Abstract
It has previously been reported that microRNA (miR)-155 is linked to the recurrence and prognosis of hepatocellular carcinoma (HCC) following liver transplantation. However, the role of miR-155 in the invasion and metastasis of HCC cells remains largely unclear. The aim of this study was to investigate the expression of miR-155 in HCC cells and its role in the invasion and migration of HCC cells in vitro. We found that the level of expression of miR-155 in HCC tissues and cells was significantly increased compared with non-tumorous adjacent tissues. Further study revealed that recombinant human transforming growth factor-β (TGF-β1) up-regulated the expression of miR-155 in HCC cells in vitro. Further, the overexpression of miR-155 in HCC cell line Huh-7 led to increased levels of cell invasion and migration compared with untreated control Huh-7 cells. MiR-155-overexpressed Huh-7 cells also exhibited altered levels of expression of certain cellular adhesion molecules related to epithelial-mesenchymal transition (EMT), including low levels of CDH1 and higher levels of FN1, SNAI1 and ZEB1, compared with control Huh-7 cells. Moreover, it was found that the overexpression of miR-155 and of TGF-β1 protein decreased the expression of E-Cadherin and increased the expression of Vimentin in Huh-7 cells. These results indicate that an increased level of miR-155 in HCC cells, possibly due to stimulation by TGF-β1, accelerates the process of EMT, promotes cellular invasion and migration in vitro, and thereby further promotes the progression of HCC.
Keywords: miR-155, hepatocellular carcinoma, EMT, TGF-β1, invasion, migration
Introduction
Hepatocellular carcinoma (HCC) currently accounts for more than 5% of all cancer cases and is the fifth leading cause of cancer related mortality worldwide [1,2]. Based on a report from the National Office for Cancer Prevention and Control in China, HCC is the fourth most common cancer in China and the third most common cause of cancer related mortality [3]. In 2015, 466,000 new HCC cases were diagnosed in China, with 422,000 recorded deaths from the disease (3). Due to the lack of symptoms during early stages of HCC and the rapid progression of the disease, about 80% of HCC patients are diagnosed at an advanced stage [4]. Liver resection and orthotopic liver transplantation are often applied as the only curative treatments for HCC, and the prognosis is poor in cases where the tumor cannot be surgically removed [5]. Despite major efforts in past decades, the precise molecular mechanisms of HCC still remain uncertain, and the available therapeutic options to retard disease progression remain limited.
During disease progression, carcinoma cells from the solid tumor can escape as a result of dedifferentiation of epithelial cells which occurs by loss of cell-to-cell contact, and these cells subsequently gain migratory and invasive abilities [6]. This phenotypical conversion of cells, generally designated as epithelial-mesenchymal transition (EMT), has been described in several different types of carcinoma cells including HCC [7-9]. There is increasing evidence to suggest that hepatocellular EMT plays a pivotal role in the metastatic progression of HCC.
MicroRNAs (miRNAs) are non-coding, single stranded RNAs of 22 nucleotides, processed from endogenous precursor RNAs with stem-loop structures that play important roles in cancer pathogenesis acting as either oncogenes or tumor suppressors [10]. MiR-155 acts as an oncogene and is up-regulated in several human cancers, including HCC [11]. It has been reported that miR-155 promotes EMT and cancer stem cell phenotypes [12,13]. However, it is still unclear whether and how miR-155 regulates EMT, invasion and metastasis of HCC cells.
Against this background, this present study was undertaken to investigate the cellular expression and effect of miR-155 on EMT-related molecules, as well as on the invasion and migration of HCC cells in vitro. It was anticipated that the results would inform and extend current understanding regarding the molecular mechanisms of HCC.
Materials and methods
Tissue collection
Fresh HCC tumor tissue samples were obtained from 32 cases (male patients: 28 cases; female patients: 4 cases) as well as paired fresh tissue samples from adjacent non-tumorous tissues, at the time of surgery. All tissues were confirmed by histopathological evaluation. Tumor dimensions were measured by a pathologist upon sampling, and the maximal tumor diameter was recorded. Informed consent was obtained from all patients prior to the outset of the study, and the study protocol was approved by the institutional ethics committee of the First Affiliated Hospital of Soochow University, Jiangsu, China.
