Abstract
WNT1-inducible signaling pathway protein-1 (WISP-1) is an extracellular matrix-related protein that plays multiple roles in cellular physiology and pathology. Accumulating evidence shows that WISP-1 is involved in the process underlying fibrotic diseases. However, the correlation between WISP-1 and renal fibrosis is unknown. In this study, we hypothesized that WISP-1 levels might be correlated with renal fibrosis and could be used as a noninvasive biomarker to screen for renal fibrosis in patients with chronic kidney disease (CKD). We first measured the WISP-1 expression levels using a transforming growth factor-β (TGF-β)-induced renal fibrosis tubular epithelial cell (TEC) model and a mouse model of obstructive nephropathy. We then evaluated the correlation between serum WISP-1 levels and fibrosis scores in biopsy-proven renal fibrosis of patients with CKD. Based on the findings from both in vivo and in vitro studies, the levels of WISP-1 and fibrotic parameters (collagen I, fibronectin and α-smooth muscle actin) were significantly increased in the fibrotic models. Consistently, patients with focal proliferative IgA nephropathy, focal segmental glomerular sclerosis and diabetic nephropathy displayed markedly elevated serum WISP-1 levels and fibrosis scores of renal biopsies compared with normal subjects and patients with minimal change disease (P<0.05). Importantly, the serum WISP-1 levels were positively correlated with fibrosis scores in the renal biopsies of these patients (r=0.475, P=0.0001). Thus, serum WISP-1 levels may be used as a potential noninvasive biomarker of renal fibrosis in patients with CKD.
Keywords: WISP-1, renal fibrosis, chronic kidney disease, biomarker
Introduction
The prevalence of chronic kidney disease (CKD) has been estimated to be 10.8% in China [1] and 11.6% in the USA [2]. CKD is becoming a major global public health issue and is causing a substantial social and economic burden globally [3,4]. According to data from the United State Renal Data System (USRDS) 2016 Annual Report, the annual expenditure on CKD among patients 65 and older is estimated to be US$50 billion, accounting for 20% of the annual Medicare costs of this age group. Moreover, an additional cost of US$32.8 billion was spent on end-stage renal disease (ESRD) (https://www.usrds.org/).
Renal fibrosis is a common pathogenesis of various CKDs, such as IgA nephropathy (IgAN) [5,6], primary focal segmental glomerular sclerosis (FSGS) [7] and diabetic nephropathy (DN) [8-12]. Massive fibrosis in renal glomeruli and/or tubules induces a deterioration of renal function, ultimately causing ESRD. Early detection of renal fibrosis may lead to better diseases outcomes for patients and reduce social and economic burdens. In current clinical practice, renal fibrosis must be examined via a renal biopsy, an inconvenient and invasive procedure. In this study, we aimed to identify a noninvasive biomarker of renal fibrosis for the early detection and monitoring of fibrotic renal diseases.
WNT1-inducible signaling pathway protein 1 (WISP-1), also known as CCN4/ELM-1, is an extracellular matrix-related protein. WISP-1 plays multiple roles in cellular and pathogenic processes including cell proliferation, migration, adhesion, angiogenesis and tumorigenesis [13,14]. Previous studies have shown that WISP-1 is strongly correlated with fibrotic diseases, such as lung, heart and liver fibrosis [15-17]. WISP-1 is a prohypertrophic and profibrotic growth factor that promotes cardiomyocyte hypertrophy, fibroblast proliferation and extracellular matrix (ECM) deposition [18]. Elevated WISP-1 levels have been observed in patients with idiopathic pulmonary fibrosis, and the blockade of the WISP-1 protein using a neutralizing antibody resulted in the remarkable attenuation of fibrosis in a mouse model of bleomycin-induced pulmonary fibrosis [16]. Moreover, the blockade of WISP-1 using a neutralizing monoclonal antibody attenuated chronic liver injury and hepatic fibrosis in a carbon tetrachloride (CCl4)-treated mouse model [19]. However, whether WISP-1 is correlated with renal fibrosis in patients with CKD is unknown. In the present study, we hypothesize that the serum WISP-1 levels are increased in patients with renal fibrotic diseases and potentially serves as a biomarker of renal fibrosis. Indeed, both the in vivo and in vitro studies revealed that WISP-1 expression was elevated with increased levels of fibrotic parameters, including collagen I, fibronectin and α-smooth muscle actin. We also found that the serum WISP-1 levels were positively correlated with the severity of renal fibrosis in patients with CKD. Our study indicates that WISP-1 is a potential noninvasive biomarker for the detection of renal fibrosis.
Materials and methods
Patients and controls
Renal biopsies were obtained from a cohort of 96 patients with CKD who were hospitalized in the Department of Nephrology of Sichuan Provincial People’s Hospital between 2010 and 2015. Seventy-seven patients with CKD were diagnosed with renal fibrosis based on a renal biopsy, including 35 patients with focal proliferative IgAN, 18 with FSGS, and 24 with DN. Nineteen biopsies showing minimal change diseases (MCD) without renal fibrosis were used as the minimal positive controls, and 18 healthy age- and gender-matched volunteers served as the normal controls. According to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines (http://kdigo.org/home/guidelines/), CKD is defined as abnormalities of kidney structure or functions that are present for ≥3 months with implications for health. The renal biopsies were independently examined by two senior pathologists in Sichuan Provincial People’s Hospital. The included patients had no history of receiving corticosteroids or other immunosuppressive therapy for kidney diseases when they underwent the biopsy examinations and had no systemic diseases, such as rheumatoid arthritis, pulmonary fibrosis or liver cirrhosis. The study protocol was approved by the Medical Ethics Committee of the Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital (No. 2014 Natural Science (04)).
Demographic and laboratory data
Patient demographic data, including gender, age, and blood pressure were collected at admission. Serum and urine samples were obtained from the patients and healthy volunteers. Laboratory data, including the hemoglobin, glucose, albumin, blood urea nitrogen (BUN), serum creatinine (Scr), and uric acid levels, 24-hour urinary protein excretion and the estimated glomerular filtration rate (eGFR) were examined at the Clinical Laboratory Department of Sichuan Provincial People’s Hospital. Serum creatinine levels were measured using an enzymatic method and recalibrated to standardized creatinine measurements. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation [2].
WISP-1 ELISA analysis
The serum WISP-1 levels in each group were determined using an ELISA kit (Abcam, Cambridge, UK), according to the manufacturer’s instructions. Prior to examination, all serum samples from each group were collected and stored at -80°C. Each sample was measured in duplicate simultaneously.
