Abstract
Stimulatory and inhibitory effects of Notch signaling pathway on osteogenesis were both widely reported, questioning the effectiveness of small molecules targeting the Notch pathway for prevention or treatment of bone loss diseases. Here we showed that Notch signaling is activated in osteocytes embedded within the mineralized matrix and in late stages of bone marrow mesenchymal cell osteogenic cultures. Inhibition of Notch signaling markedly reduced mineralization activities of bone marrow mesenchymal cells and inhibited expressions of mineralization-associated genes when Notch ligand Jagged1 was conditionally deleted, confirming the essential roles of Notch signaling in mineralization stages of osteoblast differentiation. Moreover, intermittent activation of Notch signaling showed significant increases of bone formation in mice, rats and ovariectomized rats. A two-phase action model of Notch signaling in osteogenesis is proposed, where activation of Notch signaling in early stages of osteoblast differentiation results in proliferation of immature preosteoblast lineage cells and activation in late stages promotes differentiation of osteoblasts into osteocytes. Moreover, valproic acid is a strong activator of Notch signaling, and yearly administration of valproic acid daily showed little side effects, indicating that long term and intermittent activation of Notch signaling will be a safe and ideal way to promote anabolic bone formation for treatment of osteoporosis. Therefore, Notch signaling pathway is a good therapeutic target for bone loss diseases, and valproic acid, resveratrol and other Notch activators are promising therapeutic molecules for promoting anabolic bone formation when administered intermittently.
Keywords: Notch, osteogenesis, bone, anabolic, valproic acid, resveratrol
Introduction
Notch signaling pathway is an evolutionary conserved pathway regulating cell proliferation, differentiation, cell fate determination in both embryonic and adult organs [1-4]. In mammals, the Notch transmembrane receptor family consists of four members, Notch1-4. The Notch ligands identified, Jagged1/2 (Jag1/2) and delta-like 1/3/4 (Dll1/3/4), are type I transmembrane proteins [1-4]. Notch intracellular signaling is initiated upon ligand binding followed by two sequential proteolytic cleavage events mediated by tumor necrosis factor (TNF)-α converting enzyme (TACE) or γ-secretase, releasing the Notch extracellular domain and Notch intracellular domain (NICD). Subsequently NICD translocates into the nucleus, regulating expressions of target genes [1-4]. Deletion or mutation of any one or more of genes in Notch signaling pathway showed severe skeletal phenotypes in humans and in mouse models [1,2,5-7]. However, contradictory results were reported for roles of the Notch signaling pathway on skeletogenesis [1-4,8-16]. In in vitro cell cultures, activation of Notch signaling was reported to either promote [8,9] or inhibit [10,11] osteoblast differentiation and mineralization, while inhibition of Notch signaling also showed to promote [11] or inhibit [12] differentiation and mineralization of osteoblasts. In animal models, loss of Notch function resulted in radiodense [13,14] or osteoporotic [13-15] bones, while gain of Notch function was reported to have either osteoporotic [11,16] or osteosclerotic [12,15,16] phenotypes. Therefore, it is difficult to interpret functions of Notch signaling pathway in osteogenesis using a single-action model.
Here we show that Notch signaling pathway is strongly activated in osteocytes and intermittent activation of Notch signaling showed significant increases of bone formation in mouse and rat models. A two-phase model was then proposed, explaining Notch actions on osteogenesis and therapeutic potentials of intermittent activation of Notch signaling for bone loss diseases.
Materials and methods
Animals
All mouse and rat experiments were approved by the Institutional Animal Care and Use Committees of Wuhan University, and all applicable institutional and/or national guidelines for the care and use of animals were followed. The Jag1f/f mice [17], TNR mice [18] and Mx1Cre mice [19] were from The Jackson Laboratory. To induce the expression of Cre recombinase in Mx1Cre mice, female Jag1f/f; Mx1-Cre (J1MX1) mice were treated with 250 μg of polyinosinic-polycytidylic acid (pIpC) intraperitoneally every other day for three times at six weeks of age and sacrificed at 8 weeks for analysis.
For in vivo study, mice, rats and ovariectomized rats were treated with valproic acid (VPA, 100 mg/kg of body weight dissolved in PBS) or vehicle control (PBS) intraperitoneally for three months. Four groups of studies were performed. Group A: Three-month-old female C57BL/6 mice (n=8 for each subgroup). A regimen of daily treatment of VPA was continued for 0, 2, 3, 4 or 7 days followed by 7, 5, 4, 3 or 0 days of control (PBS) treatment each week. This weeklong paradigm was repeated 13 times. Group B: Five-month-old female C57BL/6 mice (n=12 for each subgroup). A regimen of daily treatment of VPA was continued for 0, 2, 3, 4 or 5 days followed by 5, 3, 2, 1 or 0 days of control (PBS) treatment each week. This weeklong paradigm was repeated 13 times. Group C: Six-month-old female Sprague-Dawley rats (n=12 for each subgroup). A regimen of daily treatment of VPA was continued for 0, 2, 3, 4 or 5 days followed by 5, 3, 2, 1 or 0 days of control (PBS) treatment each week. Daily treatment of PTH (100 µg/kg of body weight) subcutaneously for 5 consecutive days each week was as a positive control. This weeklong paradigm was repeated 13 times. Group D: Four-month-old female Sprague-Dawley rats (n=12 for each subgroup) were bilaterally ovariectomized (OVX) or sham operated (SHAM). Treatment regimen started at six months of age. A regimen of daily treatment of VPA in OVX rats was continued for 0, 2, 3, 4 or 5 days followed by 5, 3, 2, 1 or 0 days of control (PBS) treatment each week. Daily treatment of PTH (100 µg/kg of body weight) in OVX rats or PBS in SHAM rats for 5 consecutive days each week were as controls. This week long paradigm was repeated 13 times.
