Abstract
CD47 is an antiphagocytic signal that cancer cells employ to inhibit macrophage-mediated destruction. CD47 is overexpressed in various human malignancies. However, the expression and functional significance of CD47 in high-grade serous ovarian carcinoma (HGSOC) has not been completely understood. In this study, we reported that CD47 was commonly overexpressed in HGSOC. Higher CD47 expression was significantly correlated with poor prognosis of HGSOC patients. Functional investigations revealed that CD47 overexpression in ovarian cancer cells significantly promoted migration and invasion. Moreover, CD47 induced epithelial-mesenchymal transition (EMT) through modulating E-cadherin and N-cadherin. Our findings suggest that up-regulation of CD47 is correlated with ovarian cancer progression and it might be a potential biomarker for predicting clinical outcomes.
Keywords: CD47, prognosis, high-grade serous ovarian carcinoma, invasion, EMT
Introduction
Ovarian cancer is one of most common gynecological cancer and the leading cause of death all around the world. There was estimated to 52,100 new cases and 22,500 deaths from ovarian cancer in China in 2015 [1]. High-grade serous ovarian carcinomas (HGSOCs), accounting for up to 60% of ovarian epithelial carcinoma, are genetically and histologically heterogeneous tumors [2]. Despite the advances in the research and treatment, the molecular pathogenesis of HGSOC has not been fully characterized. This is due to the fact that the vast majority of cases are not detected until late-stage. Ovarian cancer is traditionally thought to be derived from ovarian surface epithelium (OSE) [3]. Recent studies suggest that ovarian cancer may arise from precursor lesions located in the fallopian tubal epithelium (FTE) [4]. The origin and molecular pathogenesis of HGSOC are largely unknown.
CD47 is a widely expressed transmembrane protein that interacts with several molecules including integrins, thrombospondins and signal regulatory protein alpha (SIRPα) [5]. Upon binding CD47, the protein SIRPα that expressed on macrophages and dendritic cells initiates a signaling cascade and results in the inhibition of phagocytosis [5]. Increasing data show that CD47 is nearly expressed on all cancers, and blockade of its function leads to tumor cell phagocytosis and elimination. In addition to serving as a potent signal to aid escape from immune surveillance, CD47 also plays a role in cellular proliferation, apoptosis, adhesion and migration. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway [6]. The role of CD47 in cell migration was first demonstrated for neutrophils, where CD47 blocking antibodies inhibited transmigration of neutrophils and monocytes through the endothelium [7] and blocking CD47 function by neutralizing antibodies has been shown to inhibit migration and metastasis in tumor models [8].
CD47 was first identified as a tumor antigen on human ovarian cancer in the 1980s [9] and the increasing circulating level in serum was present with increasing tumor burden and higher histological grade, especially for ovarian cancer patients with serous and unclassified adenocarcinomas [10]. CD47 expression was amplified in 15/316 (5%) ovarian serous cancers in the TCGA database. Patients with CD47 low-expression had a better treatment response to standard therapy, and trended towards improved overall survival in ovarian cancer [11].
In the present study, we investigated the clinical significance and functional role of CD47 in ovarian cancer. We found CD47 was commonly overexpressed in HGSOC. High expression of CD47 was correlated with poor prognosis in HGSOC patients. Functional investigations revealed that CD47 overexpression in ovarian cancer cells significantly promoted invasion and induced epithelial-mesenchymal transition (EMT).
Materials and methods
Patients and tissue samples
All evaluated HGSOC and fallopian tube tissues were collected in Qilu Hospital of Shandong University between November 2005 and December 2012. This study included 153 cases of HGSOC and 70 cases of fallopian tube tissues. HGSOC tissues were obtained from primary ovarian cancer patients receiving no surgery or chemotherapy previously. Fallopian tube tissues were from patients who received a total hysterectomy and bilateral salpingo-oophorectomy for uterine diseases or for benign neoplastic adnexal pathologic changes. The tissue sections were collected and prepared for tissue microarray (TMA) as described previously [12]. Ethics Committee of Shandong University approved the study and all participants gave written informed consent.