Histology and immunohistochemistry
Histological and immunohistochemical analyses were performed on methyl Carnoy’s-fixed, paraffin-embedded, 3-μm-thick tissue sections as previously described [9,10,20-23]. Changes in renal morphology were assessed by periodic acid-Schiff (PAS) and Masson’s trichrome staining (Trichrome stain kit, ScyTek Laboratories, West Logan, UT, USA) according to the manufacturer’s instructions. A microwave-based antigen retrieval technique was used to immunostain the sections. Primary antibodies against WISP-1 (Abcam, Cambridge, UK), collagen I (Abcam, Cambridge, UK), fibronectin (Abcam, Cambridge, UK) and α-smooth muscle actin (α-SMA) (Proteintech, Chicago, IL, USA) were incubated with the tissue sections overnight at 4°C. An isotype-matched rabbit IgG (R&D Systems, Minneapolis, MN, USA) was used as a negative control throughout the study. After washing with PBS, the tissue sections were incubated with horseradish peroxidase-labeled secondary antibodies (Dako, Carpinteria, CA, USA) at room temperature for 1-2 hours, followed by the development of a brown color using diaminobenzidine. All tissue sections were counterstained with hematoxylin and eosin to visualize the nuclei. Changes in both histology and immunohistochemistry were quantitatively analyzed using the Image-Pro Plus 7.0 quantitative imaging program (Media Cybernetics, Bethesda, MD, USA) as previously described [9,10,20-23]. Positive signals were quantitatively measured in both the glomeruli and the tubulointerstitium area and are expressed as percentages of the examined areas.
Cell culture and treatment
A normal rat tubular epithelial cell line (TEC) (NRK52E, ATCC, USA) was maintained in Dulbecco’s Modified Eagle’s Medium with low glucose (DMEM/LG) culture medium containing 5% fetal bovine serum and a 0.1% antibiotic-antimycotic solution (Gibco®, Life Technologies, Carlsbad, CA, USA) in 75-cm2 flasks in a 37°C incubator with 5% CO2. To study WISP-1 expression during renal fibrosis in vitro, TECs were treated with 2 ng/ml human transforming growth factor (TGF)-β1 (R&D Systems, Minneapolis, MN, USA) in serum-free medium for 0, 6, 12 and 24 hours, based on the results of our preliminary studies [22,23]. The WISP-1 and renal fibrosis markers levels were also analyzed by real-time RT-PCR and western blotting.
Mouse model of obstructive kidney disease
Twenty male C57BL/6 mice (20-22 g body weight, 10 weeks old) were purchased from the Laboratory Animal Services Center, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China. A unilateral ureteral obstruction (UUO) renal fibrosis model was created by left ureteral ligation as previously described [20,22,23]. A group of ten age-matched mice was used as the sham control group. Sham mice received the same procedure as the mice in the UUO group, but with the exception that the left ureter was not ligated. All mice were housed under controlled environmental conditions (23°C) under a 12-hr light/dark cycle. The experimental mice were sacrificed on day 7 after UUO, and the serum and kidney tissues were collected for further analysis. The experimental procedures were approved by the medical ethics committees of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China, and the experiments were performed in accordance with the guidelines provided by the National Institutes of Health for the use of animals in laboratory experiments.
Real-time (RT)-PCR and gene expression analysis
Total RNA was isolated from the cultured cells and renal cortex using the TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized using a reverse transcriptase kit (Takara, Japan). The mRNA expression levels of fibrotic markers (collagen I, fibronectin, and α-SMA) were measured by real-time PCR. The WISP-1 primers used were as follows: forward: 5’-CGCCCGAGGTACGCAATAG-3’ and reverse: 5’-GCAGTTGGGTTGGAAGGACT-3’. Other primers were used as previously described [20,22,23]. The housekeeping gene GAPDH was used as the internal control. The expression levels of the target genes were calculated using the ΔΔCt method. The results are presented as the mean ± standard error of the mean (SEM).
Western blot analysis
Protein was extracted from the mouse kidney tissues and experimental cells using radioimmunoprecipitation assay (RIPA) lysis buffer, and western blot analysis was performed using a previously described method [9,10,20-23]. Primary antibodies, against WISP-1 (Abcam, Cambridge, UK), collagen I (Abcam, Cambridge, UK), fibronectin (Abcam, Cambridge, UK), α-SMA (Proteintech, Chicago, IL, USA) and GAPDH (MAB374, Millipore, Billerica, MA, USA) were used in the study. After incubation with the primary antibody at 4°C overnight, the blotting membrane was stained with the secondary antibody at room temperature for 2 hours. The protein signals were captured using an Image Quant LAS 4000 mini system and quantitatively analyzed using the ImageJ software (NIH). The protein levels were analyzed based on the ratio of the target proteins to GAPDH. The data are presented as the mean ± SEM.
Statistical analysis
Continuous variables are presented as the mean ± SEM or the median (interquartile range), and differences between groups were compared using Student’s t-test or one-way analysis of variance (ANOVA) with the S-N-K or Mann-Whitney U test. Categorical data were compared using the Chi square test or Fisher’s exact test. Spearman’s or Pearson’s correlation analysis was used to analyze the correlations between serum WISP-1 expression, the renal fibrosis scores in biopsy tissues and the patient clinical parameters. A P<0.05 was considered significant. The SPSS 19.0 statistical software was used to analyze the data.
Results
WISP-1 levels and fibrosis are increased in TGF-β-treated TEC
We examined the role of WISP-1 in the TGF-β-induced fibrosis in TECs in vitro. Based on the results of the real-time PCR analysis, 2 ng/ml TGF-β induced fibrosis, as indicated by the increases in the levels of the fibrotic markers collagen I, fibronectin and α-SMA. As expected, WISP-1 expression was increased in a time-dependent manner after TGF-β treatment and reached a maximum at 24 hours (Figure 1A-D). Consistent with this finding, the western blot analysis confirmed that the levels of both WISP-1 and the fibrotic markers collagen I and fibronectin were increased after 24 hours of TGF-β treatment (Figure 1E-H). Together, these results suggest that WISP-1 expression may correlate with fibrotic events.
Figure 1.