Mouse bone marrow mesenchymal cell culture
Primary BMSCs were obtained from mouse femurs or tibia and cultured as previously described [12]. Briefly, bone marrow was flushed from the femurs or tibia and cultured in basal medium (α-modified Eagle’s minimal medium (αMEM) supplemented with 10% FBS and 1% penicillin/streptomycin) at a cell density of 1-2×107/ml. For osteogenic differentiation, BMSCs were maintained in basal medium for 7 days, and changed to osteogenic medium (basal medium supplemented with 10-8 M dexamethasone, 8 mM β-glycerophosphate, and 50 μg/ml L-ascorbate) for additional 14 days. For DAPT treatment experiments, BMSCs were cultured in media supplied with various concentration of DAPT or DMSO as indicated. Media were replaced every three days.
Gene expression analysis
Total RNA was extracted using TRIzol reagent (Invitrogen) followed by DNase treatment. First-strand cDNA was synthesized using oligo(dT) primer and Superscript II reverse transcriptase. Quantitative real-time PCR was performed in triplicate and the relative amount of mRNA was normalized to the expression of cyclophilin A.
Trabecular bone analysis
Femurs from mice and rats were scanned using μCT50 as previously described [12]. Briefly, the bones were dissected, cleaned, fixed in 10% formalin, transferred to 75% ethanol, loaded into scanning tubes, and imaged with the following parameters: 70 kV, 114 μA, 0.5 mm Al filter, integration time 300 ms, 1000 projections/180 degree, resolution 6 μm for mouse samples and 15 μm for rat samples. Trabecular bones were extracted and analyzed using custom scripts. Data is available upon request and custom scripts can be downloaded from www.bomomics.com.
Statistical analysis
All values were reported as means ± standard deviations (SD). Intergroup comparisons between paired control and experimental groups were analyzed using the Student’s t-test. All data were analyzed using IBM SPSS 22.0 software.
Results
Activation of Notch signaling in late stages of osteoblastogenesis
Transgenic Notch reporter (TNR) mice were developed for monitoring Notch activities in neural [20] and hematopoietic cells [18], where activated Notch signaling is associated with strong green fluorescence. As reported, we observed activated Notch signaling in osteoblasts and osteocytes of trabecular and cortical bones from femurs and vertebrates of TNR mice [12]. Similarly, all nucleated cells in calvaria of TNR mice showed strong green fluorescence (Figure 1A), indicating that Notch signaling is activated in osteocytes embedded within bone matrices. During in vitro osteoblastic differentiation of bone marrow mesenchymal cells (BMSCs), BMSCs firstly undergo proliferation, then differentiate into osteoblasts and finally differentiate into osteocytes embedded in the mineralized matrix (Figure 1B). In vitro osteoblastic differentiation of BMSCs from TNR mice showed that green fluorescence was undetectable in early stages of cultures, but showed low level expression of green fluorescence at around day 17 and strong expression at around day 21 in cells within mineralized nodules (Figure 1B).
Figure 1.
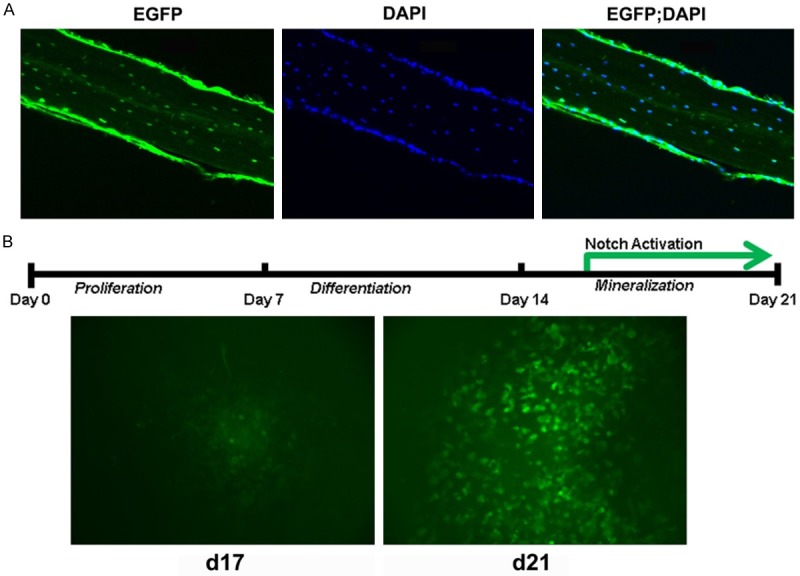
Notch signaling activation in late stages of osteoblast differentiation. A: Osteocytic expression of active Notch (EGFP) and DAPI staining of cell nuclei (DAPI) in calvaria from 8-week-old female TNR mice. B: Notch activation in late stages of in vitro osteoblast differentiation. Low level of GFP expression was detected at day 17 and strong GFP expression at day 21.