Cell culture
Human HCC cell lines Huh-7 and SMMC-7721, along with Human liver HL-7702 cells, were purchased from the cell bank of the Chinese Academy of Sciences, Shanghai, China. Human HCC cell line HepG2 was donated by the Life Sciences Department of Soochow University. Cells were cultured in the recommended medium supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was performed to determine the expression levels of miR-155 and EMT related genes (CDH1, FN1, SNAI1 and ZEB1). Total RNA was isolated and extracted from tissues or cells, and reverse transcribed into cDNA using a miScript II RT Kit (Invitrogen, USA) according to the manufacturer’s instructions. The qRT-PCR reactions were performed using an ABI7500 real-time RCR System (Applied Biosystems, Foster City, CA, USA) and miScript SYBR Green PCR Kit (Qiagen, USA). The relative expression levels of miR-155, CDH1, FN1, SNAI1 and ZEB1 were analyzed using the 2-ΔΔCT relative quantification method with human U6 as an internal control in the case of miR-155, and β-actin as an internal control for the EMT related genes. The sequences of the primers were described in Table 1. All assays were performed in triplicate.
Table 1.
Sequences of the primers
| Gene | Primer sequence |
|---|---|
| MiR-155 | Forward, 5’-UUAAUGCUAAUCGUGAUAGGGGU-3’; |
| Reverse, 5’-CCCUAUCACGAUUAGCAUUAAUU-3’ | |
| U6 | Forward, 5’-CTCGCTTCGGCAGCACA-3’; |
| Reverse, 5’-AACGCTTCACGAATTTGCGT-3’ | |
| CDH1 | Forward, 5’-TGGGCCAGGAAATCACATCC-3’; |
| Reverse, 5’-CCCCGTGTGTTAGTTCTGCT-3’ | |
| FN1 | Forward, 5’-CCCAATTGAGTGCTTCATGCC-3’; |
| Reverse, 5’-AACTCCCAGGGTGATGCTTG-3’ | |
| SNAI1 | Forward, 5’-ACCCCAATCGGAAGCCTAAC-3’; |
| Reverse, 5’-TGGCTTCGGATGTGCATCTT-3’ | |
| ZEB1 | Forward, 5’-CTGCTGGGAGGATGACACAG-3’; |
| Reverse, 5’-ATGACCACTGGCTTCTGGTG-3’ | |
| β-actin | Forward, 5’-CTGGGACGACATGGAGAAAA-3’; |
| Reverse, 5’-AAGGAAGGCTGGAAGAGTGC-3’ |
Enzyme-linked immunosorbent assay (ELISA)
HL-7702, Huh-7, SMMC-7721 and HepG2 cells (1×105 cells/well) were seeded in 24-well plates for 48 h. The cell culture supernatants were then harvested, centrifuged to remove cellular debris, and then stored at -80°C until their analysis by ELISA. The TGF-β1 secretion levels of the supernatants were detected by human TGF-β1 ELISA kits (Shanghai ExCell Biology, Inc, Shanghai, China) according to the manufacturer’s instructions. All experiments were repeated three times.
Treatment with TGF-β1
Huh-7 cells (2×105 cells/well) were incubated with or without recombinant human TGF-β1 protein (100 ng/ml, R&D Systems, USA) for 48 h. The cells were then collected for qRT-PCR analysis or flow cytometry (FCM) assay to analyze expression of miR-155 and cell adhesion molecules as indicators of EMT (E-Cadherin and Vimentin).
Overexpression of miR-155 in Huh-7 cells
The miR-155 mimic lentivirus (miR-155) and its corresponding control miRNA lentivirus (C-miRNA: Negative ctrl) were constructed by GenePharma (Shanghai, China). Overexpres-sion of miR-155 in cells, and the corresponding control stable cell lines were then established. The efficiency of overexpression of miR-155 in cells was verified by qRT-PCR.
Wound healing assays
After Huh-7 (Negative ctrl or miR-155 group) cells had been grown to approximately 100% confluence in a 6-well cell culture plate, wound healing assays were performed using a 200-μl sterile pipette tip to make a scratch in the confluent monolayer of cells. The previous culture medium was then replaced with serum-free medium. The cells were maintained at 37°C in 5% CO2. Cells were counted under a microscope in five marked fields of view at 0 and 24 h, to assess the rate of gap closure.