Immunohistochemical staining
Immunohistochemical staining was performed on 4-µm paraffin tissue sections.
The tissue sections were deparaffinized in xylene and rehydrated in a graded series of ethanol. Antigen retrieval was performed by microwave irradiation in citrate retrieval solution (PH 6.0). Nonspecific binding was blocked with donkey serum and sections were then incubated with anti-CD47 antibody (1:200, Abcam, Cambridge, UK) overnight at 4°C. The signal was developed by DAB detection system and counterstained with hematoxylin. The staining results were independently evaluated by two investigators blinded to the clinical data.
Cell lines and cell cultures
T29 (Normal ovarian surface epithelial, OSE) and FTE187 (fallopian tube secretory epithelial, FTSE) cell lines were from Jinsong Liu’s lab and maintained in cell culture medium as previously described [13]. HEK293T and ovarian cancer cell lines A2780, SKOV3, HEY and OVCAR3 were originally purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). A2780 and OVCAR3 cells were cultured in RPMI-1640 medium (Gibco, Invitrogen). SKOV3, HEY and HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen). The media were supplemented with 10% FBS (CST, USA), 100 U/ml penicillin and 100 μg/ml streptomycin. All cells were cultured at 37°C with 5% CO2 in a humidified incubator (Thermo Fisher Scientific).
Plasmid construction and lentivirus production
To generate CD47 overexpression construct, the ORF sequence of CD47 was cloned into the pLenti-C-Myc-DDK-IRES-Puro (PCMV) vector (Origene, USA). Lentivirus expressing CD47 were produced in HEK293T cells packaged with psPAX2 and pMD2.G. A2780 cells were infected for 24 hours and then selected for one week in RPMI-1640 medium containing 2 μg/ml puromycin (Merck Millipore, USA) to acquire stable cell lines NC-A2780 and CD47-OVE-A2780. Primers for plasmid construction were shown in Supplementary Table 1.
siRNA transfection
siRNAs for CD47 knockdown were purchased from GenePharma (Shanghai, China). A2780 and SKOV3 cells were transfected with siRNA by Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocol. siRNA sequences were listed in Supplementary Table 1.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using TRIZOL reagent (Invitrogen, USA) and cDNA was synthesized using PrimeScript RT reagent Kit (Takara, Japan). qRT-PCR was performed using SYBR-Green qPCR master mix (Takara, Japan). Further details were shown in Supplementary Table 1.
Western blots
Protein samples were separated by SDS-PAGE and electrotransferred onto PVDF membrane (Millipore). After blocking with 5% non-fat milk, the membrane was incubated overnight at 4°C with the primary antibody and then with horseradish peroxydase-coupled secondary antibody. Signal was detected with enhanced chemiluminescence (ECL; GE, USA). Antibodies in this study included anti-CD47 (Abcam, USA), anti-E-cadherin, anti-N-cadherin (CST, USA) and anti-β-actin (Sigma-Aldrich).
Clonogenic assay
Single-cell suspensions were plated on 6-well plates (300-500 cells/well) and cultured for 2-3 weeks. The colonies were fixed with methanol and stained with 0.1% crystal violet. Colonies of greater than 50 cells were counted. The data presented is the mean ± standard error (SE) and represents three independent experiments.
Cell cycle analysis
After serum starvation for 12 h, the medium was replaced with complete medium for another 12 hours and cells were harvested and fixed with 75% ice-cold ethanol overnight. Cell suspensions were then stained with propidium iodine (50 µg/mL) and analyzed by flow cytometry (BD Biosciences, USA).
Migration and invasion assays
Migration and invasion assays were performed using transwell system (24-well, 8 μm pore size; BD falcon). Invasion assay was performed in the same way as the migration assay except that the membrane was coated with Matrigel (BD Biosciences, San Jose, CA, USA). Briefly, 1.5×105 cells were seeded into the insert with serum-free medium and the bottom medium containing 20% FBS as a chemo-attractant. After incubating at 37°C for 12 to 48 h depending on the cell lines, the inserts were fixed with methanol and stained with 0.1% crystal violet. Invaded cells were scanned and counted in at least 6 different microscopic fields.