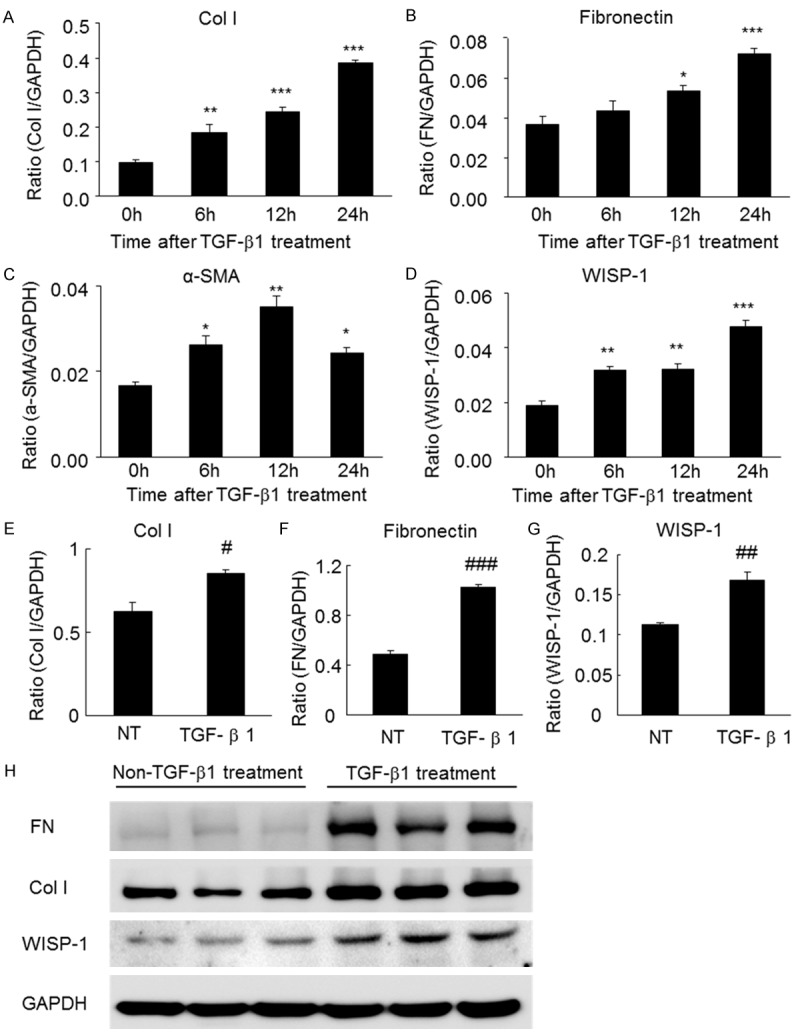
The levels of WISP-1 and fibrosis markers in TGF-β-treated TECs were assessed using real-time PCR and western blot analyses. (A) The levels of collagen I, (B) fibronectin, (C) α-SMA and (D) WISP-1 are increased in TECs in response to the TGF-β treatment (2 ng/ml for 24 hours), as determined by real-time PCR analyses. (E) The protein levels of collagen I, (F) fibronectin and (G) WISP-1 are increased in TECs in response to the TGF-β treatment (2 ng/ml for 24 hours), as determined by western blot analyses. (H) Western blots of collagen I, fibronectin, α-SMA and WISP-1 in TECs treated with or without TGF-β (2 ng/ml for 24 hours). α-SMA, α-smooth muscle actin; Col I, collagen I; FN, fibronectin; NT, not treated with TGF-β; TGF, transforming growth factor; TECs, tubular epithelial cells. *P<0.05, **P<0.01, ***P<0.001 compared with 0 h. #P<0.05, ##P<0.01, ###P<0.001 compared with NT.
WISP-1 levels and renal fibrosis are increased in the mouse model of obstructive kidney disease
To confirm the above findings, we performed an in vivo study in the UUO renal fibrotic disease model. As shown in Figure S1, the histological morphology of the UUO mice demonstrated typical hydronephrosis, fibrosis and inflammatory cell infiltration. According to the immunohistochemical staining, the collagen I, fibronectin, α-SMA and WISP-1 levels were significantly increased in mice with obstructive kidney diseases compared to the sham mice, as shown in Figure S2. We then examined the protein levels of fibrotic markers and WISP-1 in the diseased and normal kidneys using real-time PCR and western blot analyses. As shown in Figure 2A-I, the levels of the collagen I, fibronectin and α-SMA mRNAs and proteins were significantly increased in the mice with obstructive kidney disease at 7 days compared with the sham mice. The elevated WISP-1 levels were consistently and positively correlated with the changes in the levels of fibrotic markers levels in the mice, suggesting that WISP-1 might be correlated with renal fibrosis in patients with fibrotic kidney diseases. Next, we examined whether the serum WISP-1 levels correlated with the renal fibrosis scores in CKD patients.
Figure 2.
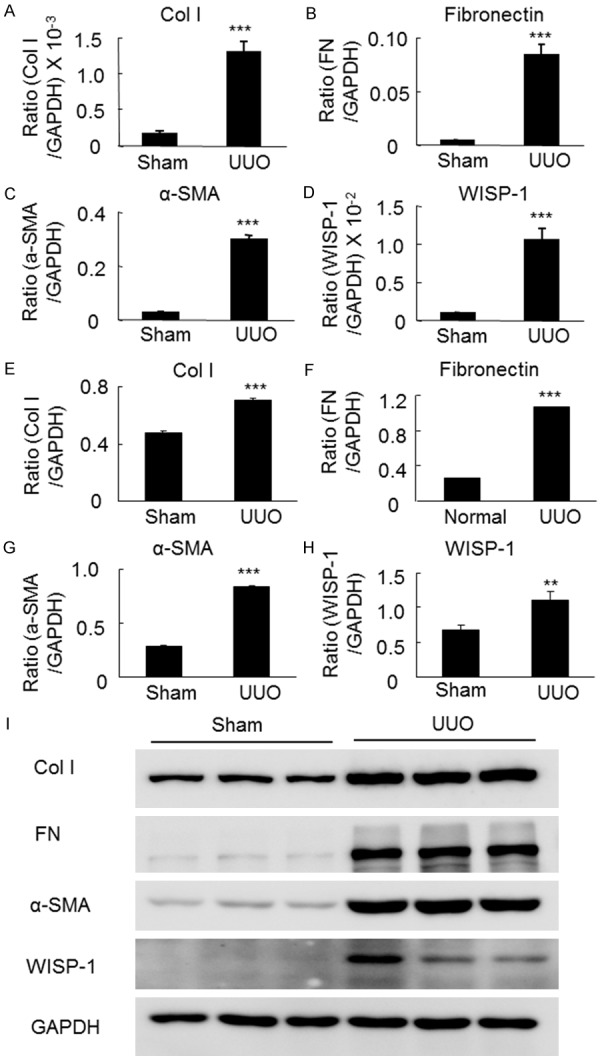
Levels of WISP-1 and fibrotic markers in the obstructive kidney disease model were assessed using real-time PCR and western blot analyses. (A) Levels of collagen I, (B) fibronectin, (C) α-SMA and (D) WISP-1 mRNAs are significantly increased in UUO mice after 7 days compared to the sham mice according to the real-time PCR analysis. (E) Levels of collagen I, (F) fibronectin, (G) α-SMA and (H) WISP-1 proteins are significantly increased in the UUO mice after 7 days compared with the sham mice, as shown by western blot analyses. (I) Western blots for collagen I, fibronectin, α-SMA and WISP-1. α-SMA, α-smooth muscle actin; Col I, collagen I; FN, fibronectin; UUO, unilateral ureteral obstruction. **P<0.01, ***P<0.001 compared with the sham mice.