Inhibition of Notch signaling impairs in vitro mineralization
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a γ-secretase inhibitor (GSI), blocks the intracellular proteolytic cleavage of Notch receptor and the release of NICD, efficiently preventing activation of Notch signaling pathway [12]. In osteogenic cultures of BMSCs from TNR mice (Figure 2A), low concentration (10 or 20 μM) of DAPT showed little effects on the expression of green fluorescence, however, high concentration of DAPT (50 or 100 μM) markedly inhibited the expression of green fluorescence, indicating efficient inactivation of Notch signaling pathway. Furthermore, no significant difference between treated and control groups was observed when BMSCs were cultured in proliferating media before day 7, however, mineralized nodular formation was greatly inhibited by DAPT in late stages of BMSC cultures starting from day 14 when cultured in osteogenic media (Figure 2B), indicating that notch inhibition by GSI impaired mineralization of BMSCs in osteogenic media. As reported, expressions of mineralization-associated genes, such as matrix extracellular phosphoglycoprotein (Mepe), dentin matrix protein-1 (Dmp1), sclerostin (Sost) and phosphate regulating endopeptidase homolog X-linked (Phex), were greatly inhibited by DAPT in late stages but not in early stages of BMSC osteogenic cultures [12].
Figure 2.

Inhibition of Notch Signaling impairs mineralizing activities of BMSCs. A: BMSCs from TNR mice were cultured in basal media for seven days and then changed to osteogenic media supplemented with various concentrations of γ-secretase inhibitor DAPT. GFP expression was monitored at day 21. B: BMSCs from C57BL/6 mice were cultured in basal media for seven days and then changed to osteogenic media supplemented with 50 μM DAPT or DMSO for additional 14 days. ALP staining (d7) and von kossa stainings (d14, d17 and d21) were performed. Upper wells: DMSO (Vehicle control); lower wells: DAPT. Mineralization areas were quantified using ImageJ software and analyzed using unpaired t-test. *, P<0.05.
Jag1 is an important Notch ligand playing essential roles in skeletogenesis, and conditional deletion of Jag1 impaired in vitro mineralization of BMSCs [12]. Similar to the effects of Notch pathway inhibition by DAPT [12], conditional deletion of Jag1 resulted in markedly reduced expression of mineralization-associated genes (Figure 3) in late stages of BMSC osteogenic cultures, indicating essential roles of Notch signaling pathway in regulating the mineralization of osteoblast differentiation.
Figure 3.
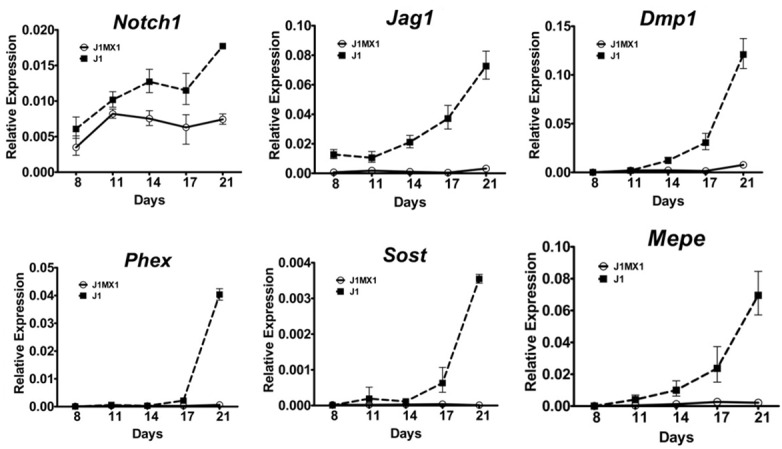
Impaired Notch signaling inhibited expressions of osteocytic mineralization-associated gene markers. Female Jag1f/f (J1) and Jag1f/f; Mx1-Cre (J1MX1) mice were treated with pIpC at six weeks of age and sacrificed at 8 weeks. BMSCs from pIpC treated mice were cultured in basal media for seven days and then in osteogenic media for additional 14 days. Total RNA was extracted at specific time point and quantitative PCR performed for expression of osteocytic mineralization markers (n=3 wells per group in quadruplicate).
Intermittent activation of Notch signaling stimulates bone formation
As restricted activation of Notch signaling in osteocytes showed profound bone formation [12,15,16,21], Notch signaling pathway is a potential therapeutic target for bone loss diseases. However, restricted activation of Notch signaling in osteocytes by small molecules is challenging. In addition, constitutive activation of Notch signaling displayed osteosclerosis by Col3.2, Col2.3 or Dmp1 promoters [12,15,16,21], and osteoporotic phenotypes by Col3.6 or Osteocalcin promoters [11,16], while conditional inactivation of Notch signaling by Prx1 or Col2.3 promoters showed age-dependent bone loss in old mice [13-15], indicating that Notch activation has inhibitory roles at early stages of osteoblast differentiation and stimulatory roles at late stages of osteoblast differentiation. Therefore, continuous activation or inactivation of Notch signaling pathway by small molecules is not optimal for stimulating bone formation, as such molecules tend to activate Notch signaling pathway in all cells of osteoblastic lineages instead of to restrict to osteocytes.
