Abstract
Mechanotransduction is the conversion of extracellular mechanical stimuli into intracellular biochemical signals, and plays an important role in heart responses to its own mechanical environment. Piezo1 as a distinct stretch-activated channel (SAC) in mammal involves in not only vascular remodeling during embryonic development but also arterial remodeling upon to hypertension at adult stage. In the present study, the expression of Piezo1 was up-regulated in failure heart induced by myocardial infarction (MI) by real-time PCR, Western blot and immunohistochemistry analysis. Expression of Piezo1 mRNA and protein was enhanced by AngiotensinII (AngII) in neonatal rat ventricular myocytes via AT1 receptor depended methods. Furthermore, the Piezo1 expression was attenuated by Erk1/2 chemical inhibitor (U0126) only, but not by p38 MAPK inhibitor (SB203580), or JNK inhibitor (SP600125). Finally, systolic function improvement followed by chronic treatment with angiotensin receptor blocker (ARB) losartan prevented Piezo1 up-regulation in failure heart in vivo. In conclusion, our studies linked mechanotransduction which involved renin-angiotensin system that mediated up-regulation of Piezo1 to a clinically relevant heart failure.
Keywords: Piezo1, stretch-activated channels, mechanotransduction, neonatal rat ventricular myocytes, angiotensin II, heart failure
Introduction
The heart can intrinsically sense and respond to its own mechanical environment to maintain hemodynamic stability known as mechanotransduction [1]. Mechanotransduction in heart serves as adaptive compensatory mechanisms under transient limited pressure or volume load for improving output [1,2]. However, under prolonged and chronic overload, the regulatory processes turn into maladaptive structural remodeling and lead to heart failure [3]. SACs are directly activated by mechanical force and identified as candidates for cardiac mechanotransducers [4,5]. In a recent tremendous progress, Piezo1 and Piezo2 were verified as distinct SACs in mammal and critical for mechanical responses in cells [6-9]. Heterologous cells overexpressing Piezo1 confer similar mechanically sensitive currents [6]. Importantly, purified Piezo1 reconstituted into artificial bilayers performed cation channels characteristics, thereby validating Piezo1 as an authentic ion channel in fidelity [10].
Piezo1 is gated and tuned by cellular membrane bilayer tension directly, but not by cytoskeleton [11,12]. Piezo1 opens upon a various of mechanical stimuli, including pipette poking, cell swelling, stretch, or shear stress, and mediate K+, Na+, Ca2+ and Mg2+ influx with a slight preference for Ca2+ [13]. A synthetic small molecule Yoda1 sensitizes Piezo1 by slowing the inactivation phase of channel, while protonation inhibits Piezo1 by stabilizing the inactivated state [14,15].
Piezo1 is widespread in various tissues such as bladder, colon, kidney, lung, and skin, suggesting that it played a potential and general role in these organs [6]. The functional researches showed that Piezo1 mediated SACs activity in tubule epithelial cells and overcrowding triggered extrusion without apoptosis in live epithelial cells [16]. In addition, knockdown of Piezo1 in bronchial epithelial cells reduced integrin-dependent adhesion and increased migration [17]. In translational medicine, gain-of-function mutations in Piezo1 was associated with dehydrated hereditary stomatocytosis [18-20].
Recently, it was highlighted that Piezo1 was expressed in mammalian embryonic vascular endothelial cells and played an essential role in vascular remodeling during embryonic development [21,22]. While Piezo1 is dispensable for the arterial myogenic response at the adult stage, but involed in arterial remodeling upon AngII infused hypertension [23]. Increased opening of Piezo1 independent of hypertension in smooth muscle cells influences arterial remodeling, including both diameter and wall thickness, and mediates adaptive responses to hypertension [23].
After all, mechanical stress in myocardium and vascular wall are rhythmically changed with cardiac cycle. Hence, we hypothesized that Piezo1 channels may participate in the pathophysiological processes in heart, such as heart failure. In the present study, we attempted to explore whether the expression of SACs Piezo1 was altered in failure heart, and the underlying pathway mechanism.
Material and methods
Animal model of myocardial infarction
Animals were supplied by the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). All experiments with animals were performed in accordance with the Guidelines and Policies for Laboratory Animals approved by the Sun Yat-sen University Animal Care and Use Committee. Male Sprague Dawley rats (body weight ~200 g) were anesthetized with 10% chloral hydrate (0.35 ml/100 g, intraperitoneal injection), then rapidly intubated and mechanically ventilated (tidal volume, 1 ml/100 g body weight; ventilation rate, 60 strokes/min) by a constant volume animal ventilator (HX-300S, Techman Apparatus). A left thoracotomy was performed at the fourth intercostal space, myocardial infarction (MI) was induced by ligation of the LAD coronary artery by a 5-0 silk suture. Sham-operated animals underwent the same surgical procedure without ligation of LAD coronary artery. The rats survived through day 1 post-MI were randomly assigned to 4 weeks and 8 weeks post-MI groups. To explore the effects of losartan on the expression of Piezo1, rats surviving 24 h after MI were administered daily with placebo or losartan (40 mg/kg, Merck) by oral gavage for 8 weeks.
