Abstract
Ventricular arrhythmia (VA) in structurally normal heart is considered as benign. However, these arrhythmias have been recently reported to induce left ventricular (LV) dysfunction. Up to now, there is no efficacious method to detect abnormal myocardial systolic function in VA patients. Therefore, in the current study, we used cardiac magnetic resonance feature tracking (CMR-FT) on balanced steady state free precision (SSFP) cine images to investigate LV myocardial strain in 42 VA patients without known heart disease as well as in 29 normal volunteers. As compared with controls, VA patients had lower peak values of radial and circumferential strain (RS, CS) in both basal and middle parts of LV and the peak value of longitudinal strain (LS) in VA patients was also decreased in middle LV. Moreover, as revealed by LV myocardial segmental peak strain, reduced RS, CS and LS peaks in VA were more significant in anterior, inferoseptal and anterolateral segments, especially for the patients with frequent premature ventricular complexes. Our results suggested that VA in normal heart is associated with abnormal segmental wall motion, which can be efficaciously detected by CMR-FT derived strain analysis. And early clinical management should be considered to prevent further significant symptoms in these patients.
Keywords: Ventricular arrhythmia, cardiac magnetic resonance
Introduction
Ventricular arrhythmia (VA) in the absence of structural heart disease predominantly originates from ventricular outflow tract, which accounts for about 10% of all VA cases with most of the patients experiencing a benign course and developing favorable prognosis [1,2]. However, in some cases, VA in normal heart can lead to severe symptoms and sudden death. Recent studies have reported that VA in patients without overt cardiovascular disease can cause adverse left ventricular (LV) remodeling and dysfunction, especially in those with frequent premature ventricular complexes (PVCs) [3,4]. These evidences indicate a potential abnormal LV myocardial function associated with VA. Theoretically, disorders of electrical activation initiated by VA may result in significant ventricular systolic dyssynchrony [5,6]. However, most of VA patients without structural heart diseases have normal LV ejection fraction (LVEF), and a more efficacious method is thus in need to evaluate the myocardial systolic function for VA in normal hearts.
Cardiac magnetic resonance feature tracking (CMR-FT) has been recently proposed to quantitatively evaluate myocardial function [7]. It allows the tracking of tissue voxel motion through CMR balanced steady state free precession (SSFP) cine images, and myocardial mechanisms derived from CMR-FT don’t rely on additional sequences, which largely reduces post-processing time [8]. Recently, CMR-FT has been successfully applied to detect the abnormalities of regional ventricular wall motion in patients with cardiac diseases, such as ischemic heart diseases and cardiomyopathies [9-13], however, little evidence is available regarding the feasibility of CMR-FT in detecting myocardial deformation in VA patients without structural heart diseases. In the current study, we therefore aim to evaluate whether CMR-FT is sensitive enough to detect abnormal LV myocardial strains as well as regional wall systolic dyssynchrony in VA patient with normal LVEF.
Methods
Patient population
The study population included 42 consecutive VA subjects without structural heart disease, which were diagnosed by cardiovascular physicians in the Second Affiliated Hospital of Nanchang University from 2012 to 2016. The following inclusion and exclusion criteria were applied: 1) VA patients were diagnosed by invasive radiofrequency catheter ablation and intracardiac electrophysiological examinations; 2) PVCs and VT were determined by 24-h Holter monitoring. Frequent PVC was defined as more than 1000 PVCs over 24 hours, and non-sustained VT was defined as three or more consecutive ventricular arrhythmias with tachycardia frequency more than 100 times/min; 3) patients had malignant or congenital idiopathic ventricular arrhythmia without overt cardiovascular disease were excluded, such as polymorphic VT, Brugada wave; 4) patients with structural heart diseases, ischemic cardiomyopathy and significant coronary atherosclerosis were excluded; 5) patients with familial history of heart diseases, particularly the arrhythmogenic right ventricular cardiomyopathy, were excluded; 6) patients with familial history of sudden death were; 7) patients with renal impairment, systemic hypertension, or diabetes mellitus were excluded.