Immunofluorescence analysis
Cells were seeded on coverslips in the 24-well plates and cultured for 24 h. Coverslips were then fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were blocked with 10% goat serum in PBS and then incubated with anti-N-cadherin antibodies (1:200) at 4°C overnight. Followed by incubating with rhodamine-labeled secondary antibody (Beyotime Biotechnology) for 1 hour at room temperature in the dark, the cells were counterstained with DAPI (Life Technologies). Finally, the slides were mounted with anti-fading reagent and images were captured under a fluorescence microscope (Olympus, Tokyo, Japan).
Statistical analysis
Statistical analysis was carried out using SPSS V17.0. Survival curves were calculated by Kaplan-Meier methods, with comparisons performed by the log-rank test. Chi-square test was used to analyze the differences of clinical characteristics. Student’s t-test was chosen to analyze the differential expression of quantitative data. Statistically significance was considered as: *P≤0.05 or **P≤0.01.
Results
CD47 is highly expressed in epithelial hyperplasia of the fallopian tube (FT)
Recent evidences suggest most high-grade ovarian serous carcinomas originate in the FTs [14,15]. To evaluate CD47 expression in FT tissues, we immunohistochemically examined CD47 expression in normal (n=36) and hyperplasia (n=34) FTs. As shown in Figure 1A and 1B, CD47 showed weak staining in normal FTs (25/36, 69.4%) and while showed moderate or strong staining on hyperplasia FTs (29/34, 85.3%). Interestingly, in the same FT sample, CD47 was highly expressed in multiple layer area than single layer area (Figure 1C). These findings suggested that CD47 may be used for detection of secretory cell hyperplasia of FT.
Figure 1.
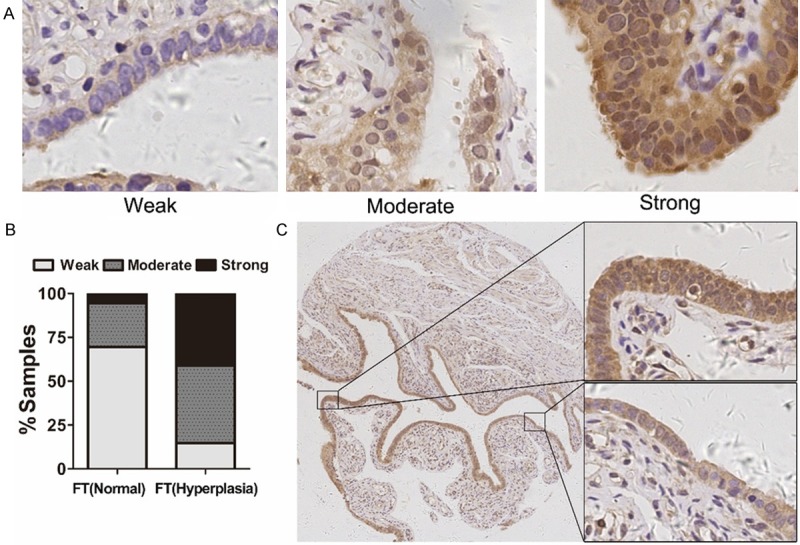
CD47 expression in epithelial hyperplasia and normal fallopian tube. A: Immunohistochemical staining of CD47 expression in normal (n=36) and hyperplasia (n=34) FTs. B: The histogram indicates the CD47 expression level distribution in normal and hyperplasia FTs. C: CD47 expression in the same FT sample including multiple layer area and single layer area.
CD47 is overexpressed in HGSOC
To investigate CD47 expression level in HGSOC patients, we first performed qPCR to measure CD47 expression in normal FT (n=30) and HGSOC (n=39) tissues. We observed that CD47 mRNA level in HGSOC group was significantly higher than in the normal FT group (Figure 2A). To confirm CD47 overexpression in HGSOC, we conducted western blot for CD47 innormal FT (n=12) and HGSOC (n=19) tissues. As illustrated in Figure 2B, CD47 protein level was increased in most HGSOC tissues. Consistently, immunohistochemical staining revealed a significant increase of CD47 level in HGSOC group compared with FT group (Figure 2C). We further measured CD47 expression in ovarian cancer cell lines and normal cell lines and found ovarian cancer cell lines exhibited higher CD47 expression levels than normal cell lines (Figure 2D). These findings indicated that CD47 overexpression was common in HGSOC.