Matrigel invasion assay
The invasive potential of Huh-7 (Negative ctrl or miR-155 group) cells across matrigel was evaluated objectively in an invasion chamber. Briefly, the cell inserts (8-um pore size, 6.5-mm diameter, Corning, USA) coated with 20 uL of matrigel (BD, USA) were placed in a 24-well plate. A total of 2 ×104 cells/well was seeded in the upper chamber (media containing 1% charcoal stripped fetal calf serum (FCS)). The lower chamber was filled with 800 uL of culture medium containing 5% charcoal stripped FCS. The cells were then incubated at 37°C for 48 h. The inserts were then removed, washed in PBS, and the non-invading cells together with the matrigel were removed from the upper surface of the filter by wiping with a cotton bud. The inserts were then fixed in methanol for 10 min at room temperature and stained with hematoxylin. The cells were observed under an Olympus BX51+DP70 microscope (Olympus, Tokyo, Japan). The cells that had migrating to the lower surface of the insert were counted in five predetermined fields, at a magnification of ×200. The invasion index for each group was calculated as the ratio of the numbers of cells that had migrated to the lower surface of the insert, to the number in the vehicle control. Each experiment was carried out in triplicate, and repeated three times.
Western blotting
Cells were harvested and lysed at room temperature in RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific, Waltham, MA). Samples containing equal amounts of protein were separated by 12.5-15% SDS-PAGE electrophoresis, followed by electrotransferrenced onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, USA). The nitrocellulose blots were then incubated with antibodies under the manufacturer’s recommended conditions. The primary antibodies used were anti-CDH1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-SNAI1 (Santa Cruz Biotechnology), anti-ZEB1 (Santa Cruz Biotechnology), and anti-β-actin (Cell Signal Technology, USA). Horseradish peroxidase-conjugated or anti-rabbit antibody (Santa Cruz Biotechnology) was used as the secondary antibody. Signals were detected using an enhanced chemiluminescence system (Millipore, Billerica, MA).
FCM
The Huh-7 (Negative ctrl or miR-155 group) cells were cultured, or Huh-7 cells were treated with or without TGF-β1 protein (100 ng/ml, R&D Systems) for 48 h. The cells were then collected, washed with phosphate buffer saline (PBS), and then labeled with mouse anti-human E-Cadherin and Vimentin antibodies (BD, USA). Isotypic control antibodies were used. After incubation in darkness for 30 min at room temperature, cells were analyzed immediately by a flow cytometer (FACS Calibur, BD, USA).
Statistics
All values are presented as means ± SEM. The data were analyzed with GraphPad Prism version 5 using t-tests or one-way ANOVAs to detect differences between groups. Differences were considered to be statistically significant when P<0.05.
Results
Expression of miR-155 in HCC tissues and cell lines was significantly increased
We first analyzed the expression levels of miR-155 in HCC tissues and cell lines. As shown in Figure 1, expression of miR-155 was significantly increased in HCC tissues compared with adjacent normal (non-tumorous) tissues (P<0.01) (Figure 1A). In addition, expression of miR-155 was significantly higher in the three HCC cell lines (Huh-7, SMMC-7721 and HepG2) compared within human liver HL-7702 cells (P<0.05 or P<0.001) (Figure 1B), especially in the case of SMMC-7721 and HepG2 cells (P<0.001) (Figure 1B). These data indicate that the level of expression of miR-155 is associated with the biological regulatory behavior of HCC cells. According to the relatively low level of expression of miR-155 in Huh-7 cells, these cells were selected for further study in vitro.
Figure 1.
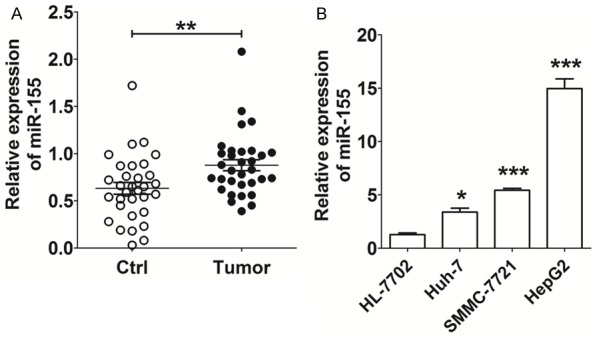
Expression of miR-155is elevated in HCC tissues and cell lines. A: qRT-PCR analysis of miR-155 expression in HCC tissues (n=32) and paired adjacent normal tissues. Ctrl: adjacent normal tissues; Tumor: HCC tissues. B: qRT-PCR analysis of miR-155 expression in HCC cell lines (Huh-7, SMMC-7721 and HepG2) and human liver cell line HL-7702. The data are expressed as means ± SEM. *P<0.05, **P<0.01 and ***P<0.001.