As a control, 29 healthy volunteers were also recruited from medical examinations. None of the healthy controls had history of cardiovascular diseases, valvular heart disease, renal impairment, systemic hypertension, or diabetes mellitus. All controls had normal electrocardiography (ECG) or sinus tachycardia or sinus bradycardia.
All procedures in the present study were performed with the approval of the institutional review board and ethics committee of the Second Affiliated Hospital of Nanchang University in accordance with the 1964 Helsinki declaration. Written informed consent was obtained from all participating individuals prior to the study.
Cardiac magnetic imaging
All CMR examinations were performed on a 1.5T and 3.0T scanner (GE Signa Excite HD Twinspeed) with a cardiac phased-array 8 channel coil. ECG was used for cardiac gating and breath holding [14]. Following a three-plane or real-time localizer, balanced steady state free precession (SSFP) cines were acquired in the short-axis (SAX) and long axis (vertical and horizontal planes, LAX). Contiguous short axis cines extending from the atrioventricular valve plane to the apex were obtained to cover the entire left and right ventricles (6 mm parallel slices with 2 mm gap). The four-chamber planes were acquired to cover the LV and RV chambers (5 mm slice thickness with 1 mm gap). Typical scan parameters were applied: 1) 1.5T: NEX 1, FOV 35 cm, a matrix of 224×224, TR/TE 3.8/1.6 ms, 20 phases per cardiac cycle; 2) 3.0T: NEX 1, FOV 35 cm, a matrix of 224×160, TR/TE 3.5/1.6 ms, 20 phases per cardiac cycle [15].
Image analysis
CMR-FT analysis was performed on Circle Cardiovascular Imaging (Tissue Tracking, CVI42, Circle Cardiovascular Imaging, Calgary, Alberta, Canada). SAX and LAX cine images were uploaded into the software which reconstructed a 3D model and derived peak radial, circumferential and longitudinal strain [16]. End-diastolic and end-systolic phases were selected, endo/epi contours were drawn manually, and papillary muscles were excluded from the endocardial contour. Then the software automatically tracked the defined LV slices through the cardiac cycle. The segmental peak strain values were displayed in a modified 16-segment LV model according to the standard 17-segment model of the American Heart Association [17]. As a global functional evaluation, LVEF was also assessed via standardized protocols using a semi-automated commercially available software (CVI42) [14,17,18].
Reproducibility
Previous studies demonstrated that the circumferential global strain and peak strain values displayed excellent reproducibility [19-21]. Therefore, in the present study, we randomly selected CMR images from 14 subjects for the assessment of inter- and intra-observer variability of strain values.
Statistical analysis
Continuous variables were assessed for normality distribution using the one sample Kolmogorov-Smirnov test and presented as mean ± SD. Comparisons of CMR parameters between controls and VA patients, frequent PVC and non-sustain VT were performed using independent samples t-Test or Wilcoxon signed-rank test, as appropriate. One-way ANOVA was applied to compare the regional LV peak strain in VA patients as well as in healthy controls. Agreement was tested by calculating mean bias from Bland-Altman analysis. All statistical analysis was performed using the SPSS v20.0 (SPSS Inc., Chicago, IL, USA) and Bland-Altman analysis was performed with Medcalc v 16.4.1 (MedCalc Software bvba, Ostend, Belgium). A two-sided P<0.05 was considered statistically significant.
Results
Patient characteristics
29 healthy controls and 42 VA patients were enrolled in the current study. As shown in Table 1, no significant difference was observed in age, gender and heart rate between VA patients and controls, and LV function indexes (Cardiac Output, End-diastolic volume, LVEF) also remained normal in VA patients. All VA patients had clinical symptoms, such as palpitation, chest distress. 52.5% of them (22 patients) had frequent premature ventricular complexes (PVCs), 26% (11 patients) had non-sustained ventricular tachycardias (VT), and the rest 21.5% (9 patients) had both or others. Through catheter ablation and electrophysiological examinations, ectopic origin was confirmed in 20 VA patients, including 13 patients originating from right ventricular outflow tract, 2 from left outflow tract and 5 from other positions (Table 1).