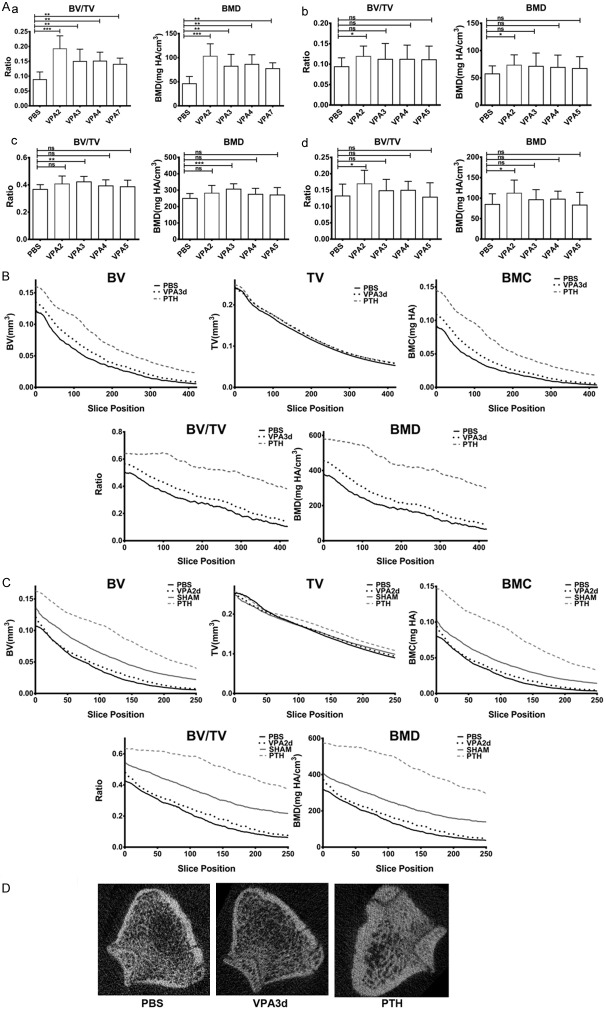
To evaluate the therapeutic potential of Notch signaling pathway on bone loss diseases, effects of intermittent activation of Notch signaling pathway on bone formation were tested using mouse and rat models. Valproic acid (VPA) is known to activate Notch signaling pathway in various tissues [22-25] and showed enhanced mineralization and ossification in in vitro cultures [26-31]. As shown, VPA strongly stimulated trabecular bone formation in mice, rats and ovariectomized rats (Figure 4A) only when administrated intermittently, while long duration of VPA treatment showed little anabolic bone formation effects. Here, optimal stimulation of bone formation by VPA in mice and ovariectomized rats was the regimen of two-day consecutive VPA treatment followed by five-day control treatment each week, while consecutive treatment of VPA more than 2 days each week showed less profound bone formation effects. In six-month-old SD rats, optimal treatment regimen was three-day consecutive treatment of VPA followed by four-day control treatment each week, indicating that intermittent rather than continuous activation of Notch signaling by VPA is essential to promote bone formation.
Figure 4.
Intermittent activation of Notch signaling enhanced trabecular bone formation. A: Mice or rats were treated with saline (PBS) or VPA (VPA2-VPA7 or VPA2-VPA5) for three months, and trabecular bones from distal femurs were analyzed. Data were represented as a mean ± SD. a. Three-month-old female C57BL/6 mice (n=8 for each group) was treated with VPA for 2, 3, 4 or 7 consecutive days or PBS each week. b. Five-month-old female C57BL/6 mice (n=12 for each group) was treated with VPA for 2, 3, 4 or 5 consecutive days or PBS each week. c. Six-month-old female Sprague-Dawley rats (n=12 for each group) was treated with VPA for 2, 3, 4 or 5 consecutive days or PBS each week. d. Female Sprague-Dawley rats were bilaterally ovariectomized (OVX) at four months of age and treatment started at six months of age. OVX rats (n=12 for each group) was treated with VPA for 2, 3, 4 or 5 consecutive days or PBS each week. Student’s t-test was used for between group analyses. *P<0.05, **P<0.01, ***P<0.001. B: Layer-by-layer analysis of femurs from six-month-old rats treated with PBS, VPA (for 3 consecutive days each week) or PTH. Slice position is relative to the growth plate reference position of rat distal femurs. Data were represented as a mean at each layer. C: Layer-by-layer analysis of femurs from six-month-old OVX or SHAM rats treated with PBS, VPA (for 2 consecutive days each week) or PTH. Slice position is relative to the growth plate reference position of rat distal femurs. Data were represented as a mean at each layer. D: Representative images of rat femurs treated with PBS, VPA or PTH respectively 1.5 mm (100 layers) away from growth plate reference positions.
Layer-by-layer analysis showed that intermittent activation of Notch signaling by VPA promoted small but significant trabecular bone formation in SD rats and ovariectomized SD rats (Figure 4B and 4C), and such an increase of bone formation showed no structural irregularities (Figure 4D), while profound bone formation by PTH administration was observed associated with significant changes of trabecular microarchitectures (Figure 4D), indicating that Notch signaling pathway is a good anabolic drug target for prevention or treatment of bone loss diseases.
Model of Notch signaling in osteoblast differentiation
In view of the fact that Notch signal pathway is not activated in early stages of BMSC osteogenic differentiation but strongly activated in late stages of differentiation, the following model of Notch’s roles on osteoblastogenesis was proposed (Figure 5): Notch signaling is not activated during the differentiation of mesenchymal stem cells (MSCs) into osteoblasts, and activation of Notch signaling in this stage inhibits the differentiation of MSCs into osteoblasts, resulting in accumulation of immature preosteoblast lineage cells. Notch signaling is activated during the differentiation of osteoblasts into osteocytes and activation of Notch signaling in this stage promotes osteoblasts differentiation and mineralization into osteocytes. Moreover, inhibition of Notch signaling in early stages of MSC osteogenic differentiation promotes the differentiation of MSCs into osteoblasts resulting in accumulation of mature osteoblasts, while inhibition of Notch signaling pathway in late stages of osteoblast differentiation inhibits differentiation and mineralization of mature osteoblasts into osteocytes.
Figure 5.