TGF-β1 up-regulates the level of miR-155 in Huh-7 cells
Recent research has showed that TGF-β1 can regulate miR-155 expression in cancer stem cells [12]. To explore the possible regulatory relationship between TGF-β1 and miR-155 in HCC, we analyzed the expression level of TGF-β1 in HCC cells by ELISA. As shown, the secretion level of TGF-β1 from cells of all three HCC cell lines (Huh-7, SMMC-7721 and HepG2) was higher than that from human liver HL-7702 cells (P<0.05, P<0.01 or P<0.001) (Figure 2A). Further analysis revealed that there was a significant positive correlation between the miR-155 expression level and TGF-β1 secretion (R2=0.485, P<0.001) (Figure 2B). In addition, TGF-β1 protein markedly increased the expression of miR-155 in Huh-7 cells (P<0.01) (Figure 2C). These data suggest that the observed up-regulation of miR-155 in HCC cells may be mediated by high expression of TGF-β1.
Figure 2.
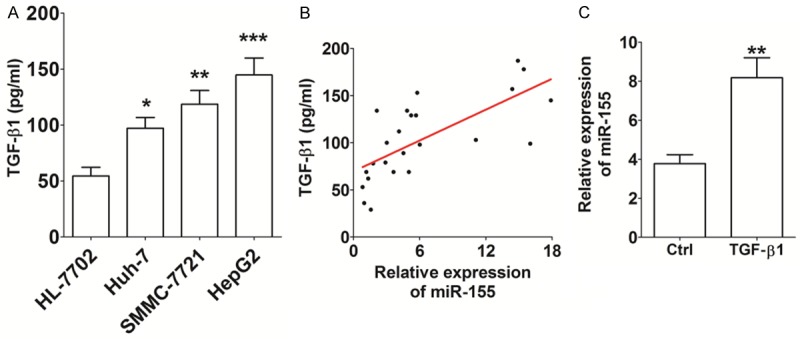
TGF-β1 up-regulates the level of miR-155 expression in Huh-7 cells. A: The secretion level of TGF-β1 from HCC cell lines (Huh-7, SMMC-7721 and HepG2) and human liver cell line HL-7702, as determined by ELISA. B: The correlation between the miR-155 expression and TGF-β1 secretion in Huh-7 cells (R2=0.484). C: After stimulation with recombinant human TGF-β1 protein (100 ng/ml) for 48 h, qRT-PCR was used to analyze miR-155 expression in Huh-7 cells. TGF-β1: recombinant human TGF-β1 protein. The data are expressed as means ± SEM. *P<0.05, **P<0.01 and ***P<0.001.
MiR-155 promotes the invasion and migration of Huh-7 cells in vitro
To explore whether miR-155 is involved in regulating the invasion and metastasis of HCC cells, we constructed miR-155-overexpressed Huh-7 cells by transfection with miR-155 mimic. Expression of miR-155 was found to be significantly higher in the transfected Huh-7 cells compared with the negative control cells, confirming that overexpression had been achieved (P<0.001) (Figure 3A). Further analysis revealed that this overexpression of miR-155 significantly enhanced the invasiveness of Huh-7 cells when compared with the control group (P<0.001) (Figure 3B). Moreover, the overexpression of miR-155 led to a high level of migration ability of Huh-7 cells in vitro (P<0.001) (Figure 3C). These results suggest that the elevated level of miR-155 expression gives rise to an increase in the invasion and migratory ability of HCC cells.
Figure 3.