Table 1.
Patient demographics and the LV function parameters
| Variable | VA group (n=42) | Control group (n=29) | p-value |
|---|---|---|---|
| Demographic data | |||
| Age, y | 36±15.5 | 35±16.8 | 0.797 |
| Male/Female, n (%) | 26 (62)/16 (38) | 16 (55)/13 (45) | 0.571 |
| Heart rate (/min) | 77.45±15.61 | 79.36±13.92 | 0.598 |
| LV function | |||
| Cardiac Output (ml/min) | 6.21±1.33 | 6.57±1.65 | 0.313 |
| End-diastolic volume (ml) | 124.13±28.10 | 132.67±30.49 | 0.228 |
| LVEF (%) | 66.45±7.27 | 69.84±7.52 | 0.061 |
| Clinical Symptom | Palpitation, chest distress | NA | |
| Arrhythmia type, n (%) | |||
| Frequent PVCs | 22/(52.5) | NA | |
| Non-sustained VT | 11 (26) | NA | |
| Other | 9 (21.5) | NA | |
| Catheter ablation, n (%) | 20 (48) | NA | |
| Ectopic origin type, n (%) | |||
| Right ventricular outflow tract | 13 (31) | NA | |
| Left ventricular outflow tract | 2 (5) | NA | |
| Other | 5 (12) | NA |
LV CMR-FT
Representative LV CMR-FT images were shown in Figure 1A-C. Circumferential strain (CS) was determined from LV short-axis at end-diastole (Figure 1A), while longitudinal strain (LS) was defined from both 4- and 2-chamber cines (Figure 1B and 1C). The distribution of segmental CS and LS from a 38-year old male volunteer and a 39-year old male VA patients were also shown in Figure 1D-I. In healthy condition, the distribution CS and LS curves from different segments are consistent (Figure 1D and 1E), while in VA patient, the CS and LS curves varied a lot from different segments, with most of the segments displaying a reduced strain value (Figure 1G and 1H). As shown in the 16-segment maps of peak LS, VA patient had much reduced peak LS at basal level, especially at basal anterior segment, which displayed a reserved value (Figure 1F and 1I). These results suggested reduced myocardial strain at different LV levels and at different segments. And our next step is to figure out the deregulated levels and segments.
Figure 1.
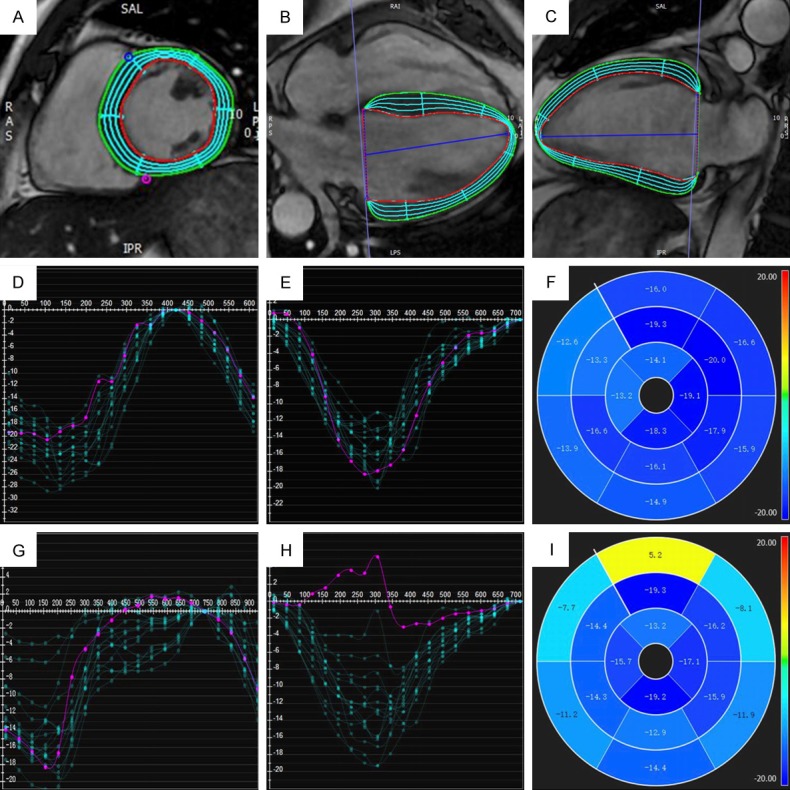
LV CMR-FT. (A) Circumferential strain from LV short-axis. Longitudinal strain from 4-chamber (B) and 2-chamber (C) cines. (D-F) are the representative segmental CS curves (D), LS curves (E) and 16-segmental map of peak LS (F) from a healthy volunteer, while (G-I) are segment CS curves (G), LS curves (H) and 16-segmental map of peak LS (I) from a VA patient.