Measurement of cardiac function, blood pressure and heart rate
Transthoracic echocardiographic images of hearts from all groups of unconscious rats (anesthetized by 10% chloral hydrate, 0.25-0.3 ml/100 g, intraperitoneal injection) were obtained using a 12-MHz ultrasound probe (Philips), left ventricular inner diameters in diastole (LVIDd) and systole (LVIDs), LVFS and LVEF were assessed. Blood pressure and heart rate were measured using the non-invasive tail cuff system (Softron BP98A). Then, rats were euthanized for the studies outlined below.
Isolation and culture of neonatal rat ventricular myocytes
The hearts of one or two-day-old rats were rapidly removed from the chest cavity under anesthesia, atrial tissue of the hearts were trimmed off. The ventricles were washed in ice-cold phosphate-buffered saline (PBS), cut into small pieces of approximately 1 mm3 and digested with 0.125% trypsin (Sigma) in a water bath at 37°C for 5 min and 0.006% collagenase I (Sigma) at 37°C for 2 h successively. Cells were harvested after digestion and re-suspended in (DMEM)/F12 (1:1) (GIBCO) supplemented with 10% (v/v) foetal bovine serum (FBS, Hyclone). The cells were preplated in uncoated plates for 1 h at 37°C incubator to reduce the contamination of cardiac fibroblasts. The neonatal rat ventricular myocytes (NRVMs) were collected and cultured in plating medium containing 10% FBS and 1% Brdu.
Quantitative real time polymerase chain reaction
Total RNAs were extracted from the rat heart tissues after blood drained and lysed NRVMs with TRIzol reagent kit (Invitrogen, USA) according to the manufacturer’s instructions and the concentration was quantified by NanoDrop 2000 (Thermo Scientific). Total 500 ng RNA was reverse transcribed to cDNA with the PrimeScript RT Reagent Kit (TaKaRa, Japan) using the manufacturer’s protocol. Real time polymerase chain reaction (RT-PCR) for Piezo1 (forward 5’-ATGGAGCCGCACGTGCTG-3’ and reverse 5’-CTACTCCCTCTCACGTGTCCA-3’) and GAPDH as the internal control (forward 5’-TATGTCGTGGAGTCTACTGG-3’ and reverse 5’-AGTGATGGCATGGACTGTGG-3’) was carried out by the LightCycler 480 Real Time PCR system (Roche Diagnostics) using SYBR Premix Ex Taq (TaKaRa, Japan) over 40 cycles.
Western blot
Rat heart tissue and lysed NRVMs were collected for Western blot analysis. Samples were homogenized in RIPA buffer (Beyotime Biotechnology) with protease inhibitor (Roche). Protein concentration was determined by using the bicinchoninic acid assay (BCA assay). Samples (50 μg/lane) were mixed with loading buffer and separated by using 8% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked in 5% skim milk for 2 hours at room temperature and incubated overnight at 4°C with the primary antibodies as follows: anti-Piezo1 (1:1000 Proteintech, USA), anti-GADPH (1:1000 Cell Signaling Technology, USA), Erk1/2 (Cell Signaling Technology, USA), p-Erk1/2 (1:1000 Cell Signaling Technology, USA). Membranes were then washed and incubated in HRP-labeled second antibodies at 1:5000. Immunoreactive bands were detected with Gel Documentation and Analysis System (G-box, Syngene, UK) by Western Chemiluminescent HRP Substrate (Millipore Corporation, Billerica, USA). The intensity of protein bands were analyzed by ImageJ software.
Immunohistochemical analyses
For immunohistochemical analyses, sections obtained from formalin-fixed, paraffin-embedded specimens were incubated with primary antibody anti-Piezo1 (Proteintech, USA) and sequentially incubated with a secondary antibody and stained with 3,3-diaminobenzidine (DAB). The sections were digitized and analyzed under a microscope (Digital Nikon Camera DXM 1200, Japan) with Image-Pro Plus software version 6.0. The fields were chosen at random in a blind manner.
Statistical analyses
Data were expressed as mean ± SEM, and were analyzed using the SPSS statistical analysis package (venison 13.0). Statistical comparisons were made using one-way ANOVA, followed by a Bonferroni post hoc analysis. P<0.05 was considered statistically significant.