Demographic and laboratory characteristics
Demographic and laboratory data from the 96 patients with biopsy-proven kidney diseases (35 with IgAN, 18 with FSGS, 24 with DN and 19 with MCD) were analyzed. Data from 18 healthy age- and gender-matched volunteers were used as the normal control group. The demographic and laboratory characteristic of subjects are shown in Table 1. The systolic pressure was significantly higher in the patients with IgAN, DN and FSGS than in patients with MCD (P<0.01 or P<0.05) and the normal controls (P<0.01). Patients with IgAN, DN, and FSGS had remarkably lower hemoglobin levels than patients with MCD (P<0.01). Patients with DN had significantly higher blood glucose levels than patients with MCD (P<0.01) and the normal controls (P<0.01). The serum albumin levels were significantly lower in the patients with MCD, IgAN, DN and FSGS than in the normal controls (P<0.01) and were significantly higher in the patients with IgAN and DN compared to the patients with MCD (P<0.01). Patients with IgAN, DN and FSGS had significantly higher uric acid (P<0.01), BUN (P<0.01) and Scr levels (P<0.01), and a decreased eGFR (P<0.01) than the normal controls. The IgAN patients had significantly lower 24-hour urinary protein excretion levels than the MCD (P<0.01), FSGS (P<0.01) and DN (P<0.01) patients. The laboratory data indicated that the clinical manifestations of the patients were consistent with the biopsy-proven diagnoses. Next we examined WISP-1 levels in the serum and biopsy specimens from patients with CKD.
Table 1.
Demographic and laboratory characteristic
| Normal control (n=18) | MCD (n=19) | IgAN (n=35) | DN (n=24) | FSGS (n=18) | Low fibrosis group | High fibrosis group | |
|---|---|---|---|---|---|---|---|
| Fibrosis <25%, (n=43) | Fibrosis ≥25%, (n=34) | ||||||
| Gender (M/F) | 10/8 | 13/6 | 16/19 | 13/11 | 13/5 | 23/20 | 19/15 |
| Age (years) | 34 (28, 57) | 22 (18, 36)** | 37 (25, 44)## | 66 (59, 80)**,##,cc | 28 (19, 47)aa | 39 (23, 51)## | 34 (24, 43)# |
| SBP (mmHg) | 112 (103, 123) | 118 (104, 127) | 130 (124, 152)**,## | 164 (129, 180)**,# | 136 (130, 146)**,## | 132 (124, 141)**,## | 145 (127, 155)**,## |
| DBP (mmHg) | 68±10 | 74±12 | 84±14**,# | 71±18bb | 91±15**,## | 82±15**,# | 90±15**,## |
| Hemoglobin (g/l) | 143±15 | 151±23 | 134±19## | 111±22**,##,cc | 131±26##,aa | 132±22## | 129±22*,## |
| Glucose (mmol/l) | 4.82 (4.73, 5.15) | 5.29 (4.37, 6.45) | 5.09 (4.78, 5.71) | 13.2 (9.29, 22.1)**,##,cc | 5.17 (4.62, 6.22)aa | 5.26 (4.77, 6.79) | 5.09 (4.76, 6.15) |
| Albumin (g/l) | 45.4 (43.9, 48.5) | 20.9 (16.4, 26.2)** | 39.6 (37.0, 43.7)**,## | 36.0 (32.9, 41.5)**,##,c | 27.8 (16.9, 40.3)**,cc | 39.2 (28.1, 41.5)**,## | 37.0 (33.6, 43.6)**,## |
| BUN (mmol/l) | 4.8 (4.1, 6.1) | 5.4 (3.9, 6.1) | 6.3 (5.5, 8.3)**,## | 10.9 (8.3, 23.5)**,##,cc | 8.8 (5.6, 10.7)**,##,a | 6.1 (5.3, 8.4)**,# | 9.2 (6.4, 10.8)**,##,&& |
| Scr (μmol/l) | 65.5 (58.2, 77.5) | 68.9 (61.1, 84.8) | 90.3 (63.0, 134.6)**,## | 126.8 (95.2, 257.7)**,##,cc | 143.7 (93.7, 215.4)**,##,c | 87.8 (62.5, 104.9)*,# | 148.9 (116.1, 199.2)**,##,&& |
| UA (mmol/l) | 285 (244, 351) | 381 (285, 468)* | 385 (335, 521)** | 422 (362, 597)** | 464 (338, 495)** | 367 (317, 470)** | 472 (393, 523)**,#,&& |
| Urine Protein (g/24 hours) | -- | 3.47 (2.23, 5.48) | 1.41 (0.96, 2.97)##,a | 4.61 (2.97, 9.34)cc | 4.69 (2.28, 14.68)cc | 2.12 (1.00, 4.61) | 2.36 (1.36, 8.50) |
| eGFR (ml/min/1.73 m2) | 102.3 (92.6, 114.2) | 124.1 (86.2, 140.3) | 65.9 (48.2, 104.9)**,##,a | 47.9 (21.0, 55.4)**,##,cc | 51.7 (29.1, 88.7)**,## | 88.9 (53.5, 106.0)*,## | 45.1 (32.5, 59.6)**,##,&& |
SBP, systolic blood pressure; DBP, diastolic blood pressure; BUN, blood urea nitrogen; Scr, serum creatinine; UA, uric acid; eGFR, estimated glomerular filtration rate; MCD, minimal change disease; IgAN, IgA nephropathy; DN, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis.
p<0.05 compared with the normal controls;
p<0.01 compared with the normal controls.
p<0.05 compared with patients with MCD;
p<0.01 compared with patients with MCD.
p<0.05 compared with patients with DN;
p<0.01 compared with patients with DN.
p<0.01 compared with patients with FSGS.
p<0.05 compared with patients with IgAN;
p<0.01 compared with patients with IgAN.
p<0.01 compared with the low fibrosis group (fibrosis <25%).