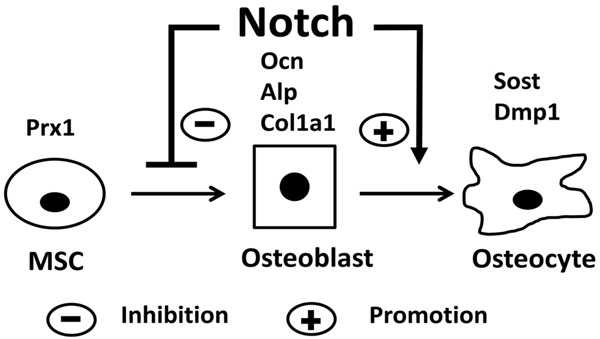
Model of notch signaling in osteoblast differentiation. Notch inhibits the differentiation of MSCs into osteoblasts and promotes the differentiation of osteoblasts into osteocytes.
Discussion
During osteoblastogenesis, mesenchymal stem cells (MSCs) differentiate into functional osteoblasts. Subsequently, functional osteoblasts undergo matrix maturation and differentiate into osteocytes, which are embedded in the mineralized matrix. Here we reported that Notch signaling pathway is activated in osteocytes and inhibition of Notch signaling impairs in vitro mineralization of BMSCs. Enhanced bone formation by intermittent activation of Notch signaling was observed in mouse and rat models and a dimorphic Notch action model on osteoblastogenesis was then proposed.
Conflicting results of Notch effects on osteoblastogenesis were reported, however our dual action model of Notch signaling pathway in osteoblastogenesis could explain nearly all reported inconsistencies for in vivo and in vitro data. As reported, forced activation of Notch signaling in preosteoblasts resulted in accumulation of immature osteoblasts [32,33], and Notch activation in early stages of osteoblast differentiation (Prx1Cre; Rosa-NICDf/+ or Col3.6-NICD) showed enhanced proliferation and suppressed differentiation of mesenchymal progenitor cells (MPC) in the developing limb [34] and features of osteopenia in adult mice [11], while Notch activation in late stages of osteoblast differentiation (Col2.3Cre; Rosa-NICDf/+, Dmp1Cre; Rosa-NICDf/+, Col3.2CreERT2; Rosa-NICDf/+ or Col2.3-NICD) resulted in increased bone formation [12,15,16]. Such gain of Notch function results in osteoblastogenesis can be well explained by our dimorphic Notch action model: continuous activation of Notch signaling in early stages of osteoblast differentiation inhibits the differentiation of MSCs (or MPCs) into osteocytes, resulting in proliferation of MPCs and decreasing of osteocyte numbers, thus features of osteopenia were observed in adult mice due to impaired differentiation of MSCs into osteocytes. However, continuous activation of Notch signaling in late stages of osteoblast differentiation promotes differentiation of osteoblasts into osteocytes, resulting in temporarily reduced osteoblast numbers and increased osteocyte numbers. To keep the balance between MSCs and osteoblasts, more MSCs were promoted to differentiate into more osteoblasts. Hence, the net outcome of continuous activation of Notch signaling in late stages of osteoblast differentiation is to promote more MSCs to differentiate into more osteocytes, resulting in increased bone formation. Similarly, Notch inactivation in both early and late stages of osteoblast differentiation (Prx1Cre; Notch1-/fNotch2f/f, Prx1Cre; Notch2f/f, Prx1-Cre; RBPjkf/f or Col2.3Cre; Psen1f/fPsen2-/-) showed age-dependent bone loss [13-15], and marked increases of radiodensity and double-labeled surfaces within the trabecular bones were observed in young mice when Notch signaling was conditionally inactivated in early stages of osteoblast differentiation [13]. According to our Notch action model, inactivation of Notch signaling in early stages of osteoblast differentiation promotes the differentiation of MSCs into mature osteoblasts, but inhibits the differentiation and mineralization of mature osteoblasts into osteocytes due to the inactivation of Notch signaling, resulting in accumulation of osteoblasts, upregulation of osteoblast activities and reduction of osteocytes. Subsequently, proliferations and accumulations of osteoblasts resulted in marked increases of bone mass in young mice, possibly by induced ectopic mineralization of osteoblasts independent of Notch signaling pathway or incomplete inactivation of Notch signaling mediated by Cre recombinase. When Notch is inactivated in late stages of osteoblast differentiation, differentiation of osteoblasts into osteocytes was inhibited and severe age-related bone loss was observed in old mice as expected. Furthermore, results from in vitro loss of Notch function or gain of Notch function studies using primary bone marrow mesenchymal cells [8,9,12], osteoblastic cell lines [8] or cell lines derived from bone marrow mesenchymal cells [10] were all consistent with our proposed Notch action model. However, in the context of BMP-2 treatment, conflicting results were reported about Notch’s roles on osteoblastogenesis [8,35-38]. As BMP-2 has profound bone stimulation effects and such effects may be independent of Notch signaling pathway, the controversial results of Notch actions in osteoblasteogenesis under BMP-2 treatment condition will not affect the validity of our proposed Notch action model.
Here, intermittent activation of Notch signaling by valproic acid (VPA) showed significant increases of trabecular bone formation in mice, rats or ovariectomized rats. Such observations can be explained by our model as well. During the phase of Notch activation by VPA, osteoblasts were promoted to differentiate into osteocytes, resulting in decreased osteoblast numbers and increased osteocyte numbers. In the meanwhile, osteoprogenitor cells fail to differentiate into osteoblasts when Notch is activated by VPA, leading to increased osteoprogenitor numbers and decreased osteoblast numbers. During the phase of normal Notch activity when No VPA is present, increased osteoprogenitors numbers and decreased osteoblast numbers promote more osteoprogenitors to differentiate into more osteoblasts. Hence, the net outcome of intermittent activation of Notch pathway by VPA is promoting more osteoprogenitors to differentiate into more osteocytes, leading to increased bone formation.