Figure 2.
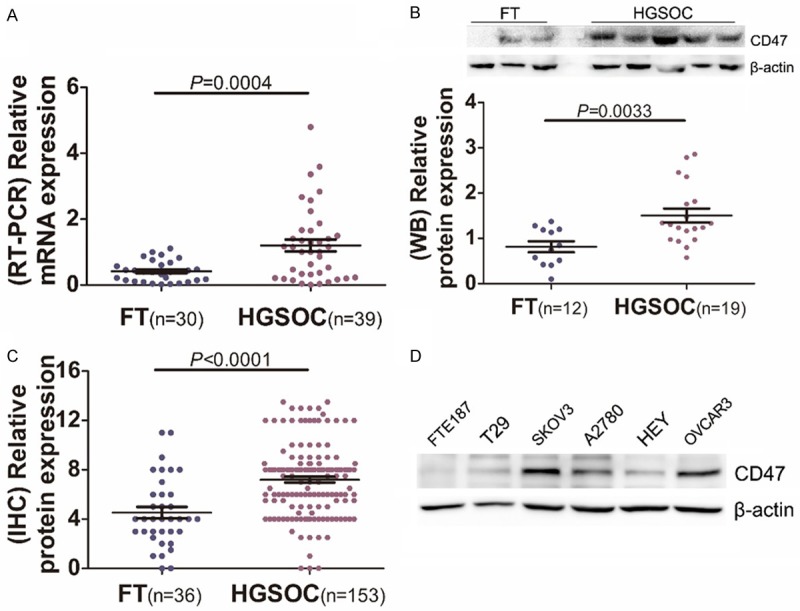
Expression analysis of CD47 in high-grade serous ovarian carcinoma. A: Relative expression of CD47 in HGSOCs (n=39) and FTs (n=30) measured by Real-time PCR. B: Western blot analysis of CD47 in HGSOCs (n=19) compared to FTs (n=12). C: Quantitative immunohistochemical analysis of CD47 expression in HGSOCs (n=153) and FTs (n=36). D: CD47 expression in ovarian cancer cell lines and two normal cell lines measured by western blot.
Correlation between CD47 expression and clinic-pathological parameters
We next analyzed clinicopathological and prognostic significance of CD47 expression in HGSOC. Interestingly, we found elevated CD47 expression was associated with high level of CA125 in HGSOC patients (Table 1). While Elevated CD47 expression was not associated with other clinic-pathological factors. We further evaluated the association between CD47 expression and overall survial of patients with HGSOC by Kaplan-Meier method. Patients with higher CD47 expression had a significantly shorter overall survival time than did patients with low CD47 expression (Figure 3B). These observations indicate that upregulation of CD47 was closely associated with progression and poor prognosis in HGSOC patients.
Table 1.
Characteristics and CD47 expression (IHC) in ovarian cancer patients
| Clinicopathological Variables | Number | CD47 expression | P valuea | |
|---|---|---|---|---|
|
| ||||
| Low level | High level | |||
| Age (years) | ||||
| <56 | 74 | 29 | 45 | 0.7425 |
| ≥56 | 79 | 34 | 45 | |
| Stage | ||||
| Early (stages I) | 36 | 13 | 23 | 0.5631 |
| Advanced (stages III-IV) | 117 | 50 | 67 | |
| CA125 in serum (U/mL) | ||||
| <600 | 63 | 33 | 30 | 0.0205 |
| ≥600 | 90 | 30 | 60 | |
| Lymph nodes metastasis | ||||
| Negative | 27 | 10 | 17 | 0.7822 |
| Positive | 27 | 12 | 15 | |
Independent-samples t-test.
Figure 3.