Distribution of peak strain in the LV levels
As shown in Figure 2, both healthy controls and VA patients had the highest radial strain (RS) peak existed at apical levels of LV and lowest peak RS at middle levels. In contrast, the highest peaks of CS and LS existed at middle levels, while apical levels had the lowest CS and LS peaks. As compared with controls, VA patients had significantly lower RS and CS peaks at both basal and middle levels of LV (RS-basal: 35.63±13.57% vs. 42.61±10.32%, P=0.022; RS-mid: 28.69±10.43% vs. 36.04±7.54%, P=0.002; CS-basal: -15.23±3.38% vs. -17.50±1.95%, P=0.001; CS-mid: -15.38±4.19% vs. -18.05±2.61%, P=0.002), and the LS peak in the middle was also decreased in VA (LS-mid: -14.59±4.44% vs. -17.64±2.64%, P=0.001). No differential myocardial strains were observed at apical levels.
Figure 2.
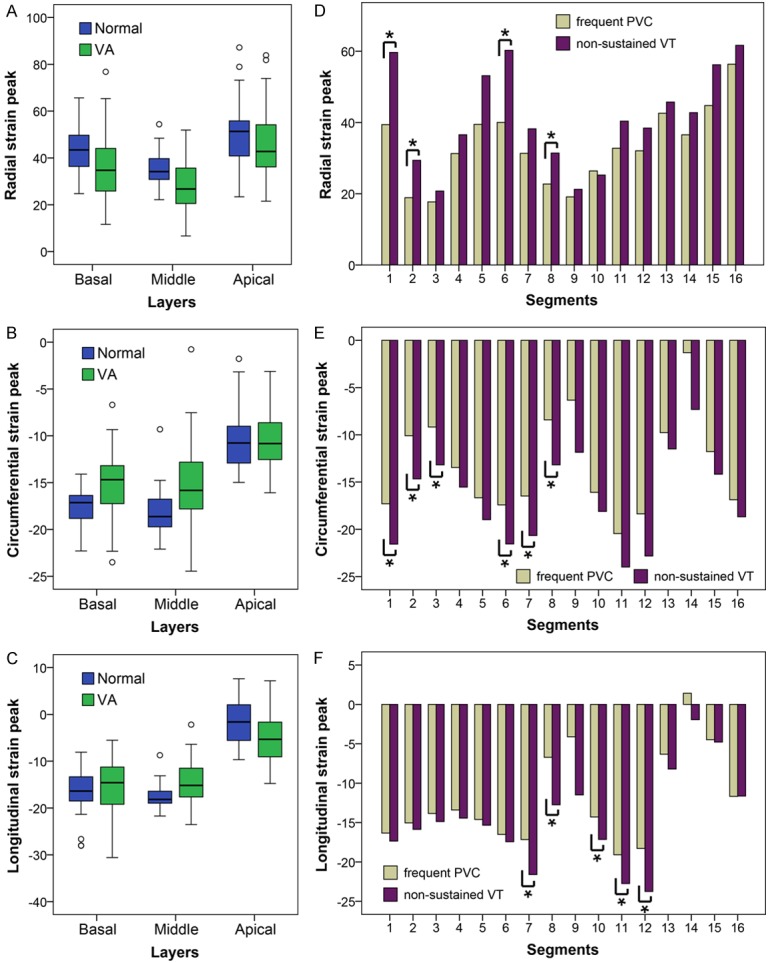
The distribution of regional peak strain by groups in the radial (A), circumferential (B) and longitudinal (C) directions. Blue and green boxplots correspond to control subjects and VA cohorts respectively. Bar charts represented the segmental peak radial (D), circumferential (E) and longitudinal (F) strain values of frequent PVCs (brown) and non-sustained VT (purple).