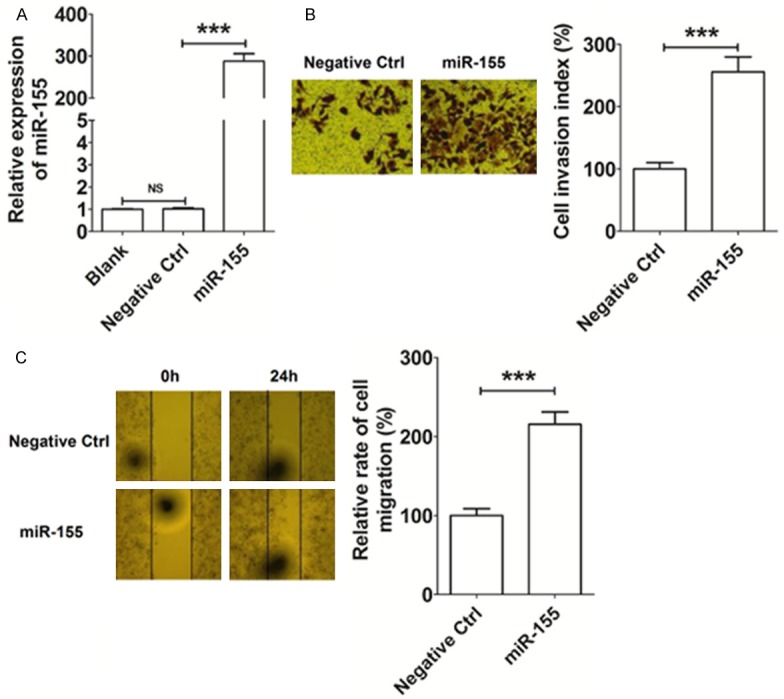
MiR-155 promotes the invasion and migration of Huh-7 cells in vitro. A: The construction of miR-155 overexpression in Huh-7 cells by transfection. B: Matrigel invasion assay analysis of Huh-7 cells after transfection. C: Wound healing assay results for Huh-7 cells after transfection. Blank: Huh-7 cells transfected without miRNA lentivirus; Negative Ctrl: Huh-7 cells transfected with the control miRNA lentivirus; miR-155: Huh-7 cells transfected with the miR-155 mimic lentivirus. The data are expressed as means ± SEM. ***P<0.001. NS: no statistically difference.
TGF-β1/miR-155 signaling promotes EMT of HCC cells
To identify the possible mechanisms by which miR-155 regulates the invasion and migratory abilities of HCC cells, we analyzed the expression of EMT related molecules in Huh-7 cells. As shown in Figure 4, our results demonstrated that the overexpression of miR-155 down-regulated the transcription level of CDH1 (P<0.01) (Figure 4A), and up-regulated the transcription levels of FN1 (P<0.01) (Figure 4B), SNAI1 (P<0.001) (Figure 4C) and ZEB1 (P<0.001) (Figure 4D) in Huh-7 cells, compared with the negative control group. The results of the Western Blotting analysis were consistent with this, showing that miR-155-overexpressed Huh-7 cells had a visibly lower level of expression of CDH1, and a higher expression of SNAI1, than the control group cells (Figure 4E).
Figure 4.
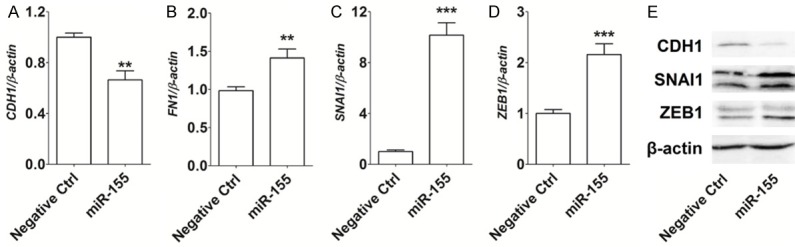
MiR-155 promotes EMT of HCC cells in vitro. A-D: qRT-PCR analysis of the expression of EMT related genes CDH1, FN1, SNAI1 and ZEB1 in Huh-7 cells; E: Western Blotting analysis of CDH1, SNAI1 and ZEB1 protein levels in Huh-7 cells. Negative Ctrl: Huh-7 cells transfected with the control miRNA lentivirus; miR-155: Huh-7 cells transfected with the miR-155 mimic lentivirus. The data are expressed as means ± SEM. **P<0.01 and ***P<0.001.
Finally, FCM was performed to evaluate the effect of TGF-β1/miR-155 expression on the expression of the EMT related cell adhesion molecules E-Cadherin and Vimentin, in Huh-7 cells. We found that expression of TGF-β1 protein (as a positive control) in the TGF-β1 treated Huh-7 cells, led to a marked decrease in the expression of E-Cadherin (P<0.001) (Figure 5A-D), and a significant increase in the expression of Vimentin (P<0.001) (Figure 5A-D), compared with untreated negative control group cells. Similarly, the overexpression of miR-155 also resulted in a marked decrease in expression of E-Cadherin and an increase in the expression of Vimentin expression in Huh-7 cells (P<0.05 or P<0.01) (Figure 5A-D). These findings suggest that TGF-β1/miR-155 signaling promotes EMT of HCC cells, and further triggers the invasion and migration of HCC cells in vitro.
Figure 5.