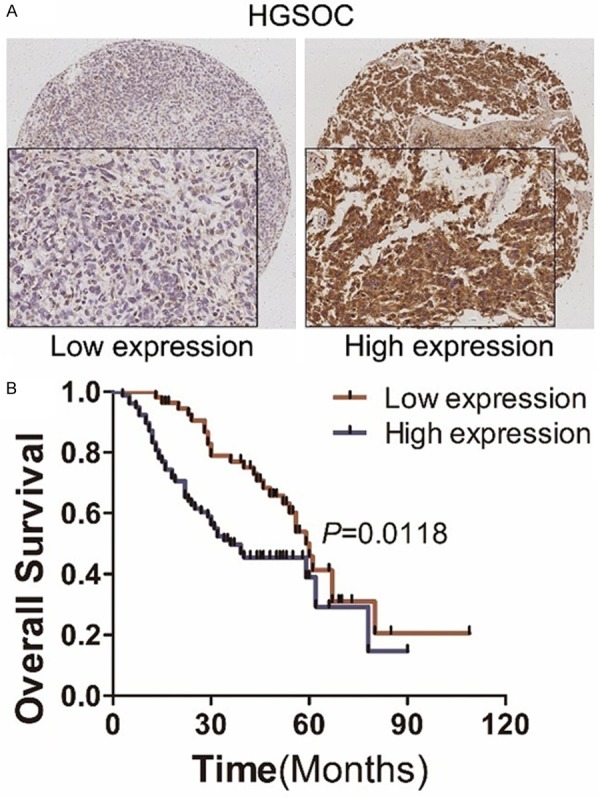
Immunohistochemical analysis of CD47 expression and its correlation with prognosis of HGSOC patients. A: Representative images of immunohistochemical analysis of CD47 expression in HGSOCs. B: Kaplan-Meier survival analysis of CD47 high-expression group versus low-expression group.
Generation of ovarian cancer cells with CD47 overexpression or knockdown
To investigate the potential function of CD47 in ovarian cancer, we established stable cell line with CD47 overexpression (CD47-OVE-A2780). We also introduced siRNAs to knockdown CD47 expression in A2780 and SKOV3 cells. Real-time PCR and western blot were performed to prove the successful generation of ovarian cancer cells with CD47 overexpression or knockdown (Figure 4A, 4B).
Figure 4.
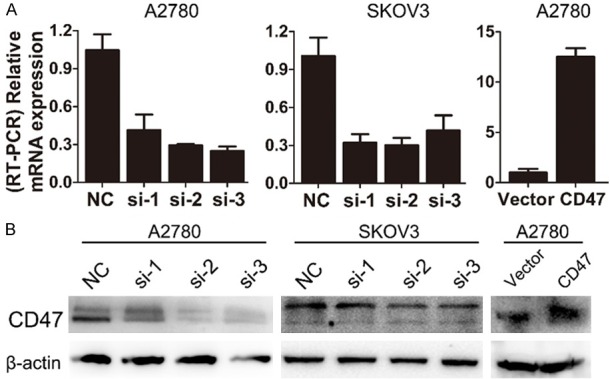
CD47 expression in ovarian cancer cells with CD47 overexpression and knockdown. A: Relative CD47 mRNA level in CD47 overexpression and knockdown cell lines. B: Western blot analysis of CD47 in CD47 overexpression and knockdown cell lines.
CD47 promotes migration and invasion of ovarian cancer cells
To determine the effect of altered CD47 expression on metastasis of ovarian cancer cells, migration and invasion abilities were first measured by transwell assay. A2780 cells with CD47 overexpression significantly enhanced the migration and invasion potentials compared with control cells (Figure 5A). While A2780 and SKOV3 cell lines with CD47 knockdown showed reduced migration and invasion ability (Figure 5B, 5C). These data indicate that CD47 promotes migration and invasion of ovarian cells in vitro.
Figure 5.