16-segment LV myocardial peak strain
Through modified 16-segment LV model, the peak of radial, circumferential and longitudinal strain can be acquired for each single LV segment (basal segments: 1~6; mid segments: 7~12; apical segments: 13~16). As listed in Table 2, VA patients had significantly reduced peak PS in segments 1, 6, 7, 9, 10, 12, 13 as compared with healthy controls, and peak CS of segments 1, 5, 6, 7, 9, 12 and peak LS of segments 7, 12, 13 were also dramatically decreased in VA patients (P<0.05). These results suggested abnormal motion within anterior, septal and anterolateral walls in VA patients without structural heart diseases.
Table 2.
3D segmental peak strain for controls and VA cohorts (S, %)
| Segments | RS | CS | LS | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Controls | VA | P | Controls | VA | P | Controls | VA | P | |
| Basal | |||||||||
| 1 anterior | 64.02±18.40 | 45.53±23.64 | 0.001 | -20.90±3.17 | -18.33±5.36 | 0.014 | -17.41±5.81 | -16.96±6.49 | 0.768 |
| 2 anteroseptal | 26.18±10.80 | 22.51±11.53 | 0.181 | -13.21±3.27 | -11.02±6.94 | 0.08 | -16.72±5.41 | -15.55±6.18 | 0.46 |
| 3 inferoseptal | 20.33±8.60 | 19.51±11.28 | 0.742 | -12.47±3.39 | -10.65±5.16 | 0.1 | -15.48±4.87 | -14.31±5.38 | 0.35 |
| 4 inferior | 34.94±12.32 | 34.15±16.44 | 0.825 | -15.71±2.75 | -14.31±4.13 | 0.115 | -14.88±5.24 | -13.94±6.13 | 0.505 |
| 5 inferolateral | 46.83±16.95 | 44.74±24.89 | 0.674 | -20.02±3.52 | -17.86±4.69 | 0.039 | -16.94±5.37 | -15.57±6.87 | 0.373 |
| 6 anterolateral | 63.36±23.09 | 47.32±24.20 | 0.007 | -22.69±3.67 | -19.24±6.35 | 0.01 | -17.87±5.20 | -17.36±6.22 | 0.72 |
| Middle | |||||||||
| 7 anterior | 47.75±18.28 | 32.63±14.21 | <0.001 | -20.47±3.38 | -17.05±7.57 | 0.012 | -21.32±3.20 | -18.23±6.74 | 0.012 |
| 8 anteroseptal | 27.62±10.14 | 24.25±13.05 | 0.247 | -10.93±5.81 | -8.86±9.11 | 0.283 | -10.16±6.19 | -7.53±10.06 | 0.178 |
| 9 inferoseptal | 23.06±3.95 | 19.65±6.97 | 0.011 | 11.49±8.48 | -7.87±10.42 | 0.013 | -10.46±8.01 | -6.21±11.53 | 0.058 |
| 10 inferior | 32.33±9.29 | 27.01±10.99 | 0.037 | -18.30±3.22 | -16.91±4.28 | 0.142 | -16.87±2.84 | -14.78±5.83 | 0.079 |
| 11 inferolateral | 42.53±15.64 | 35.39±17.40 | 0.081 | -23.74±4.31 | -21.63±5.58 | 0.091 | -23.05±4.34 | -20.42±5.70 | 0.04 |