Expression and localization of WISP-1 in patients with biopsy-proven CKD
The levels of glomerulosclerosis, tubulointerstitial fibrosis and collagen ECM deposition were measured using PAS and Masson’s trichrome staining. Biopsies of nontumor kidney tissue from patients with renal cell carcinoma served as negative controls. Renal fibrosis was observed in biopsies from patients with IgAN, DN and FSGS, but not in those from patients with MCD or the negative controls, as shown in Figure 3. WISP-1 levels were significantly elevated in the biopsy tissues from patients with IgAN, DN and FSGS compared to the biopsy tissues from the negative controls and patients with MCD (Figure 3). Additionally, the expression of WISP-1 was mainly localized in the tubulointerstitial areas where fibrosis occurred. These results suggested that WISP-1 was significantly expressed in renal fibrosis biopsy tissues and might be correlated with kidney fibrosis in CKD patients.
Figure 3.
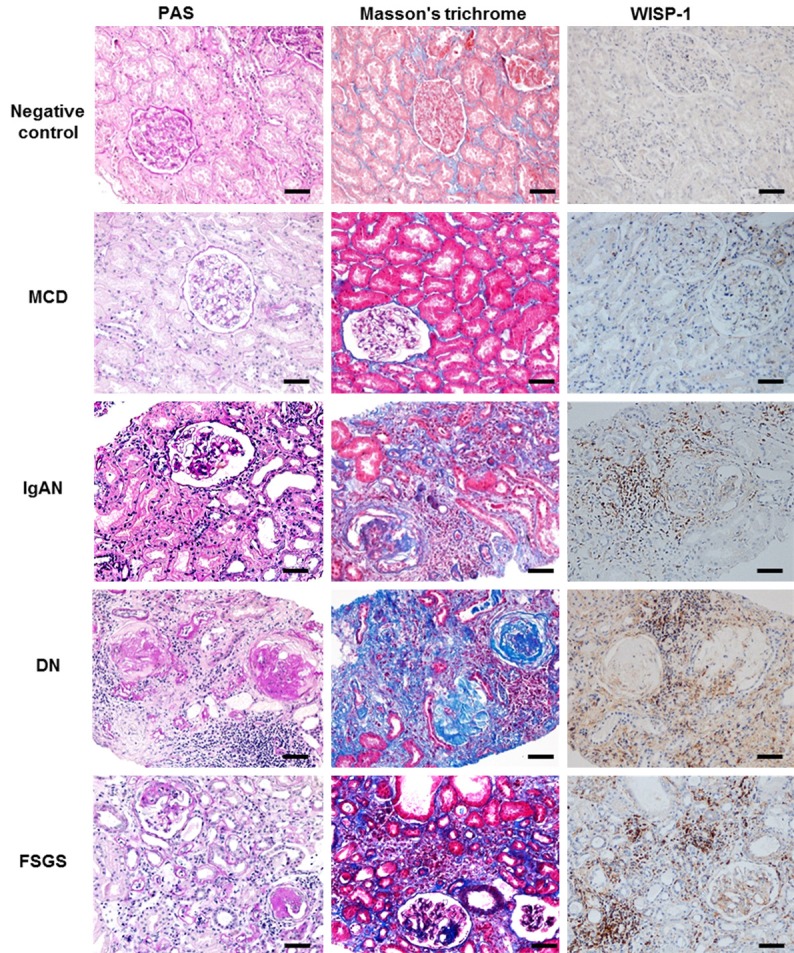
Extracellular matrix deposition and WISP-1 expression in kidney tissues from patients with MCD, IgAN, DN and FSGS and the negative controls. Representative images of histological and immunohistochemical staining images. Renal fibrosis indices and WISP-1 levels are increased in renal biopsy tissues from patients with fibrotic diseased diseases (IgAN, DN, and FSGS) compared with patients with MCD (minimal positive control) and negative controls. DN, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease; negative control, normal kidney tissues from patients with renal cell carcinoma; PAS, periodic acid-Schiff. Bars =50 μm.
Serum WISP-1 levels are elevated in CKD patients
Because urinary WISP-1 levels are not detectable in the healthy subjects and patients with CKD, according to our preliminary results and other studies [24], we evaluated the serum WISP-1 levels in patients with CKD. As shown in Figure 4A, the serum WISP-1 levels in patients with IgAN (645.45 (503.95, 934.26) pg/ml), DN (700.36 (280.45, 840.52) pg/ml) and FSGS (664.81 (448.35, 893.74,) pg/ml) were significantly higher than the levels observed in patients with MCD (506.82 (132.60, 612.52) pg/ml) and healthy subjects (377.93 (233.12, 657.20) pg/ml) (all, P<0.05). However, no significant differences in the serum WISP-1 levels were observed in the patients with IgAN, DN or FSGS (P>0.05). Based on these findings, serum WISP-1 levels do not represent a disease-specific marker (for IgAN, DN or FSGS), but instead are a potential biomarker that could be used in renal fibrosis screening. Therefore, we analyzed the correlation between the serum WISP-1 levels and the fibrotic scores in these CKD patients.
Figure 4.
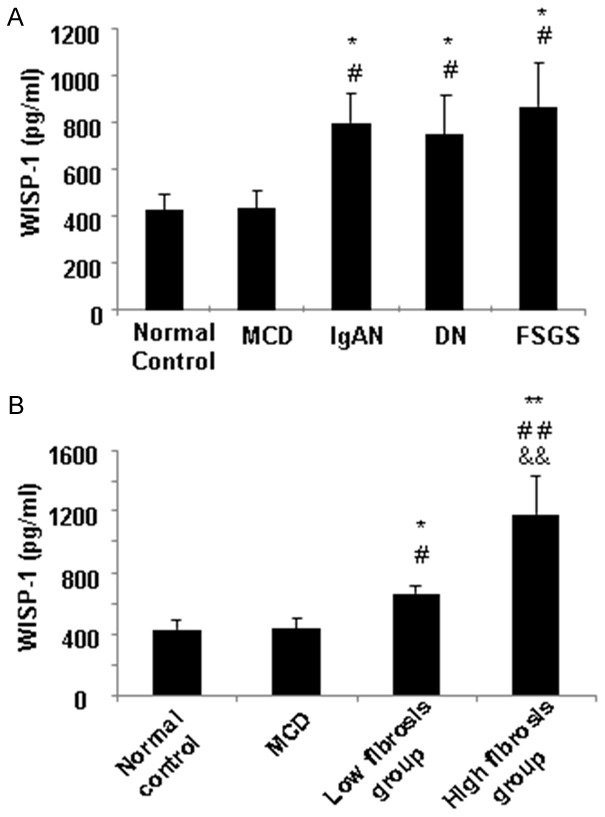
Serum WISP-1 levels in patients with renal fibrotic diseases. A. The WISP-1 concentration is significantly higher in the sera from patients with IgAN, DN and FSGS than in the sera from patients with MCD and healthy subjects (the normal controls). B. The WISP-1 levels in the high fibrosis group (over 25% of fibrosis) were significantly increased compared with the low fibrosis group (less than 25% of fibrosis). *P<0.05, **P<0.01 compared with the normal controls. #P<0.05, ##P<0.01 compared with the MCD patients. &&P<0.01 compared with the high fibrosis group. DN, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease. Low fibrosis group, patients had renal fibrosis in less than 25% of the tissue. High fibrosis group, patients had fibrosis in greater than 25% of the tissue.