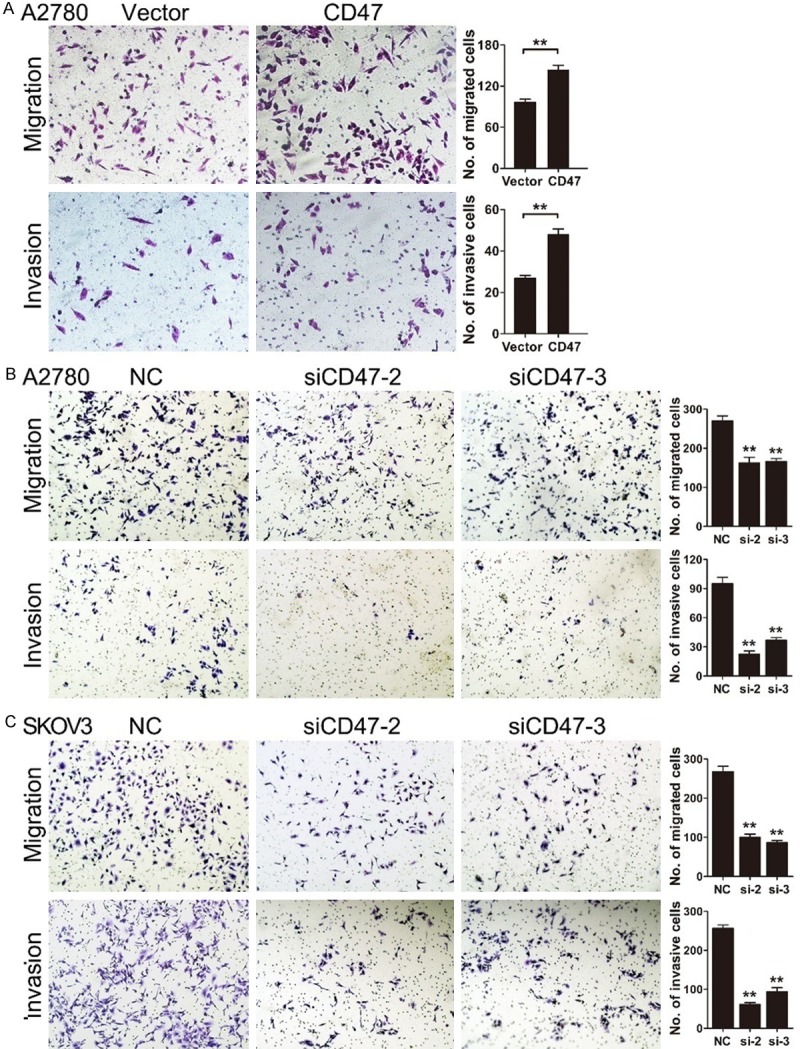
CD47 promotes migration and invasion of ovarian cancer cells. A: Ectopic expression of CD47 enhanced migration and invasion ability in A2780 cells. B and C: A2780 and SKOV3 cell lines with CD47 knockdown showed reduced migration and invasion ability. The data shown here are from a representative experiment repeated three times with similar results. Data are presented as means ± S.D. *P<0.05, **P<0.01.
CD47 induces epithelial-mesenchymal transition (EMT) of ovarian cancer cells
We observed CD47-OVE-A2780 cells exhibited a more mesenchymal phenotype compared to control cells. While CD47 knockdown cells displayed a more epithelial phenotype compared to control cells (Figure 6A). We then assessed the expression of EMT markers by western blot and found CD47 overexpression increased level of N-cadherin and decreased level of E-cadherin. On the contrary, the attenuation of CD47 reduced the expression of N-cadherin and the expression of E-cadherin increased obviously (Figure 6B). We further confirmed N-cadherin expression level by immunofluorescence staining in CD47-OVE-A2780 cells compared with their corresponding control cells (Figure 6C). These data suggest that CD47 was involved in EMT-related gene regulation and may contribute to invasive ability of HGSOC.
Figure 6.