Result
Expression of Piezo1 is up-regulated during heart failing process
Whereas the expression of Piezo1 channels in normal heart has been determined in low level compared with bladder, colon, kidney, lung, and skin [6]. During pathophysiological process, weather Piezo1 expression is regulated in failure heart has not been clarified. To investigate the expression of Piezol during heart failing process, a heart failure model was established by the induction of LAD coronary artery ligation for MI in rats (Figure 1A) [24]. Left ventricular diameter and systolic function in sham and MI rats were assessed with echocardiography. LVIDd and LVIDs were gradually dilated after MI (Figure 1B), which were associated with marked systolic dysfunction as reflected by LVEF and FS decline (Figure 1C). Strikingly, compared with sham-operated control, an increase of the piezo1 transcription in right ventricle (RV), left ventricular infarction remote zone (RZ) and left ventricular infarction border zone (BZ) in failure heart was observed 4 weeks after MI, and lasted to 8 weeks after MI (Figure 2A). Subsequently, the Piezo1 globally up-regulating in failure heart was confirmed by western blot analysis in protein level (Figure 2B). Immunostaining results showed that Piezo1 was distributed widely but low in ventricular myocytes in sham-operated group (Figure 2C). After 4 and 8 weeks, significant increase of Piezo1 density was displayed in MI-induced heart failure compared with sham controls (Figure 2D). Base on the above significant results, Piezo1 channel was up-regulated during heart failing process after MI.
Figure 1.
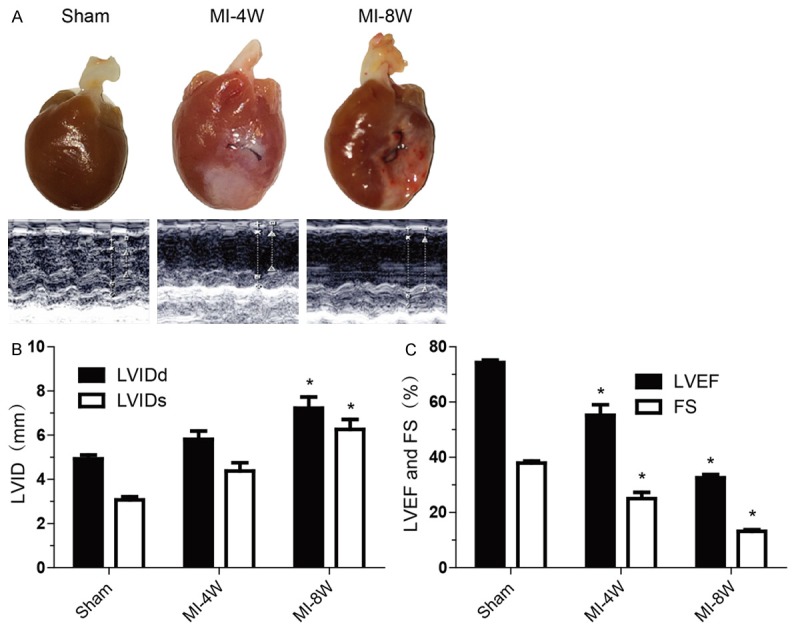
Heart failure model was established by MI. A: Representative photographs (top) and echocardiographys (bottom) of hearts from Sham group, post-MI 4 weeks (MI-4W) group and post-MI 8 weeks (MI-8W) group. B: Left ventricular inner diameters in diastole (LVIDd) and systole (LVIDs) measured by echocardiography in Sham group (n=7), MI-4W group (n=6) and MI-8W group (n=6). C: Left ventricular ejection fractions (LVEF) and fractional shortening (FS) measured by echocardiography in Sham group (n=7), MI-4W group (n=6) and MI-8W group (n=6). Data are presented as mean ± SEM. *P<0.05 vs Sham. Oneway ANOVA analysis with Bonferroni test was performed among all groups.
Figure 2.
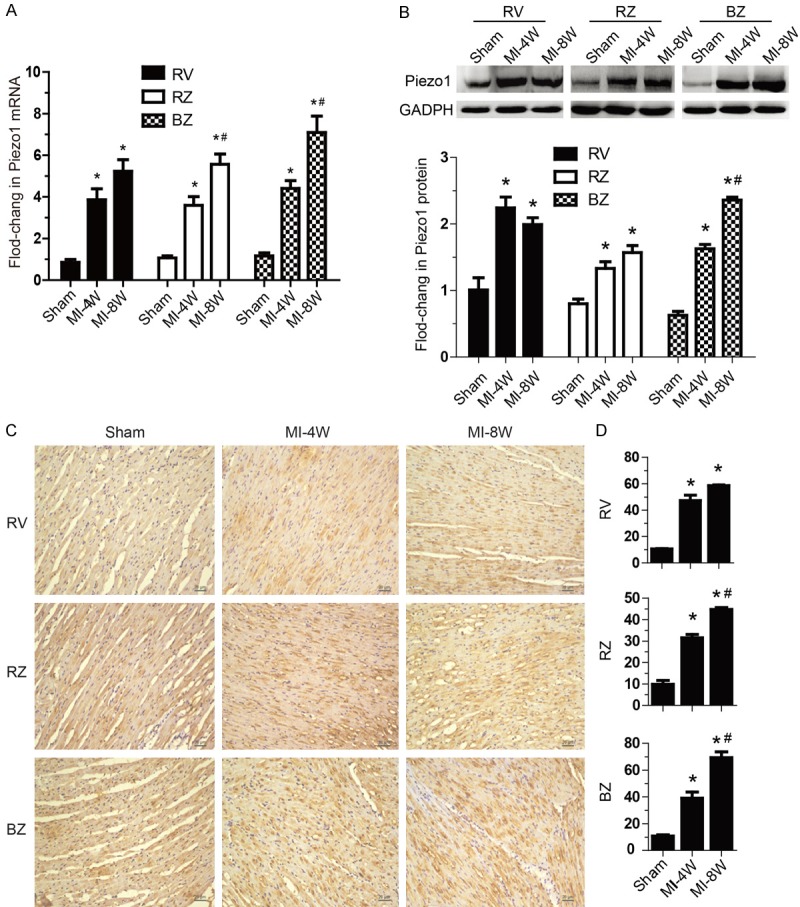
Expression of Piezo1 was up-regulated during heart failing process. A: mRNA levels of Piezo1 relative to GAPDH mRNA in right ventricle (RV), left ventricular infarction remote zone (RZ) and left ventricular infarction border zone (BZ) of hearts from Sham group, MI-4W group and MI-8W group (n=6 hearts per group). B: Protein levels of Piezo1 in RV, RZ and BZ of hearts from Sham group, MI-4W group and MI-8W group (n=6 hearts per group). C and D: Representative Piezo1 immunohistochemical staining sections and semiquantitative Piezo1 density of RV, RZ and BZ of hearts from Sham group, MI-4W group and MI-8W group (n=6 hearts per group). Data are presented as mean ± SEM. *P<0.05 vs Sham; #P<0.05 vs MI-4W. Oneway ANOVA analysis with Bonferroni test was performed among all groups.