Serum WISP-1 levels are correlated with the renal fibrosis severity in CKD biopsy tissues
To explore whether the serum WISP-1 levels were associated with the fibrosis severity, 77 CKD patients (35 with IgAN, 18 with FSGS, and 24 with DN) with biopsy-proven differential renal fibrosis scores were analyzed. As shown in Table 1, 43 patients displayed renal fibrosis in less than 25% of the tissue (low fibrosis group), and 34 patients displayed fibrosis in greater than 25% of the tissue (high fibrosis group). Laboratory parameters, including the BUN, Scr and uric acid levels and the eGFRs, significantly differed among groups 1 and 2 and the controls. Thus, the severity of fibrosis significantly affected the renal function parameters (BUN, Scr and eGFR), consistent with the clinical observations. Next, we examined the serum WISP-1 levels in the two groups and the correlation with their fibrosis scores. As shown in Figure 4B, the WISP-1 levels in the high fibrosis group (754.76 (498.78, 1149.62) pg/ml) were significantly increased compared with the low fibrosis group (645.45 (449.32, 902.84) pg/ml). Therefore, we further assessed the correlations between the serum WISP-1 levels, the severity of fibrosis in the biopsy tissues and the laboratory parameters (including gender, age, blood pressure, hemoglobin, glucose, albumin, BUN, Scr, and uric acid levels, 24-hour urinary protein excretion and the eGFR) using Spearman’s or Pearson’s correlation analysis. The serum WISP-1 levels were positively correlated with the renal fibrosis scores (r=0.475, P=0.001) (Figure 5). No significant associations were observed between the serum WISP-1 levels and the other parameters. Thus, the serum WISP-1 levels are closely correlated with the severity of renal fibrosis in patients with CKD.
Figure 5.
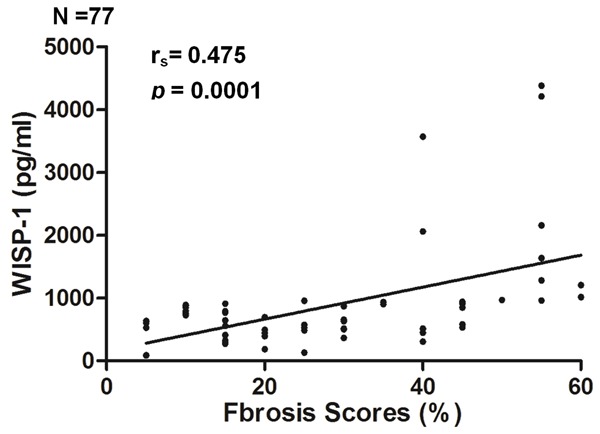
The serum WISP-1 levels are positively correlated with the renal fibrosis scores in CKD patients. The WISP-1 expression levels in the serum were positively correlated with the renal fibrosis scores in the renal biopsy tissues from the CKD patients.
Discussion
Renal fibrosis is a landmark of CKD and is characterized by the excessive deposition of ECM in renal tissues, including marked increases in the levels of collagens I, III and IV, fibronectin and α-SMA. Currently, substantial efforts are devoted to monitoring the development and progression of renal fibrosis in patients with CKD. However, the assessment of renal fibrosis in patients with CKD requires a renal biopsy. Noninvasive biomarkers that can be used to screen for fibrosis in patients with CKD are urgently needed. The Wnt/β-catenin pathway is known to be linked to the pathogenesis of fibrotic disorders [25,26]. WISP-1 is a downstream molecule of Wnt-1 and β-catenin that is induced by the Wnt-1 protein [13,14]. In this study, we report for the first time that the WISP-1 levels are elevated in the fibrotic kidney in vivo and in TGF-β-induced TEC fibrosis in vitro. Importantly, the WISP-1 levels in the sera are correlated with the severity of renal fibrosis in CKD patients. Our findings are consistent with other publications reporting increased WISP-1 levels in fibrotic organs, including the lung, heart and liver [15-17]. Thus, WISP-1 represents a potential biomarker of fibrosis patients with various types of tissue or organ fibrosis.
Patients with CKD, including IgAN, DN and FSGS, are generally recruited to test the correlation of the serum WISP-1 level with the fibrosis severity in renal biopsies. Progressive renal fibrosis is very common in patients with IgAN, DN and FSGS [5-12]. Conversely, patients with MCD have no fibrosis in the kidney but present large amounts of albumin in their urine. Therefore, in our study, the kidney tissues from patients with MCD, served as the minimal positive controls for renal fibrosis [27], and nontumor kidney tissues from patients with renal cell carcinoma served as the negative controls. First, WISP-1 levels were significantly elevated in biopsy-proven fibrotic renal tissues (IgAN, DN and FSGS) compared to that in MCD and nontumor tissues (the negative controls). Second, WISP-1 was primarily expressed in the tubulointerstitium in the biopsied kidney tissues, which exhibited inflammatory cells infiltration during the fibrotic process. Renal fibrosis is a condition of repair and scarring, which usually accompanies an inflammatory response during kidney injury [9-12,23,28,29], WISP-1 has been shown to play an important role in inflammation in obese animals [30] and in animals with hepatic injuries [31]. Consistent with the findings from patients with CKD, high WISP-1 levels were observed in the area with severe tubulointerstitial fibrosis in the UUO mouse model. Interestingly, WISP-1 expression was induced by exogenous TGF-β1 in rat tubular epithelial (NRK52E) cells in vitro. The profibrotic growth factor TGF-β1 induces the expression of α-SMA and other collagen matrix components in TECs by promoting epithelial-mesenchymal transition (EMT) in NRK52E cells [32,33]. In this study, the observed significant increases in WISP-1 and α-SMA expression in the TECs indicate that WISP1 expression in tubular cells may depend on TGF-β1 via the EMT. Based on these findings, the fibrosis-related protein WISP-1 is correlated with renal fibrosis events in vitro, and in vivo in patients with CKD.