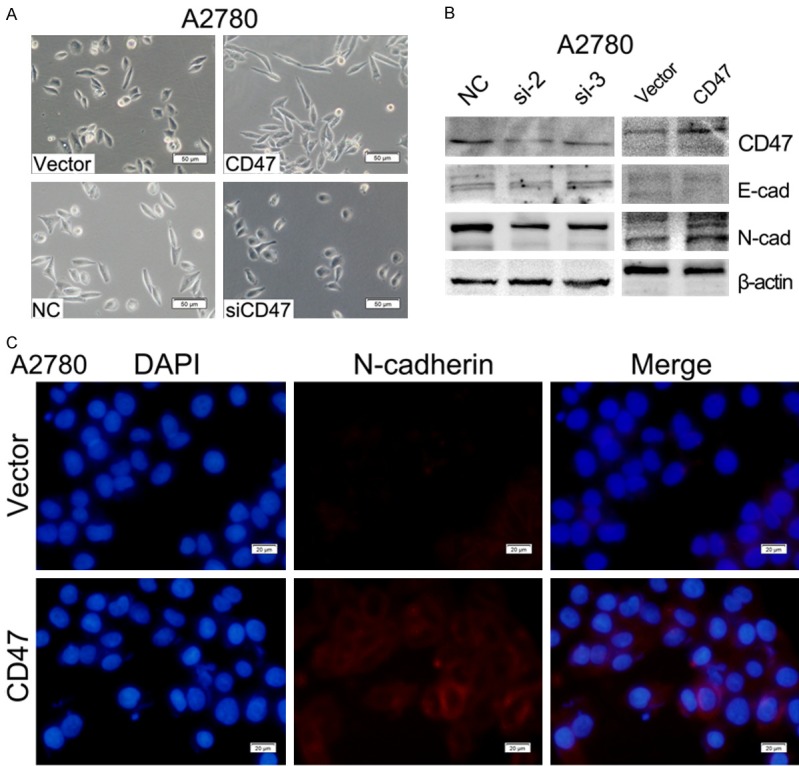
CD47 induces epithelial-mesenchymal transition (EMT) of ovarian cancer cells. A: Morphological changes in A2780 cells with CD47 overexpression or knockdown. B: E-cadherin and N-cadherin expression measured by WB in ovarian cancer cells with CD47 overexpression or knockdown. C: Immunofluorescence staining of N-cadherin in CD47-OVE-A2780 cells compared to the control.
Discussion
High-grade serous carcinoma is the most malignant form of ovarian cancer and accounts for up to 70% of all ovarian cancer cases. The majority of HGSOC have recently been found to originate in the FT, not the ovary [16]. In this study, we reported that CD47 is commonly overexpressed in HGSOC compared to FT. High expression of CD47 indicates poor prognosis in HGSOC patients. CD47 is up-regulated in many cancers, and its high expression is a negative prognostic indicator for several cancers [17]. Consistently, CD47 is reported to be overexpressed in epithelial ovarian cancer. Patients with CD47 low expression had a better treatment response to standard therapy [11]. CD47 expression was significantly high in ovarian clear cell carcinoma and correlated with patient surgical stage, chemotherapy resistance and prognosis [18].
CD47 is an antiphagocytic signal that cancer cells employ to inhibit macrophage-mediated destruction [19]. CD47 promotes tumor invasion and metastasis in non-small cell lung cancer [20]. Activation of CD47 receptors causes proliferation of human astrocytoma via an Akt-dependent pathway [6]. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells [21]. Our data revealed that CD47 overexpression in ovarian cancer cells significantly promoted migration and invasion in vitro. Moreover, CD47 induced epithelial-mesenchymal transition (EMT) through modulation of E-cadherin and N-cadherin. However, we found that CD47 did not influence on proliferation and cell cycle progression (Supplementary Figure 1).
When we analyzed CD47 expression in FT, we found CD47 was highly expressed in epithelial hyperplasia of the FTs (29/34, 85.3%). Interestingly, in the same FT sample, CD47 was highly expressed in multiple layer area than single layer area (Figure 1C). Clinical and pathological data showed elevated CD47 expression was associated with high level of CA125 in HGSOC patients. These findings suggested that CD47 may be used for detection of secretory cell hyperplasia of FT and for early stage detection of HGSOC. Future studies are needed to elucidate mechanisms regulating the overexpression of CD47 in HGSOC.
In conclusion, our results showed that CD47 was commonly overexpressed in hyperplasia of the FT and HGSOC. High expression of CD47 was associated with poor prognosis and high level of CA125 in HGSOC patients. Our findings indicate that CD47 is a strong candidate biomarker and therapeutic target in high-grade serous ovarian carcinoma.