AngII increased Piezo1 expression in NRVMs
To understand how Piezo1 expression was enhanced during heart failure process, a set of in vitro experiments were performed. To screen the stimulating factors that regulated piezo1 expression in heart, NRVMs were continuously treated with interleukin-6 (IL-6), hydrogen peroxide (H2O2), AngII or cyclic stretching. Of interest, AngII, the well-known neurohormonal factor that promotes myocardial remodeling dramatically increased Piezo1 in mRNA level (Figure 3A). As displayed in Figure 3B and 3C, western blotting affirmed that AngII augmented Piezo1 expression in protein level. It should be noted that the Piezo1 expression was peaked after 24 hours at the concentration of 10-6 mol/L exposed to AngII. These findings demonstrate that Piezo1 is up-regulated in NRVMs by AngII which has been proposed as a deteriorating factor during heart failure.
Figure 3.

AngII increased Piezo1 expression in NRVMs. A: Piezo1 mRNA levels in NRVMs treated with interleukin-6 (IL-6 10 μmol/L), hydrogen peroxide (H2O2 100 μmol/L), AngII (1 μmol/L) and 10% cyclic stretching, respectively (n=5). B: Piezo1 protein was analyzed by Western blot in cultured cells treated with AngII (1 μmol/L) at the indicated time points (n=4). C: Piezo1 protein was analyzed by Western blot in cultured cells treated with AngII at indicated concentrations for 24 hours (n=4). The relative expression level of Piezo1 was calculated as a foldchange normalized by GADPH. Data are presented as mean ± SEM. *P<0.05 vs control; #P<0.05 vs AngII 24 hours. Oneway ANOVA analysis with Bonferroni test was performed among all groups.
Piezo1 is elevated by AngII through AT1 receptors-Erk1/2 pathway
AngII type 1 receptor (AT1) and AT2 take the overwhelming majority of responsibility of biological effects exerted by AngII. As shown in Figure 4A, the AT1 blocker (losartan), rather than AT2 blocker (PD123319), effectively inhibited Piezo1 expression. These results indicated that AngII elevated Piezo1 principally through AT1 receptor. AT1 receptor activated MAPKs pathway which has been demonstrated to regulate a diverse range of genes. To test the possibility of AngII-elicited MAPK signaling also controlling Piezo1 expression, cells were pretreated with Erk1/2 inhibitor (U0126), p38 MAPK inhibitor (SB203580), or JNK inhibitor (SP600125) for 1 hour and followed by AngII exposure for 24 hours. It turned out that only U0126, but not SB203580 or SP600125, abrogated the up-regulation of Piezo1 in protein level induced by AngII (Figure 4B). Taking together, the data hinted that Piezo1 expression was elevated by AngII as a consequence of AT1 receptor-mediated Erk1/2 pathway. Moreover, Erk1/2 kinase activities were increased in NR-VMs after exposure to AngII for 30 min, and inhibited by losartan pre-treatment, but not by PD123319 (Figure 4C). These findings demonstrated that the expression of Piezo1 is controlled by AngII-AT1 receptor-Erk1/2 signal pathway in NRVMs.
Figure 4.