In theory, alternate activation and inhibition of Notch signaling will have profound bone formation effects as well, and such a view was supported by published report [39] indirectly. In the mouse model of systemic lupus erythematosus (MRL/lpr mice), high expressions of Notch1, Notch2, Jag1 and NICD proteins in BMSCs [39] and severe osteoporotic phenotype were observed due to continuous activation of Notch signaling in BMSCs. When Notch signaling in MRL/lpr mice was intermittently inhibited by DAPT, markedly increased bone formation was observed. In this treatment regimen, Notch inhibitor DAPT was administered subcutaneously on a daily basis for three consecutive days (Notch inhibition) to MRL/lpr mice followed by 4-days without treatment each week (Notch activation). Similar to the scenario of intermittent activation of Notch signaling, such a treatment regimen of alternating activation and inhibition of Notch signaling resulted in extensive bone formation as expected.
Osteoporosis is a metabolic bone disease with impaired bone strength and increased risk of fracture, affecting up to 50% postmenopausal women older than 50 years [40,41]. Currently, most approved drugs for treatment of osteoporosis are antiresorptive agents with potential adverse effects on long time usage [40,41], and PTH and anti-sclerostin antibodies (romosozumab and blosozumab) are the only approved anabolic agents for treatment of osteoporosis [40,41], where PTH is restricted to a single 24 months regimen and long-term safety of anti-sclerostin antibodies are uncertain [40,41]. Here we showed that intermittent activation of Notch signaling has strong anabolic effects on bone formation, indicating that Notch signaling pathway is a new identified target for the treatment of osteoporosis. Valproic acid (VPA) is a strong Notch activator and has been used to treat a variety of seizure and bipolar disorders for decades [42,43]. Moreover, long term usage of VPA are not associated with severe side effects [42,43], indicating that long term activation of Notch signaling is not likely to be associated with severe side effects. Therefore, Notch signaling pathway is an ideal target for developing anabolic agents for the treatment of osteoporosis.
VPA and Resveratrol (RESV) both strongly activated Notch signaling in various cell lines [22-25,44-48] and enhanced ossification and mineralization in osteogenic cultures [26-31,49-54], consistent with their Notch activating activities in these cells. In humans, the half-life of VPA is 10-20 h, and about 6-9 h in children. Therefore, it is reasonable to see reported severe bone loss in patients with long-term use of VPA [42,43], which can be well explained by continuous activation of Notch signaling, and reported cases of enhanced bone formations in young patients [55], possibly due to shorter half-lives or changed metabolic profiles of VPA in those patients. In addition, RESV was reported to significantly promote bone formation in animal models [56-58] and in a randomized placebo-controlled clinical trial [59]. Due to the rapid metabolism and clearance of RESV [56,57,60], daily administration of RESV in animal studies and clinical trials is similar to intermittent activation of Notch signaling, thus stimulatory effects of RESV on bones were reported. Therefore, VPA, RESV and their long half-life derivatives are in theory better molecules for the treatment of osteoporosis by promoting anabolic bone formations through intermittent activation of Notch signaling pathway.
Acknowledgements
This work was funded in part by National Natural Science Foundation of China (81170806). The authors would like to thank Dr. Peng Liu for support and assistance with this project. Special thanks also go to µCT core facilities of Wuhan University School and Hospital of Stomatology for helping scan and analyze the mouse and rat femurs.
Disclosure of conflict of interest
A patent application has been filed relating to this work.
References
- 1.Yavropoulou MP, Yovos JG. The role of Notch signaling in bone development and disease. Hormones (Athens) 2014;13:24–37. doi: 10.1007/BF03401318. [DOI] [PubMed] [Google Scholar]
- 2.Tao J, Chen S, Lee B. Alteration of Notch signaling in skeletal development and disease. Ann N Y Acad Sci. 2010;1192:257–268. doi: 10.1111/j.1749-6632.2009.05307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mead TJ, Yutzey KE. Notch signaling and the developing skeleton. Adv Exp Med Biol. 2012;727:114–130. doi: 10.1007/978-1-4614-0899-4_9. [DOI] [PubMed] [Google Scholar]
- 4.Engin F, Lee B. NOTCHing the bone: insights into multi-functionality. Bone. 2010;46:274–280. doi: 10.1016/j.bone.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- 6.Huppert SS, Ilagan MX, De Strooper B, Kopan R. Analysis of Notch function in presomitic mesoderm suggests a gamma-secretaseindependent role for presenilins in somite differentiation. Dev Cell. 2005;8:677–688. doi: 10.1016/j.devcel.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Hrabe de Angelis M, McIntyre J 2nd, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- 8.Tezuka K, Yasuda M, Watanabe N, Morimura N, Kuroda K, Miyatani S, Hozumi N. Stimulation of osteoblastic cell differentiation by Notch. J Bone Miner Res. 2002;17:231–239. doi: 10.1359/jbmr.2002.17.2.231. [DOI] [PubMed] [Google Scholar]