Acknowledgements
This work was supported by National Clinical Research Center for Gynecological Oncology (2015BAI13B05) and National Natural Science Foundation of China (81572554, 81472432, 81672578, and 81401145).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Gersbach E, Zhang X, Xu X, Dong R, Lee P, Liu J, Kong B, Shao C, Wei JJ. miR-106a represses the Rb tumor suppressor p130 to regulate cellular proliferation and differentiation in high-grade serous ovarian carcinoma. Mol Cancer Res. 2013;11:1314–1325. doi: 10.1158/1541-7786.MCR-13-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurman RJ, Shih Ie M. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186:733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 6.Sick E, Boukhari A, Deramaudt T, Ronde P, Bucher B, Andre P, Gies JP, Takeda K. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia. 2011;59:308–319. doi: 10.1002/glia.21102. [DOI] [PubMed] [Google Scholar]
- 7.Sick E, Jeanne A, Schneider C, Dedieu S, Takeda K, Martiny L. CD47 update: a multifaceted actor in the tumour microenvironment of potential therapeutic interest. Br J Pharmacol. 2012;167:1415–1430. doi: 10.1111/j.1476-5381.2012.02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uluckan O, Becker SN, Deng H, Zou W, Prior JL, Piwnica-Worms D, Frazier WA, Weilbaecher KN. CD47 regulates bone mass and tumor metastasis to bone. Cancer Res. 2009;69:3196–3204. doi: 10.1158/0008-5472.CAN-08-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knauf S, Urbach GI. Identification, purification, and radioimmunoassay of NB/70K, a human ovarian tumor-associated antigen. Cancer Res. 1981;41:1351–1357. [PubMed] [Google Scholar]
- 10.Knauf S, Bast RC Jr. Tumor antigen NB/70K and CA 125 levels in the blood of preoperative ovarian cancer patients and controls: a preliminary report of the use of the NB12123 and CA 125 radioimmunoassays alone and in combination. Int J Biol Markers. 1988;3:75–81. doi: 10.1177/172460088800300201. [DOI] [PubMed] [Google Scholar]
- 11.Brightwell RM, Grzankowski KS, Lele S, Eng K, Arshad M, Chen H, Odunsi K. The CD47 “don’t eat me signal” is highly expressed in human ovarian cancer. Gynecol Oncol. 2016;143:393–397. doi: 10.1016/j.ygyno.2016.08.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, Marquez RT, Auersperg N, Yu Y, Hahn WC, Mills GB, Bast RC Jr. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–1663. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Liu J, Segura MF, Shao C, Lee P, Gong Y, Hernando E, Wei JJ. MiR-182 overexpression in tumourigenesis of high-grade serous ovarian carcinoma. J Pathol. 2012;228:204–215. doi: 10.1002/path.4000. [DOI] [PubMed] [Google Scholar]
- 15.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, Chen JY, Ohman AW, Stepule CD, Kwak S, Karst AM, Hirsch MS, Setlur SR, Crum CP, Dinulescu DM, Drapkin R. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca; Tp53; Pten models. Cancer Cell. 2013;24:751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 2013;24(Suppl 10):x16–21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 17.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Tan M, Zhang S, Li X, Gao J, Zhang D, Hao Y, Gao S, Liu J, Lin B. Expression and significance of CD44, CD47 and c-met in ovarian clear cell carcinoma. Int J Mol Sci. 2015;16:3391–3404. doi: 10.3390/ijms16023391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, Ozkan E, Fernhoff NB, van de Rijn M, Weissman IL, Garcia KC. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Wang J, Kong X, Li E, Liu Y, Du X, Kang Z, Tang Y, Kuang Y, Yang Z, Zhou Y, Wang Q. CD47 promotes tumor invasion and metastasis in non-small cell lung cancer. Sci Rep. 2016;6:29719. doi: 10.1038/srep29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, Gilkes DM, He J, Semenza GL. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A. 2015;112:E6215–6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