| 12 anterolateral | 42.95±13.44 | 33.20±18.01 | 0.016 | -23.34±4.65 | -19.99±7.26 | 0.032 | -23.99±4.86 | -20.37±7.69 | 0.028 |
| Apical | |||||||||
| 13 anterior | 52.54±15.81 | 42.58±17.40 | 0.016 | -10.42±2.90 | -9.61±5.01 | 0.434 | -2.58±6.68 | -5.88±5.29 | 0.023 |
| 14 septal | 40.11±11.24 | 37.55±13.43 | 0.403 | -1.93±8.71 | -7.13±8.35 | 0.562 | 5.43±5.82 | 0.57±7.57 | 0.003 |
| 15 inferior | 49.20±19.92 | 48.45±21.34 | 0.881 | -11.29±7.06 | -12.28±6.38 | 0.539 | -0.46±7.74 | -4.48±7.75 | 0.035 |
| 16 lateral | 60.32±26.73 | 56.54±26.81 | 0.56 | -18.38±5.24 | -17.44±5.30 | 0.461 | -8.79±5.88 | -11.10±4.90 | 0.077 |
Notably, reduced myocardial strain peak is more pronounced in patients with frequent PVCs. As shown in Figure 2D-F, as compared with non-sustained VT, VA patients with frequent PVCs had lower peak RS in segments 1, 2, 6, 8 (1: 39.40±17.57% vs. 59.65±27.67%, P=0.015; 2: 18.89±11.09% vs. 29.40±11.74%, P=0.017; 6: 40.03±20.32% vs. 60.26±22.52%, P=0.014; 8: 22.72±10.99% vs. 31.42±14.83%, P=0.042), lower peak CS in segments 1~3, 6~8 (1: -17.29±5.81% vs. -21.57±3.42%, P=0.037; 2: -10.09±7.29% vs. -14.66±4.73%, P=0.022; 3: -9.18±5.72% vs. -13.18±5.03%, P=0.022;6: -17.41±7.63% vs. -21.55±3.95%, P=0.044; 7: -16.48±5.11% vs. -20.66±2.17%, P=0.008; 8: -8.42±7.06% vs. -13.18±5.15%, P=0.041) and lower peak LS in segments 7, 8, 10~12 (7: -17.15±5.43% vs. -21.61±1.80%, P=0.005; 8: -6.71±9.31% vs. -12.74±4.81%, P=0.029; 10: -14.30±3.91% vs. -17.15±4.26%, P=0.032; 11: -19.09±5.14% vs. -22.75±5.53%, P=0.045; 12: -18.29±8.70% vs. -23.76±5.11%, P=0.027), whereas no difference was identified for any peak strain in apical segments (segments 13~16).
Reproducibility
Fourteen randomly selected cases were reanalyzed for the intra- and inter-observer feasibility and reproducibility. As shown in Figure 3C and 3E, segmental peak CS and LS had excellent agreement between measurement from intra observers (CS: ICC=0.859, 95% CI: 0.561~0.955, P=0.001; LS: ICC=0.845, 95% CI: 0.738~0.973, P<0.001), with minimal to moderate difference according to Bland-Altman analysis (Figure 3C, CS: -1.5±2.1%, 95% CI: -5.7~2.6; Figure 3E, LS: -1.7±3.0%, 95% CI: -7.6~4.1). For the measurement from different observers, segmental peak RS had excellent agreement (ICC=0.888, 95% CI: 0.653~0.964, P<0.001) with minimal difference of -0.4±8.7% (Figure 3B, 95% CI: -17.5 to 16.7).
Figure 3.