- 9.Ugarte F, Ryser M, Thieme S, Fierro FA, Navratiel K, Bornhauser M, Brenner S. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. 2009;37:867–875. e861. doi: 10.1016/j.exphem.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Shindo K, Kawashima N, Sakamoto K, Yamaguchi A, Umezawa A, Takagi M, Katsube K, Suda H. Osteogenic differentiation of the mesenchymal progenitor cells, Kusa is suppressed by Notch signaling. Exp Cell Res. 2003;290:370–380. doi: 10.1016/s0014-4827(03)00349-5. [DOI] [PubMed] [Google Scholar]
- 11.Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology. 2008;149:3890–3899. doi: 10.1210/en.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu P, Ping Y, Ma M, Zhang D, Liu C, Zaidi S, Gao S, Ji Y, Lou F, Yu F, Lu P, Stachnik A, Bai M, Wei C, Zhang L, Wang K, Chen R, New MI, Rowe DW, Yuen T, Sun L, Zaidi M. Anabolic actions of Notch on mature bone. Proc Natl Acad Sci U S A. 2016;113:E2152–2161. doi: 10.1073/pnas.1603399113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu X, Chen J, Lim J, Karner CM, Lee SY, Heisig J, Wiese C, Surendran K, Kopan R, Gessler M, Long F. Physiological notch signaling maintains bone homeostasis via RBPjk and Hey upstream of NFATc1. PLoS Genet. 2012;8:e1002577. doi: 10.1371/journal.pgen.1002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canalis E, Parker K, Feng JQ, Zanotti S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology. 2013;154:623–634. doi: 10.1210/en.2012-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 20.Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- 21.Canalis E, Adams DJ, Boskey A, Parker K, Kranz L, Zanotti S. Notch signaling in osteocytes differentially regulates cancellous and cortical bone remodeling. J Biol Chem. 2013;288:25614–25625. doi: 10.1074/jbc.M113.470492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stockhausen MT, Sjolund J, Manetopoulos C, Axelson H. Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br J Cancer. 2005;92:751–759. doi: 10.1038/sj.bjc.6602309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, Ning L, Haymart M, Kunnimalaiyaan M, Chen H. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12:942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 24.Adler JT, Hottinger DG, Kunnimalaiyaan M, Chen H. Histone deacetylase inhibitors upregulate Notch-1 and inhibit growth in pheochromocytoma cells. Surgery. 2008;144:956–961. doi: 10.1016/j.surg.2008.08.027. discussion 961-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Qian Q, Sun G, Mackey LV, Fuselier JA, Coy DH, Yu CY. Valproic acid induces NET cell growth arrest and enhances tumor suppression of the receptor-targeted peptide-drug conjugate via activating somatostatin receptor type II. J Drug Target. 2016;24:169–177. doi: 10.3109/1061186X.2015.1066794. [DOI] [PubMed] [Google Scholar]
- 26.Paino F, La Noce M, Tirino V, Naddeo P, Desiderio V, Pirozzi G, De Rosa A, Laino L, Altucci L, Papaccio G. Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: evidence for HDAC2 involvement. Stem Cells. 2014;32:279–289. doi: 10.1002/stem.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Park JR, Seo MS, Roh KH, Park SB, Hwang JW, Sun B, Seo K, Lee YS, Kang SK, Jung JW, Kang KS. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 2009;42:711–720. doi: 10.1111/j.1365-2184.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pessina A, Sisto F, Cocce V, Cavicchini L, Ciusani E, Gribaldo L, Bonomi A. A mesenchymal stromal cell line resistant to paclitaxel that spontaneously differentiates into osteoblast-like cells. Cell Biol Toxicol. 2011;27:169–180. doi: 10.1007/s10565-010-9179-x. [DOI] [PubMed] [Google Scholar]
- 29.Cho HH, Park HT, Kim YJ, Bae YC, Suh KT, Jung JS. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J Cell Biochem. 2005;96:533–542. doi: 10.1002/jcb.20544. [DOI] [PubMed] [Google Scholar]
- 30.Fu Y, Zhang P, Ge J, Cheng J, Dong W, Yuan H, Du Y, Yang M, Sun R, Jiang H. Histone deacetylase 8 suppresses osteogenic differentiation of bone marrow stromal cells by inhibiting histone H3K9 acetylation and RUNX2 activity. Int J Biochem Cell Biol. 2014;54:68–77. doi: 10.1016/j.biocel.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Wu S, Legido A, De Luca F. Effects of valproic acid on longitudinal bone growth. J Child Neurol. 2004;19:26–30. doi: 10.1177/088307380401900105011. [DOI] [PubMed] [Google Scholar]
- 32.Huang B, Wang Y, Wang W, Chen J, Lai P, Liu Z, Yan B, Xu S, Zhang Z, Zeng C, Rong L, Liu B, Cai D, Jin D, Bai X. mTORC1 prevents preosteoblast differentiation through the notch signaling pathway. PLoS Genet. 2015;11:e1005426. doi: 10.1371/journal.pgen.1005426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muguruma Y, Hozumi K, Warita H, Yahata T, Uno T, Ito M, Ando K. Maintenance of bone homeostasis by DLL1-mediated notch signaling. J Cell Physiol. 2017;232:2569–2580. doi: 10.1002/jcp.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong Y, Jesse AM, Kohn A, Gunnell LM, Honjo T, Zuscik MJ, O’Keefe RJ, Hilton MJ. RBPjkappa-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development. 2010;137:1461–1471. doi: 10.1242/dev.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sciaudone M, Gazzerro E, Priest L, Delany AM, Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–5639. doi: 10.1210/en.2003-0463. [DOI] [PubMed] [Google Scholar]
- 36.Nobta M, Tsukazaki T, Shibata Y, Xin C, Moriishi T, Sakano S, Shindo H, Yamaguchi A. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem. 2005;280:15842–15848. doi: 10.1074/jbc.M412891200. [DOI] [PubMed] [Google Scholar]
- 37.Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/betacatenin but not bone morphogenetic protein signaling. J Biol Chem. 2006;281:6203–6210. doi: 10.1074/jbc.M508370200. [DOI] [PubMed] [Google Scholar]