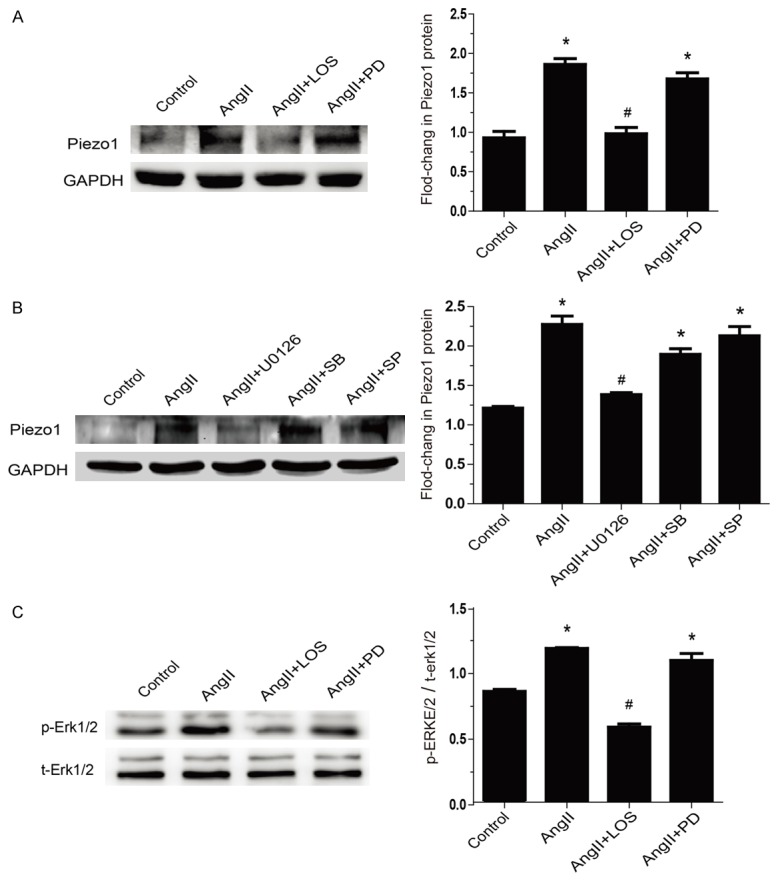
Piezo1 was elevated by AngII through AT1 receptors-Erk1/2 pathway. A: Piezo1 protein was detected by Western blot in NRVMs with 1 hour pretreatment of losartan (AT1 blocker, 10 μmol/L) or PD123319 (AT2 blocker, 10 μmol/L) before AngII exposure (1 μmol/L) for 24 hours (n=4). B: Piezo1 protein levels were measured in NRVMs pretreated for 1 hour with U0126 (Erk1/2 inhibitor, 10 μmol/L), SB203580 (p38 MAPK inhibitor, 10 μmol/L) and SP600125 (JNK inhibitor, 10 μmol/L), followed by AngII exposure (1 μmol/L) for 24 hours (n=4). c: Evaluation of the phosphorylation of Erk1/2 during AngII infusion pretreat with losartan (10 μmol/L) or PD123319 (10 μmol/L) for 30 min (n=4). Data are presented as mean ± SEM. *P<0.05 vs control; #P<0.05 vs AngII 24 hours. Oneway ANOVA analysis with Bonferroni test was performed among all groups.
Piezo1 up-regulation was offset by ARB treatment in vivo
After the in vitro observation that Piezo1 was increased by AngII activating AT1 receptor, we attempted to evaluate the inhibition effects of ARB therapy in vivo. 24 hours after LAD coronary artery ligation surgery, rats were treated with losartan or placebo for 8 weeks. Heart rate was mildly slowed down after losartan application (Figure 5A), however, with a significant lower blood pressure in losartan group (Figure 5B). Echocardiography tests showed that heart systolic function was improved after losartan treatment, particularly, reflected by LVEF reserve >50% (Figure 5C).
Figure 5.
