [PubMed] [Google Scholar]
- 6.Dai Y, Tang Y, Zhu H, Lv L, Chu Y, Zhou Y, Huo J. ZEB2 promotes the metastasis of gastric cancer and modulates epithelial mesenchymal transition of gastric cancer cells. Dig Dis Sci. 2012;57:1253–1260. doi: 10.1007/s10620-012-2042-6. [DOI] [PubMed] [Google Scholar]
- 7.Cong N, Du P, Zhang A, Shen F, Su J, Pu P, Wang T, Zjang J, Kang C, Zhang Q. Downregulated microRNA-200a promotes EMT and tumor growth through the wnt/β-catenin pathway by targeting the E-cadherin repressors ZEB1/ZEB2 in gastric adenocarcinoma. Oncol Rep. 2013;29:1579–87. doi: 10.3892/or.2013.2267. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Luo H, Shi Q, Hao Z, Ding Y, Wang Q, Li S, Xiao G, Tong S. miR-132 inhibits colorectal cancer invasion and metastasis via directly targeting ZEB2. World J Gastroenterol. 2014;20:6515–6522. doi: 10.3748/wjg.v20.i21.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Z, Brooks S, Dormoy V, Hsu C, Hsu H, Lin L, Massfelder T, Rathmell W, Xia M, Al-Mulla F, Al-Temaimi R, Amedei A, Brown D, Prudhomme K, Colacci A, Hamid R, Mondello C, Raju J, Ryan E, Woodrick J, Scovassi A, Singh N, Vaccari M, Roy R, Forte S, Memeo L, Salem H, Lowe L, Jensen L, Bisson W, Kleinstreuer N. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: focus on the cancer hallmark of tumor angiogenesis. Carcinogenesis. 2015;36(Suppl 1):S184–202. doi: 10.1093/carcin/bgv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang T, Panda H, Luo X, Chegini N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr Relat Cancer. 2012;19:541–556. doi: 10.1530/ERC-12-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XN, Ji C, Zhou PK, Wu YM. Establishments of IER5 silence and overexpression cervical cancer SiHa cell lines and analysis of radiosensitivity. Int J Clin Exp Pathol. 2016;9:6672–6682. [Google Scholar]
- 12.Hou XC, Wan WC, Wang J, Li MZ, Wang YW, Yao YB, Feng LH, Jing LJ, Lu H, Jia YJ, Peng T. Let-7a inhibits migration of melanoma cells via down-regulation of HMGA2 expression. Am J Transl Res. 2016;8:3656–3665. [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CQ, Sun HT, Gao XM, Ren N, Sheng YY, Wang Z, Zheng Y, Wei JW, Zhang KL, Yu XX, Zhu Y, Luo Q, Yang LY, Dong QZ, Qin LX. Interleukin-6 enhances cancer stemness and promotes metastasis of hepatocellular carcinoma via up-regulating osteopontin expression. Am J Cancer Res. 2016;6:1873–1889. [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu Z, Yuan W, Chen T, Zhou C, Liu C, Huang Y, Han D, Huang Q. HMGCR positively regulated the growth and migration of glioblastoma cells. Gene. 2016;576:22–27. doi: 10.1016/j.gene.2015.09.067. [DOI] [PubMed] [Google Scholar]
- 15.Fahrioğlu U, Dodurga Y, Elmas L, Seçme M. Ferulic acid decreases cell viability and colony formation while inhibiting migration of MIA PaCa-2 human pancreatic cancer cells in vitro. Gene. 2016;576:476–482. doi: 10.1016/j.gene.2015.10.061. [DOI] [PubMed] [Google Scholar]
- 16.Chiang K, Sun C, Chen M, Huang C, Hsu J, Yeh T, Chen L, Kuo S, Juang H, Takano M, Kittaka A, Chen T, Yeh C, Pang J. MART-10, the new brand of 1α, 25(OH)2D3 analog, is a potent anti-angiogenic agent in vivo and in vitro. J Steroid Biochem Mol Biol. 2016;155:26–34. doi: 10.1016/j.jsbmb.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Jridi I, Catacchio I, Majdoub H, Shahbazeddah D, El Ayeb M, Frassanito M, Ribatti D, Vacca A, Borchani L. Hemilipin, a novel Hemiscorpius lepturus venom heterodimeric phospholipase A2, which inhibits angiogenesis in vitro and in vivo. Toxicon. 2015;105:34–44. doi: 10.1016/j.toxicon.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, Wei JW, Zhou HJ, Ren N, Ye QH, Dong QZ, Qin LX. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-stat3 pathway. Hepatology. 2013;58:158–170. doi: 10.1002/hep.26305. [DOI] [PubMed] [Google Scholar]
- 19.Nesbitt H, Browne G, O’Donovan K, Byrne N, Worthington J, McKeown S, McKenna D. Nitric oxide up-regulates RUNX2 in LNCaP prostate tumours: implications for tumour growth in vitro and in vivo. J Cell Physiol. 2016;231:473–482. doi: 10.1002/jcp.25093. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Lim I, Joo H, Choi S, Choi J, Cui L, Im L, Hong S, Lim D. Sphere formation of adipose stem cell engineered by poly-2-hydroxyethyl methacrylate induces in vitro angiogenesis through fibroblast growth factor 2. Biochem Biophys Res Commun. 2015;468:372–379. doi: 10.1016/j.bbrc.2015.10.083. [DOI] [PubMed] [Google Scholar]
- 21.Yoshihara K, Tajima A, Komata D, Yamamoto T, Kodama S, Fujiwara H, Suzuki M, Onishi Y, Hatae M, Sueyoshi K, Fujiwara H, Kudo Y, Inoue I, Tanaka K. Gene expression profiling of advanced-stage serous ovarian cancers distinguishes novel subclasses and implicates ZEB2 in tumor progression and prognosis. Cancer Sci. 2009;100:1421–1428. doi: 10.1111/j.1349-7006.2009.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong Y, Syed Zanaruddin S, Lau S, Ramanathan A, Kallarakkal T, Vincent-Chong V, Wan Mustafa W, Abraham M, Abdul Rahman Z, Zain R, Cheong S. Co-expression of TWIST1 and ZEB2 in oral squamous cell carcinoma is associated with poor survival. PLoS One. 2015;10:e0134045. doi: 10.1371/journal.pone.0134045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koopmansch B, Berx G, Foidart J, Gilles C, Winkler R. Interplay between KLF4 and ZEB2/SIP1 in the regulation of E-cadherin expression. Biochem Biophys Res Commun. 2013;431:652–7. doi: 10.1016/j.bbrc.2013.01.070. [DOI] [PubMed] [Google Scholar]
- 24.Kurashige J, Kamohara H, Watanabe M, Hiyoshi Y, Iwatsuki M, Tanaka Y, Kinoshita K, Saito S, Baba Y, Baba H. MicroRNA-200b regulates cell proliferation, invasion, and migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg Oncol. 2012;19(Suppl 3):S656–664. doi: 10.1245/s10434-012-2217-6. [DOI] [PubMed] [Google Scholar]
- 25.Lima A, Mota J, Monteiro S, Ferreira R. Legume seeds and colorectal cancer revisited: protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem. 2016;197:30–38. doi: 10.1016/j.foodchem.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 26.Gamba C, Campos L, Negreiros-Lima G, Maciel-Lima K, Sousa L, Estrela-Lima A, Ferreira E, Cassali G. ZEB2 and ZEB1 expression in a spontaneous canine model of invasive micropapillary carcinoma of the mammary gland. Res Vet Sci. 2014;97:554–559. doi: 10.1016/j.rvsc.2014.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.