- 38.Viale-Bouroncle S, Gosau M, Morsczeck C. NOTCH1 signaling regulates the BMP2/DLX-3 directed osteogenic differentiation of dental follicle cells. Biochem Biophys Res Commun. 2014;443:500–504. doi: 10.1016/j.bbrc.2013.11.120. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Liu D, Chen C, Hamamura K, Moshaverinia A, Yang R, Liu Y, Jin Y, Shi S. MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab. 2015;22:606–618. doi: 10.1016/j.cmet.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gennari L, Rotatori S, Bianciardi S, Nuti R, Merlotti D. Treatment needs and current options for postmenopausal osteoporosis. Expert Opin Pharmacother. 2016;17:1141–1152. doi: 10.1080/14656566.2016.1176147. [DOI] [PubMed] [Google Scholar]
- 41.Tabatabaei-Malazy O, Salari P, Khashayar P, Larijani B. New horizons in treatment of osteoporosis. Daru. 2017;25:2. doi: 10.1186/s40199-017-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomson T, Battino D, Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016;15:210–218. doi: 10.1016/S1474-4422(15)00314-2. [DOI] [PubMed] [Google Scholar]
- 43.Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46:1323–1338. doi: 10.1016/j.clinbiochem.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Pinchot SN, Jaskula-Sztul R, Ning L, Peters NR, Cook MR, Kunnimalaiyaan M, Chen H. Identification and validation of Notch pathway activating compounds through a novel highthroughput screening method. Cancer. 2011;117:1386–1398. doi: 10.1002/cncr.25652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Li H, Liu N, Chen XY, Wu ML, Zhang KL, Kong QY, Liu J. Correlative analyses of notch signaling with resveratrol-induced differentiation and apoptosis of human medulloblastoma cells. Neurosci Lett. 2008;438:168–173. doi: 10.1016/j.neulet.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Truong M, Cook MR, Pinchot SN, Kunnimalaiyaan M, Chen H. Resveratrol induces Notch2-mediated apoptosis and suppression of neuroendocrine markers in medullary thyroid cancer. Ann Surg Oncol. 2011;18:1506–1511. doi: 10.1245/s10434-010-1488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin H, Xiong W, Zhang X, Liu B, Zhang W, Zhang Y, Cheng J, Huang H. Notch-1 activationdependent p53 restoration contributes to resveratrol-induced apoptosis in glioblastoma cells. Oncol Rep. 2011;26:925–930. doi: 10.3892/or.2011.1380. [DOI] [PubMed] [Google Scholar]
- 48.Yu XM, Jaskula-Sztul R, Ahmed K, Harrison AD, Kunnimalaiyaan M, Chen H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol Cancer Ther. 2013;12:1276–1287. doi: 10.1158/1535-7163.MCT-12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song LH, Pan W, Yu YH, Quarles LD, Zhou HH, Xiao ZS. Resveratrol prevents CsA inhibition of proliferation and osteoblastic differentiation of mouse bone marrow-derived mesenchymal stem cells through an ER/NO/cGMP pathway. Toxicol In Vitro. 2006;20:915–922. doi: 10.1016/j.tiv.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Rutledge KE, Cheng Q, Jabbarzadeh E. Modulation of inflammatory response and induction of bone formation based on combinatorial effects of resveratrol. J Nanomed Nanotechnol. 2016:7. doi: 10.4172/2157-7439.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shakibaei M, Shayan P, Busch F, Aldinger C, Buhrmann C, Lueders C, Mobasheri A. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PLoS One. 2012;7:e35712. doi: 10.1371/journal.pone.0035712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon DS, Choi Y, Choi SM, Park KH, Lee JW. Different effects of resveratrol on early and late passage mesenchymal stem cells through beta-catenin regulation. Biochem Biophys Res Commun. 2015;467:1026–1032. doi: 10.1016/j.bbrc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Dai Z, Li Y, Quarles LD, Song T, Pan W, Zhou H, Xiao Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14:806–814. doi: 10.1016/j.phymed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Danmark S, Edlund U, Finne-Wistrand A, He X, Norgard M, Blomen E, Hultenby K, Andersson G, Lindgren U. Resveratrolconjugated poly-epsilon-caprolactone facilitates in vitro mineralization and in vivo bone regeneration. Acta Biomater. 2011;7:751–758. doi: 10.1016/j.actbio.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Swoboda KJ, Scott CB, Reyna SP, Prior TW, LaSalle B, Sorenson SL, Wood J, Acsadi G, Crawford TO, Kissel JT, Krosschell KJ, D’Anjou G, Bromberg MB, Schroth MK, Chan GM, Elsheikh B, Simard LR. Phase II open label study of valproic acid in spinal muscular atrophy. PLoS One. 2009;4:e5268. doi: 10.1371/journal.pone.0005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tou JC. Evaluating resveratrol as a therapeutic bone agent: preclinical evidence from rat models of osteoporosis. Ann N Y Acad Sci. 2015;1348:75–85. doi: 10.1111/nyas.12840. [DOI] [PubMed] [Google Scholar]
- 57.Tou JC. Resveratrol supplementation affects bone acquisition and osteoporosis: pre-clinical evidence toward translational diet therapy. Biochim Biophys Acta. 2015;1852:1186–1194. doi: 10.1016/j.bbadis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Mobasheri A, Shakibaei M. Osteogenic effects of resveratrol in vitro: potential for the prevention and treatment of osteoporosis. Ann N Y Acad Sci. 2013;1290:59–66. doi: 10.1111/nyas.12145. [DOI] [PubMed] [Google Scholar]
- 59.Ornstrup MJ, Harslof T, Kjaer TN, Langdahl BL, Pedersen SB. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2014;99:4720–4729. doi: 10.1210/jc.2014-2799. [DOI] [PubMed] [Google Scholar]
- 60.Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]