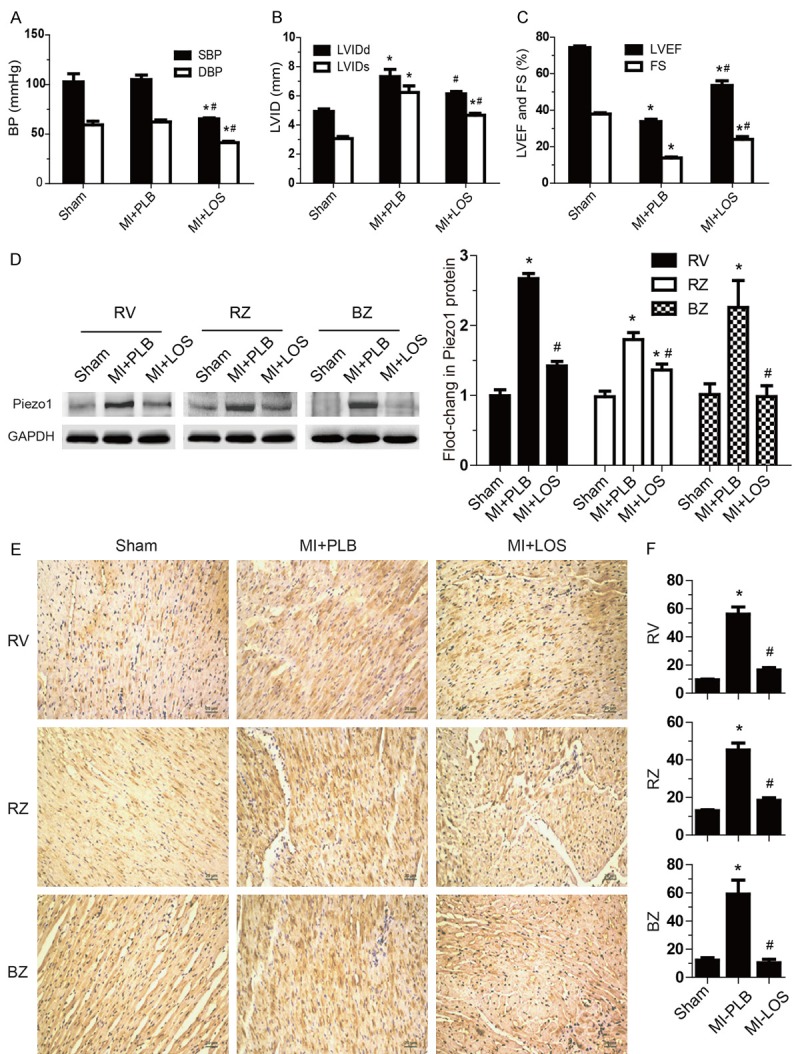
Piezo1 up-regulation is offsetted by ARB treatment in vivo. A-C: Blood pressure, LVID, LVEF, and FS were monitored in post-MI group administered daily with placebo (MI+PLB n=7) or losartan (MI+LOS n=6) for 8 weeks. Age matched- rats with Sham surgical operation was used as a control group (n=7). D: Piezo1 protein was analyzed by Western blot in RV, RZ and BZ of hearts from Sham group, MI+PLB group and MI+LOS group (n=6 hearts per group). E and F: Representative figure of Piezo1 immunohistochemical staining sections and semiquantitative Piezo1 density in RV, RZ and BZ of hearts from Sham group, MI+PLB group and MI+LOS group (n=6 hearts per group). Data are presented as mean ± SEM. *P<0.05 vs Sham; #P<0.05 vs MI+PLB. Oneway ANOVA analysis with Bonferroni test was performed among all groups.
Consistent with in vitro experiments, losartan treatment availably prevented Piezo1 protein global up-regulating in failure heart, manifested as that the levels of Piezo1 in RV, RZ and BZ of heart were all lower than those in placebo controls (Figure 5D). As expected, immunohistochemistry studies confirmed that losartan unfailingly offset increasing Piezo1 density in vivo (Figure 5E and 5F). Animal model experiments revealed that losartan could abolish elevating Piezo1 in failure heart, indicating that angiotensin system activation are responsible for Piezo1 up-regulation during heart failure.
Discussion
The present study provides evidence that the expression of Piezo1 is up-regulated in heart failure, which involved in AT1 receptor-Erk1/2 signal pathways. Firstly, we confirmed that Piezo1 is gradually and globally increased during heart failing process induced by MI. Secondly, we showed that the expression of Piezo1 is regulated by AngII-AT1 receptor-Erk1/2 signal pathway in NRVMs. Finally, with LVEF improvement, ARB chronic treatment in vivo prevents Piezo1 up-regulation in post-MI failure heart. In a conclusion, we had established the connection between mechanotransduction, involving SACs Piezo1 in cardiomyocytes, and a clinically relevant heart failure. To move forward a single step, we speculated that Piezo1 may take crucial responsibility in ventricular remodeling as the progress of heart failure.
Mechanotransduction is the conversion of extracellular mechanical stimuli into intracellular biochemical or electrical signals [25]. In the past decades, it has been identified that SACs are directly activated by cell membrane deformation involved in cardiac mechanotransduction [4,26]. And recently, Piezo1 has been emerged to be the valid SACs candidate in mammals [27]. The dying of Piezo1-knockout mice at midgestation with defects in vascular remodeling probably results from dysfunction of endothelial cell alignment conferred by Piezo1 mediating calcium influx under blood flow shear stress [21,22]. Remarkably, Piezo1 is present at the adult stage in the smooth muscle cells that participates actively in the regulation of arterial diameter and wall thickness, which might possibly be mediated by intracellular calcium [23].
Arrhythmia is one of the primary causes of death in human heart failure on account of great alterations of electrical characteristics [28]. Compared with healthy controls, a large number of ion channels gene expression are changed in failure human heart, either repressed or elevated, called ion channel remodeling [29]. In LAD ligation LV MI model, restricted necrotic myocardium loss of contractibility result in heterogeneity of stretch among different ventricular walls. We found SACs Piezo1 was gradually and globally increased during heart failing process induced by LV MI. We hypothesized that the up-regulation of Piezo1 is the manifestation of ion channel remodeling upon a complicated microenvironment, including neurohormonal factors and mechanical forces, during heart failure. However, AngII stimulates Piezo1 expression in NRVMs, while stretch is dispensable. AngII has been demonstrated to control cardiac ion channels genes profile through MAPKs pathway activation, such as K4.3, Kir6.1, and Cav3.1 [30-35]. We showed that AngII-AT1 receptor-Erk1/2 signal pathway activation augments Piezo1 expression in NRVMs, and there is a link between Piezo1-mediated mechanotransduction and AT1 receptor-dependent biochemical signal transduction.