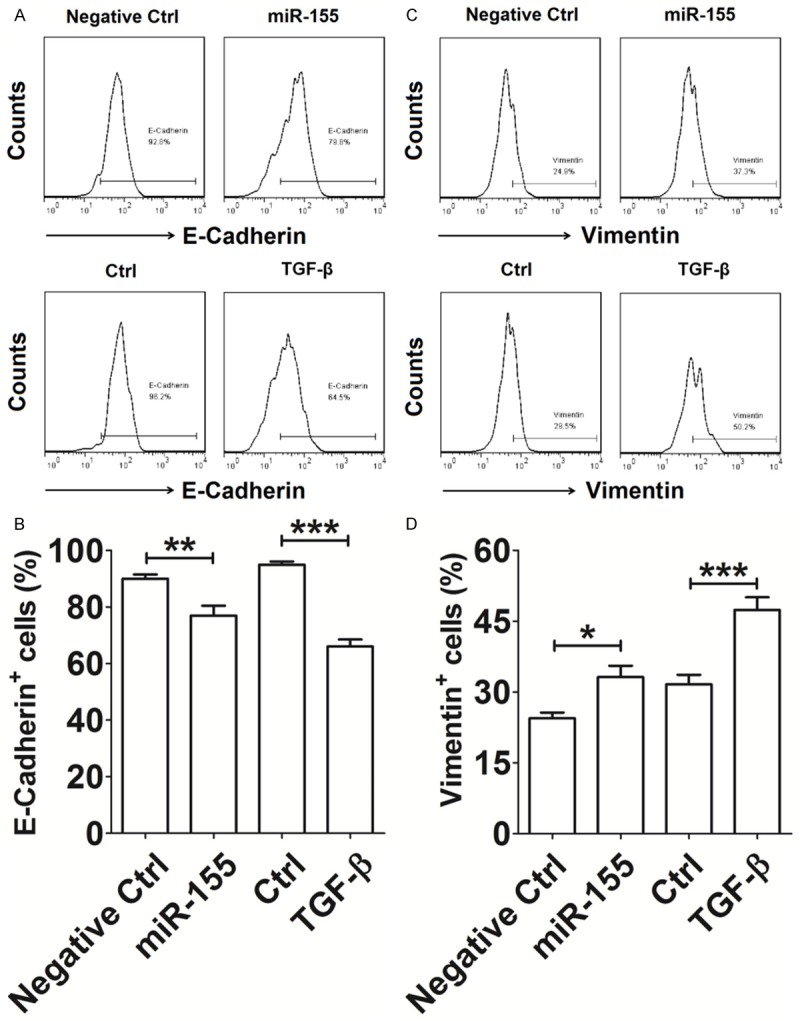
TGF-β1/miR-155 signaling promotes EMT of HCC cells in vitro. A-D: The Huh-7 cells (Negative ctrl or miR-155 group) were cultured, or Huh-7 cells were treated with or without TGF-β1 protein (100 ng/ml) for 48 h. The cells were then collected and analyzed for the expression of E-Cadherin and Vimentin by FCM. Negative Ctrl: Huh-7 cells transfected with the control miRNA lentivirus; miR-155: Huh-7 cells transfected with the miR-155 mimic lentivirus. TGF-β1: recombinant human TGF-β1 protein. The data are expressed as means ± SEM. *P<0.05, **P<0.01 and ***P<0.001.
Discussion
In recent years, miRNAs have become a major focus of research in the fields of tumor invasion and metastasis [14,15]. As a multifunctional miRNA, miR-155 plays a crucial role in a variety of physiological and pathological processes, such as hematopoietic lineage differentiation, progression of cardiovascular and cancer diseases, and diabetes [16-19]. As an oncogene, miR-155 has been shown to be overexpressed in several types of human cancers, including hepatocellular carcinoma [11], laryngeal cancer, osteosarcoma, bladder cancer, breast cancer, gastric cancer, pancreatic cancer, and is closely related to the metastasis and prognosis of malignant tumors [20-25]. In the present study, we found that the expression of miR-155 was significantly increased in HCC tissues and cell lines. In breast cancer, there are several strands of evidences that have shown that miR-155 is the key factor in breast cancer metastasis. It promotes proliferation of breast cancer cells, and is closely correlated with lymph node metastasis, as well as the stage and prognosis of the cancer [26]. In the present study, the overexpression of miR-155 led to increased invasive and migratory abilities of Huh-7 cells, suggesting that miR-155 promotes the invasion and migration of HCC cells, and may further accelerate the progression of HCC. However, the molecular mechanisms behind the regulation of miR-155 expression and its role in HCC invasion and metastasis have not yet been fully elucidated.