Indeed, the serum WISP-1 levels were significantly elevated in patients with IgAN, DN and FSGS compared to patients with MCD and the healthy subjects (the normal controls). However, no significant differences in the WISP-1 levels were found between the IgAN, DN and FSGS groups (P>0.05), suggesting that WISP-1 was not specific to the disease classification. Then, we grouped the patients according to renal fibrosis severity and examined the potential correlations between the serum WISP-1 levels, fibrosis severity, renal functions and other laboratory parameters. The serum WISP-1 levels were only positively correlated with the renal fibrosis severity. Thus, serum WISP-1 levels may serve as a noninvasive biomarker of renal fibrosis.
The discovery of biomarkers for kidney diseases has attracted increasing interest [34,35]. The serum creatinine level and the eGFR are the most common renal function indices, used in clinical practice [36,37]. These two indices are changed in patients with both acute kidney injury and CKD. Neutrophil gelatinase-associated lipocalin, cystatin C, N-acetyl-β-D-glucosaminidase and kidney injury molecule-1 are potential biomarkers of early kidney injury [38-40]. MicroRNAs (miRNAs) are also associated with kidney injury (e.g., miRNAs 15, 17, 21, 29, 31, and 192) [9,10,23,41,42]. Metabolomic and proteomic biomarkers can potentially be used to measure the progression and severity of kidney disease as replacements for the microalbumin and BUN levels [43-45]. However, the discovery of biomarkers for CKD has been slow compared to acute kidney injury. To promote the discovery and validation of biomarkers for CKD, the National Institute of Diabetes and Digestive and Kidney Diseases has established the CKD Biomarkers Consortium. Biopsy examination remains the best available approach to determine the presence and severity of renal fibrosis. However, this invasive procedure is associated with increased risks of bleeding, pain and arteriovenous fistula in patients [46-48]. Our findings indicate that serum WISP-1 is a potential biomarker that can be used to determine the severity of renal fibrosis. The use of this marker would reduce the risks associated with biopsies in patients with CKD.
This study has some limitations. We did not test the sensitivity or specificity of the serum WISP-1 levels as a biomarker of renal fibrosis. Rigorous validation of WISP-1 as a biomarker should be performed in the future. Our study did not examine whether WISP-1 was a causal mediator in fibrosis or a downstream molecule of renal fibrosis. Thus, the role of WISP-1 warrants further study.
In conclusion, to the best of our knowledge, the present study is the first to report that the serum WISP-1 levels are positively correlated with the renal fibrosis severity. Serum WISP-1 may be a potential noninvasive biomarker for the detection of renal fibrosis in CKD patients.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81300618 to Xiang Zhong), the Youth Science and Technology Creative Research Groups of Sichuan Province (Grant No. 2015TD0013 to Gui Sen Li) and GRF/RGC (No. 17113416 to HY Chen). The authors thank Prof. Shao Ping Deng and Prof. Zheng Lin Yang at the University of Electronic Science and Technology, Sichuan Academy of Medical Sciences and the Sichuan Provincial People’s Hospital for their generous provision of research platforms and technical support.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Li X, Wang H. Prevalence of chronic kidney disease in China: a crosssectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo KT, Choong HL, Wong KS, Tan HB, Chan CM. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2012;81:1044–1045. doi: 10.1038/ki.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 6.Cheung CK, Molyneux K, Boyd J, Baines R, Brunskill N, Barratt J. The role of IgA in proximal tubular cell activation and tubulointerstitial scarring in IgA nephropathy. Lancet. 2014;383:34–34. [Google Scholar]
- 7.Chun MJ, Korbet SM, Schwartz MM, Lewis EJ. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol. 2004;15:2169–2177. doi: 10.1097/01.ASN.0000135051.62500.97. [DOI] [PubMed] [Google Scholar]
- 8.You YK, Huang XR, Chen HY, Lyu XF, Liu HF, Lan HY. C-reactive protein promotes diabetic kidney disease in db/db mice via the CD32b-Smad3-mTOR signaling pathway. Sci Rep. 2016;6:26740. doi: 10.1038/srep26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HY, Zhong X, Huang XR, Meng XM, You Y, Chung AC, Lan HY. MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol Ther. 2014;22:842–853. doi: 10.1038/mt.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong X, Chung AC, Chen HY, Dong Y, Meng XM, Li R, Yang W, Hou FF, Lan HY. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56:663–674. doi: 10.1007/s00125-012-2804-x. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, Chen HY, Huang XR, Chung AC, Zhou L, Fu P, Szalai AJ, Lan HY. C-reactive protein promotes diabetic kidney disease in a mouse model of type 1 diabetes. Diabetologia. 2011;54:2713–2723. doi: 10.1007/s00125-011-2237-y. [DOI] [PubMed] [Google Scholar]
- 12.Chen HY, Huang XR, Wang W, Li JH, Heuchel RL, Chung AC, Lan HY. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes. 2011;60:590–601. doi: 10.2337/db10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlegelmilch K, Keller A, Zehe V, Hondke S, Schilling T, Jakob F, Klein-Hitpass L, Schutze N. WISP 1 is an important survival factor in human mesenchymal stromal cells. Gene. 2014;551:243–254. doi: 10.1016/j.gene.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Chiang KC, Yeh CN, Chung LC, Feng TH, Sun CC, Chen MF, Jan YY, Yeh TS, Chen SC, Juang HH. WNT-1 inducible signaling pathway protein-1 enhances growth and tumorigenesis in human breast cancer. Sci Rep. 2015;5:8686. doi: 10.1038/srep08686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jian YC, Wang JJ, Dong S, Hu JW, Hu LJ, Yang GM, Zheng YX, Xiong WJ. Wnt-Induced Secreted Protein 1/CCN4 in Liver Fibrosis Both in vitro and in vivo. Clin Lab. 2014;60:29–35. doi: 10.7754/clin.lab.2013.121035. [DOI] [PubMed] [Google Scholar]