Early in vitro experiments illustrated that mechanical stretch caused AngII release from cardiac myocytes, and acted an role in stretch-induced hypertrophy by the means of autocrine or paracrine, which established local renin-angiotensin system as a cornerstone in cardiac mechanotransduction [36,37]. The constriction of the transverse aorta-induced significant LV hypertrophy by pressure overload in ATG-deficient (ATG-/-) mice, in which AngII was not synthesized, and the attenuation by AT1 receptor antagonist candesartan, indicated that mechanical stress can induce cardiac hypertrophy in vivo through the AT1 receptor even in the absence of AngII [38]. Molecular mechanism researches revealed that the AT1 receptor was directly activated by mechanical stretch, which underwent conformational switch, and also suppressed by candesartan [39]. These results suggested that AT1 receptor mediates biological effect through both cardinal ligand-dependent and novel mechanical stress-dependent mechanisms. Our study demonstrated that AngII bounding to AT1 receptor up-regulated SACs Piezo1 in the pathophysiological procedures of heart failure. AT1 receptor probably plays a role in the upstream of mechanotransduction that modulates the expression of Pizeo1 and the subsequent correlative process of heart failure. More importantly, it helps fill up the feedback loop from the renin-angiotensin system to the Piezo1-mediated mechanotransduction.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81341010); Science and Technology Foundation of Guangdong Province (CN) (2013B021800109); Natural Science Foundation of Guangdong Province (CN) (2016A030313338).
Disclosure of conflict of interest
None.
Authors’ contribution
JLL performed most cell and animal experiments, collection of data, and manuscript writing. BSH performed molecular biology experiments, collection of data, and manuscript writing. YC, FSL, and HYZ participated in cell preparation and cultivation, immunohistochemistry, and helped to draft the manuscript. GYY and SXZ carried out echocardiographic tests. GYY, SXZ and CL carried out data analysis and interpretation, and revised the manuscript critically for important content. DFG and SXZ conceived, designed, and financial supported the study. All authors read and approved the manuscript.
Abbreviations
- SACs
stretch-activated channels
- LAD
left anterior descending artery
- AngII
angiotensin II
- LVEF
left ventricular ejection fraction
- FS
shortening fraction
- ARB
angiotensin receptor blocker
- MI
myocardial infarction
- LVIDd
left ventricular inner diameters in diastole
- LVIDs
left ventricular inner diameters in systole
- NRVMs
neonatal rat ventricular myocytes
- RV
right ventricle
- RZ
left ventricular infarction remote zone
- BZ
left ventricular infarction border zone
References
- 1.Lyon RC, Zanella F, Omens JH, Sheikh F. Mechanotransduction in cardiac hypertrophy and failure. Circ Res. 2015;116:1462–1476. doi: 10.1161/CIRCRESAHA.116.304937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz AM. Ernest Henry Starling, his predecessors, and the “law of the heart”. Circulation. 2002;106:2986–2992. doi: 10.1161/01.cir.0000040594.96123.55. [DOI] [PubMed] [Google Scholar]
- 3.Manyari DE. Prognostic implications of echocardiographically determined left ventricular mass in the framingham heart study. N Engl J Med. 1990;323:1706–1707. doi: 10.1056/NEJM199012133232413. [DOI] [PubMed] [Google Scholar]
- 4.Reed A, Kohl P, Peyronnet R. Molecular candidates for cardiac stretch-activated ion channels. Glob Cardiol Sci Pract. 2014;2014:9–25. doi: 10.5339/gcsp.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyronnet R, Nerbonne JM, Kohl P. Cardiac mechano-gated Ion channels and arrhythmias. Circ Res. 2016;118:311–329. doi: 10.1161/CIRCRESAHA.115.305043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottlieb PA, Sachs F. Piezo1: properties of a cation selective mechanical channel. Channels (Austin) 2012;6:214–219. doi: 10.4161/chan.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilius B, Honore E. Sensing pressure with ion channels. Trends Neurosci. 2012;35:477–486. doi: 10.1016/j.tins.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Xiao R, Xu XZ. Mechanosensitive channels: in touch with Piezo. Curr Biol. 2010;20:R936–R938. doi: 10.1016/j.cub.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng CA, Sachs F, Gottlieb PA, Martinac B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun. 2016;7:10366. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife. 2015:4. doi: 10.7554/eLife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honore E, Martins JR, Penton D, Patel A, Demolombe S. The Piezo mechanosensitive ion channels: may the force be with you! Rev Physiol Biochem Pharmacol. 2015;169:25–41. doi: 10.1007/112_2015_26. [DOI] [PubMed] [Google Scholar]