Metastasis is the primary cause of death in cancer patients, and EMT is predicted to be necessary for metastatic progression. Cells that have undergone EMT are endowed with the ability to transmigrate basement membranes and stromal tissues as well as to intravasate and pass through the circulatory system. In physiological terms, this process is an important event in both the early stages of embryonic development and in pathophysiological situations such as wound healing, chronic inflammation and carcinoma progression. There is a large body of evidence showing that chronic inflammation and fibrosis with increased TGF-β expression, stimulates hepatocytes to change their phenotype, functions and the EMT process in order to escape apoptosis which is the basis for hepatocarcinogenesis [27-30]. TGF-β1 levels are apparently up-regulated in colon, esophageal, gastric, hepatocellular and pancreatic cancer, and are correlated with tumor progression, metastasis and angiogenesis, which are in turn related to poor prognostic outcome [31,32]. ELISA results in the present study showed that TGF-β1 secretion levels were significantly increased in cells of HCC cell lines and that is was correlated with increased expression of miR-155, which is consistent with these previous reports suggesting a key role of TGF-β1 in cancer progression.
It has been reported that miR-155 induces the occurrence of EMT in breast cancer, and further promotes tumor metastasis. Knockdown of miR-155 in breast cancer cells can significantly inhibit EMT induced by TGF-β, and greatly reduces the metastasis rate of tumor cells. The up-regulation of miR-155 promotes the invasion and metastasis of breast cancer cells by reducing the expression of RhoA protein [33]. These studies have suggested that there is a close regulatory relationship between TGF-β and miR-155 in cancer cells. In the context of the present study, we next observed that the secretion levels of TGF-β and them RNA expression levels of miR-155 in Huh-7 cells were positively correlated. Further analysis showed that the treatment of Huh-7 cells with TGF-β1 led to a marked increase in miR-155 expression in these cells, compared with control cells. These results suggest that elevated levels of miR-155 in HCC tissues and cell lines could be mediated by an increase in the levels of secreted TGF-β1.
EMT involves the cellular loss of epithelial cell markers, such as E-cadherin accompanied by increased expression of mesenchymal markers, such as N-cadherin, a loss of tight junctions, cytoskeletal reorganization and an increase in cell migration and invasion [34]. This process is driven by transcription factors such as SNAI1. Against this background, we evaluated the effect of miR-155 and TGF-β1 on these EMT related genes, and found that the overexpression of miR-155 in Huh-7 cells significantly up-regulated the expression of FN1, SNAI1, ZEB1 and Vimentin, and down-regulated the expression of CDH1 and E-Cadherin, further promoting the process of EMT in HCC cells.
Previous studies have shown that miR-122 reduces the expression of certain mesenchymal markers such as vimentin, and decreases tumor cell migration and invasion in vitro, as well as reducing celluar proliferation, angiogenesis and intrahepatic metastasis in vivo by regulation of the target gene ADAM17 (metalloprotease 17, a sheddase) in metastatic HCC cell lines. Thus, in addition to miR-155, there may well be other miRNAs involved in the regulation of EMT of HCC cells. The specific miRNAs and their bimolecular roles in HCC progression need to be further researched.
Collectively, the findings of the present study have demonstrated that the aberrant high expression of miR-155 in HCC tissues and cell lines promotes the process of EMT, invasion and migration of HCC cells in vitro, by regulating the expression of certain EMT related molecules (CDH1, FN1, SNAI1, ZEB1, E-Cadherin and Vimentin). Under the stimulation of TGF-β1, the expression of miR-155 in HCC cells is further up-regulated, and contributes to the progression of HCC by the stimulation of EMT, invasion and metastasis. These results further add to our understanding about the biological function and role of miR-155 in HCC cells. However, there are still a number of gaps in understanding which need to be addressed by further research. In particular, the mechanism by which TGF-β1 regulates expression of miR-155 in HCC cells.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81301906) to Xiu-Min Zhou.