- 16.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Gunther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao XJ, Hua Y, Chen HM, Yang HY, Zhang T, Huang GQ, Fan HJ, Tan ZB, Huang XF, Liu B, Zhou YC. Aldehyde dehydrogenase-2 protects against myocardial infarction-related cardiac fibrosis through modulation of the Wnt/betacatenin signaling pathway. Ther Clin Risk Manag. 2015;11:1371–1381. doi: 10.2147/TCRM.S88297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colston JT, de la Rosa SD, Koehler M, Gonzales K, Mestril R, Freeman GL, Bailey SR, Chandrasekar B. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007;293:H1839–1846. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Chen Y, Ye W, Tao X, Zhu J, Wu S, Lou L. Blockade of CCN4 attenuates CCl4-induced liver fibrosis. Arch Med Sci. 2015;11:647–653. doi: 10.5114/aoms.2015.52371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Chung AC, Dong Y, Yang W, Zhong X, Lan HY. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-beta/Smad3-Azin1 pathway. Kidney Int. 2013;84:1129–1144. doi: 10.1038/ki.2013.272. [DOI] [PubMed] [Google Scholar]
- 21.Chung AC, Dong Y, Yang W, Zhong X, Li R, Lan HY. Smad7 suppresses renal fibrosis via altering expression of TGF-beta/Smad3-regulated microRNAs. Mol Ther. 2013;21:388–398. doi: 10.1038/mt.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng XM, Huang XR, Xiao J, Chen HY, Zhong X, Chung AC, Lan HY. Diverse roles of TGFbeta receptor II in renal fibrosis and inflammation in vivo and in vitro. J Pathol. 2012;227:175–188. doi: 10.1002/path.3976. [DOI] [PubMed] [Google Scholar]
- 23.Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol. 2011;22:1668–1681. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi J, Kumar C, Zhang Y, Olsen JV, Mann M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome biol. 2006;7:R80. doi: 10.1186/gb-2006-7-9-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan RJ, Zhou D, Zhou L, Liu Y. Wnt/betacatenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 2014;4:84–90. doi: 10.1038/kisup.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smeets B, Stucker F, Wetzels J, Brocheriou I, Ronco P, Grone HJ, D’Agati V, Fogo AB, van Kuppevelt TH, Fischer HP, Boor P, Floege J, Ostendorf T, Moeller MJ. Detection of activated parietal epithelial cells on the glomerular tuft distinguishes early focal segmental glomerulosclerosis from minimal change disease. Am J Pathol. 2014;184:3239–3248. doi: 10.1016/j.ajpath.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10:493–503. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- 29.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 30.Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Docke S, Keyhani-Nejad F, Gogebakan O, Osterhoff M, Kemper M, Hornemann S, Markova M, Kloting N, Stockmann M, Weickert MO, Lamounier-Zepter V, Neuhaus P, Konradi A, Dooley S, von Loeffelholz C, Bluher M, Pfeiffer AF, Rudovich N. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015;64:856–866. doi: 10.2337/db14-0444. [DOI] [PubMed] [Google Scholar]
- 31.Tong Y, Ding XB, Chen ZX, Jin SQ, Zhao X, Wang X, Mei SY, Jiang X, Wang L, Li Q. WISP1 mediates hepatic warm ischemia reperfusion injury via TLR4 signaling in mice. Sci Rep. 2016;6:20141. doi: 10.1038/srep20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns WC, Kantharidis P, Thomas MC. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells tissues organs. 2007;185:222–231. doi: 10.1159/000101323. [DOI] [PubMed] [Google Scholar]
- 34.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4:57–73. doi: 10.5527/wjn.v4.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 37.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 38.Benzer M, Alpay H, Baykan O, Erdem A, Demir IH. Serum NGAL, cystatin C and urinary NAG measurements for early diagnosis of contrast-induced nephropathy in children. Ren Fail. 2016;38:27–34. doi: 10.3109/0886022X.2015.1106846. [DOI] [PubMed] [Google Scholar]
- 39.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha V, Vence LM, Salahudeen AK. Urinary tubular protein-based biomarkers in the rodent model of cisplatin nephrotoxicity: a comparative analysis of serum creatinine, renal histology, and urinary KIM-1, NGAL, and NAG in the initiation, maintenance, and recovery phases of acute kidney injury. J Investig Med. 2013;61:564–568. doi: 10.2310/JIM.0b013e31828233a8. [DOI] [PubMed] [Google Scholar]
- 41.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGFbeta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey P, Brors B, Srivastava PK, Bott A, Boehn SN, Groene HJ, Gretz N. Microarray-based approach identifies microRNAs and their target functional patterns in polycystic kidney disease. BMC Genomics. 2008;9:624. doi: 10.1186/1471-2164-9-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kistler AD, Serra AL, Siwy J, Poster D, Krauer F, Torres VE, Mrug M, Grantham JJ, Bae KT, Bost JE, Mullen W, Wuthrich RP, Mischak H, Chapman AB. Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS One. 2013;8:e53016. doi: 10.1371/journal.pone.0053016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mischak H, Delles C, Vlahou A, Vanholder R. Proteomic biomarkers in kidney disease: issues in development and implementation. Nat Rev Nephrol. 2015;11:221–232. doi: 10.1038/nrneph.2014.247. [DOI] [PubMed] [Google Scholar]
- 45.Nkuipou-Kenfack E, Duranton F, Gayrard N, Argiles A, Lundin U, Weinberger KM, Dakna M, Delles C, Mullen W, Husi H, Klein J, Koeck T, Zurbig P, Mischak H. Assessment of metabolomic and proteomic biomarkers in detection and prognosis of progression of renal function in chronic kidney disease. PLoS One. 2014;9:e96955. doi: 10.1371/journal.pone.0096955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Misra S, Gyamlani G, Swaminathan S, Buehrig CK, Bjarnason H, McKusick MA, Andrews JC, Johnson CM, Fervenza FC, Leung N. Safety and diagnostic yield of transjugular renal biopsy. J Vasc Interv Radiol. 2008;19:546–551. doi: 10.1016/j.jvir.2007.12.447. [DOI] [PubMed] [Google Scholar]
- 47.Rollino C, Ferro M, Beltrame G, Quattrocchio G, Massara C, Quarello F, Roccatello D. Renal biopsy in patients over 75: 131 cases. Clin Nephrol. 2014;82:225–230. doi: 10.5414/CN108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulop T, Alemu B, Dossabhoy NR, Bain JH, Pruett DE, Szombathelyi A, Dreisbach AW, Tapolyai M. Safety and efficacy of percutaneous renal biopsy by physicians-in-training in an academic teaching setting. South Med J. 2014;107:520–525. doi: 10.14423/SMJ.0000000000000148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