- 14.Bae C, Sachs F, Gottlieb PA. Protonation of the human PIEZO1 ion channel stabilizes inactivation. J Biol Chem. 2015;290:5167–5173. doi: 10.1074/jbc.M114.604033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, Petrassi HM, Schumacher AM, Montal M, Bandell M, Patapoutian A. Chemical activation of the mechanotransduction channel Piezo1. Elife. 2015:4. doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–549. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHugh BJ, Murdoch A, Haslett C, Sethi T. Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS One. 2012;7:e40346. doi: 10.1371/journal.pone.0040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, Rinehart J, Gallagher PG. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120:1908–1915. doi: 10.1182/blood-2012-04-422253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, Cahalan S, Garcon L, Toutain F, Simon RP, Delaunay J, Picard V, Jeunemaitre X, Patapoutian A. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. doi: 10.1038/ncomms2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc Natl Acad Sci U S A. 2013;110:E1162–E1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YS, Chien S, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. 2014;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad KR, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A, Honore E. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015;13:1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 24.Ren Z, Raucci FJ, Browe DM, Baumgarten CM. Regulation of swelling-activated Cl(-) current by angiotensin II signalling and NADPH oxidase in rabbit ventricle. Cardiovasc Res. 2008;77:73–80. doi: 10.1093/cvr/cvm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCain ML, Parker KK. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch. 2011;462:89–104. doi: 10.1007/s00424-011-0951-4. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Sachs F. Stretch-activated ion channels in the heart. J Mol Cell Cardiol. 1997;29:1511–1523. doi: 10.1006/jmcc.1997.0392. [DOI] [PubMed] [Google Scholar]
- 27.Volkers L, Mechioukhi Y, Coste B. Piezo channels: from structure to function. Pflugers Arch. 2015;467:95–99. doi: 10.1007/s00424-014-1578-z. [DOI] [PubMed] [Google Scholar]
- 28.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DJ, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Borlak J, Thum T. Hallmarks of ion channel gene expression in end-stage heart failure. FASEB J. 2003;17:1592–1608. doi: 10.1096/fj.02-0889com. [DOI] [PubMed] [Google Scholar]
- 30.Zhang TT, Takimoto K, Stewart AF, Zhu C, Levitan ES. Independent regulation of cardiac Kv4.3 potassium channel expression by angiotensin II and phenylephrine. Circ Res. 2001;88:476–482. doi: 10.1161/01.res.88.5.476. [DOI] [PubMed] [Google Scholar]
- 31.Zhou C, Ziegler C, Birder LA, Stewart AF, Levitan ES. Angiotensin II and stretch activate NADPH oxidase to destabilize cardiac Kv4.3 channel mRNA. Circ Res. 2006;98:1040–1047. doi: 10.1161/01.RES.0000218989.52072.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isidoro TN, Philip-Couderc P, Baertschi AJ, Lerch R, Montessuit C. Angiotensin II and tumour necrosis factor alpha as mediators of ATP-dependent potassium channel remodelling in post-infarction heart failure. Cardiovasc Res. 2009;83:726–736. doi: 10.1093/cvr/cvp162. [DOI] [PubMed] [Google Scholar]
- 33.Tozakidou M, Goltz D, Hagenstrom T, Budack MK, Vitzthum H, Szlachta K, Bahring R, Ehmke H. Molecular and functional remodeling of I(to) by angiotensin II in the mouse left ventricle. J Mol Cell Cardiol. 2010;48:140–151. doi: 10.1016/j.yjmcc.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 34.Morishima M, Wang Y, Akiyoshi Y, Miyamoto S, Ono K. Telmisartan, an angiotensin II type 1 receptor antagonist, attenuates T-type Ca2+ channel expression in neonatal rat cardiomyocytes. Eur J Pharmacol. 2009;609:105–112. doi: 10.1016/j.ejphar.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Ferron L, Capuano V, Ruchon Y, Deroubaix E, Coulombe A, Renaud JF. Angiotensin II signaling pathways mediate expression of cardiac T-type calcium channels. Circ Res. 2003;93:1241–1248. doi: 10.1161/01.RES.0000106134.69300.B7. [DOI] [PubMed] [Google Scholar]
- 36.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 37.Leri A, Claudio PP, Li Q, Wang X, Reiss K, Wang S, Malhotra A, Kajstura J, Anversa P. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest. 1998;101:1326–1342. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 39.Yasuda N, Miura S, Akazawa H, Tanaka T, Qin Y, Kiya Y, Imaizumi S, Fujino M, Ito K, Zou Y, Fukuhara S, Kunimoto S, Fukuzaki K, Sato T, Ge J, Mochizuki N, Nakaya H, Saku K, Komuro I. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep. 2008;9:179–186. doi: 10.1038/sj.embor.7401157. [DOI] [PMC free article] [PubMed] [Google Scholar]