Disclosure of conflict of interest
None.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2776. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Kensler TW, Qian GS, Chen JG, Groopman JD. Translational strategies for cancer prevention in liver. Nat Rev Cancer. 2003;3:321–329. doi: 10.1038/nrc1076. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Sun VC, Sarna L. Symptom management in hepatocellular carcinoma. Clin J Oncol Nurs. 2008;12:759–766. doi: 10.1188/08.CJON.759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 7.Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, Mikulits W. Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res. 2004;566:9–20. doi: 10.1016/s1383-5742(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 8.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 9.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 10.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 11.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, Qin LX, Yang W, Wang HY, Tang ZY, Croce CM, Wang XW. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F, Kong X, Lv L, Gao J. TGF-beta1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015;359:288–298. doi: 10.1016/j.canlet.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Kong X, Lv L, Gao J. MiR-155 targets TP53INP1 to regulate liver cancer stem cell acquisition and self-renewal. FEBS Lett. 2015;589:500–506. doi: 10.1016/j.febslet.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Ma L. MicroRNA control of epithelialmesenchymal transition and metastasis. Cancer Metastasis Rev. 2012;31:653–662. doi: 10.1007/s10555-012-9368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008;5:115–119. doi: 10.4161/rna.5.3.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Ma T, Huang C, Hu T, Li J. The pivotal role of microRNA-155 in the control of cancer. J Cell Physiol. 2014;229:545–550. doi: 10.1002/jcp.24492. [DOI] [PubMed] [Google Scholar]
- 17.Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of the MIR155 host gene in physiological and pathological processes. Gene. 2013;532:1–12. doi: 10.1016/j.gene.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochimbiophy Acta. 2009;1792:497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Qin Y, Jia J, Lin T, Lin X, Chen L, Zeng H, Han Y, Wu L, Huang S, Wang M, Huang S, Xie R, Liang L, Liu Y, Liu R, Zhang T, Li J, Wang S, Sun P, Huang W, Yao K, Xu K, Du T, Xiao D. MiR-155 enhances insulin sensitivity by coordinated regulation of multiple genes in mice. PLoS Genet. 2016;12:e1006308. doi: 10.1371/journal.pgen.1006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao XD, Zhang W, Liang HJ, Ji WY. Overexpression of miR-155 promotes proliferation and invasion of human laryngeal squamous cell carcinoma via targeting SOCS1 and STAT3. PLoS One. 2013;8:e56395. doi: 10.1371/journal.pone.0056395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv H, Guo J, Li S, Jiang D. miR-155 inhibitor reduces the proliferation and migration in osteosarcoma MG-63 cells. Exp Ther Med. 2014;8:1575–1580. doi: 10.3892/etm.2014.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kono H, Nakamura M, Ohtsuka T, Nagayoshi Y, Mori Y, Takahata S, Aishima S, Tanaka M. High expression of microRNA-155 is associated with the aggressive malignant behavior of gallbladder carcinoma. Oncol Rep. 2013;30:17–24. doi: 10.3892/or.2013.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson J, Berg T, Kurzejamska E, Pang MF, Tabor V, Jansson M, Roswall P, Pietras K, Sund M, Religa P, Fuxe J. MiR-155-mediated loss of C/EBPβ shifts the TGF-β response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene. 2013;32:5614–5624. doi: 10.1038/onc.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Chen Q, Lai R, Wu X, Wu X, Liu F, Xu G, Ji Y. Elevated expression of mature miR-21 and miR-155 in cancerous gastric tissues from Chinese patients with gastric cancer. J Biomed Res. 2010;24:187–197. doi: 10.1016/S1674-8301(10)60028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habbe N, Koorstra JB, Mendell JT, Offerhaus GJ, Ryu JK, Feldmann G, Mullendore ME, Goggins MG, Hong SM, Maitra A. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8:340–346. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng SR, Guo GL, Zhang W, Huang GL, Hu XQ, Zhu J, Huang QD, You J, Zhang XH. Clinical significance of miR-155 expression in breast cancer and effects of miR-155 ASO on cell viability and apoptosis. Oncol Rep. 2012;27:1149–1155. doi: 10.3892/or.2012.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, Breitkopf K, Weng H, Mertens PR. Hepatocyte-specific Smad7 expression attenuates TGFbeta-mediated fibrogenesis and protects against liver damage. Gastroenterology. 2008;135:642–659. doi: 10.1053/j.gastro.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem. 2007;282:22089–22101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 29.Godoy P, Hengstler JG, Ilkavets I, Meyer C, Bachmann A, Müller A, Tuschl G, Mueller SO, Dooley S. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor betainduced apoptosis. Hepatology. 2009;49:2031–43. doi: 10.1002/hep.22880. [DOI] [PubMed] [Google Scholar]
- 30.Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L, Tripodi M. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res. 2008;314:143–152. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Bierie B, Moses HL. Tumour microenvironment. TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 32.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, Cheng JQ. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]