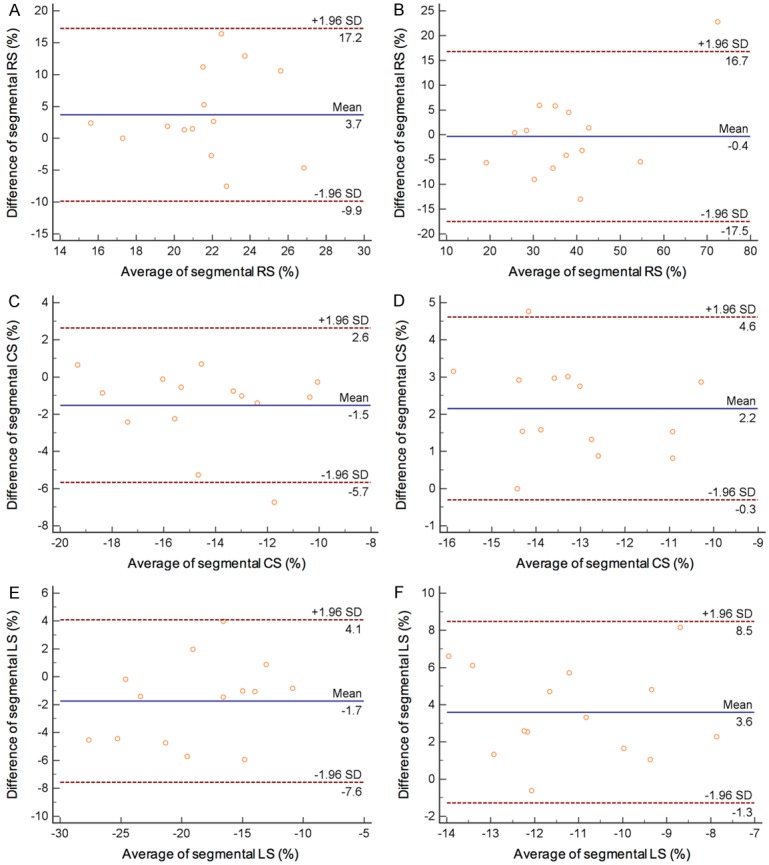
Bland-Altman analysis for peak segmental RS (A), CS (C) and LS (E) from intra-observers, and peak segmental RS (B), CS (D) and LS (F) of inter-observer.
Discussion
Myocardial strain analysis is a quantitative measurement of myocardial deformation in response to an applied force. During a cardiac cycle, the changes of myocardial fiber structure are associated with myocardial deformation in three directions: negative strain represents the myocardial segment shortening circumferentially and longitudinally, while positive strain represents radial thickening [22]. Various techniques have been used for strain analysis, including specking tracking imaging, strain-encoding and myocardial tagging with CMR [23,24]. In the current study, we used new CMR-FT approach to detect the regional wall motion abnormalities. CMR-FT is a technique that can quantify left ventricular deformation directly from SSFP cine CMR images and agrees well with other techniques. More importantly, as compared with other myocardial imaging techniques, such as cardiac computed tomography (CCT), echocardiograph, CMR has high reproducibility, high spatial, temporal resolution, no radiation exposure, and provides superior detection and quantification of segmental function [25-27]. In recent years, CMR-FT has found widespread applications in various myocardial disorders and has provided incremental values over conventional CMR imaging. To our knowledge, our study is the first to assess the feasibility of CMR-FT in detecting segmental wall motion abnormalities in VA patients without structural heart diseases.
The results from this study can be summarized as follow: 1) In general, VA with structurally normal hearts had significantly reduced 3D peak strain at the basal and middle layers of LV, while the distribution of peak strain in apical layers is similar to controls; 2) In VA patients, the 3D peak segmental strain were principally reduced in the anterior, inferoseptal and anterolateral segments, especially in the anterior segments of patients with frequent PVCs. These results indicate deregulated myocardial function in VA patients with normal global cardiac function, especially in those with frequent PVC, and also suggested careful cardiac imaging may help to detect function abnormalities in ventricular arrhythmias and aid treatment decisions.
Abnormal regional wall motion in VA has been documented in previous echocardiographic studies, occurring primarily in the early-activated regions. With strain data based on speckle tracking imaging, Yao et al evaluated circumferential strain in idiopathic frequent PVCs and found that peak CS was reduced significantly in the anterior, anteroseptal and septal segments, and the distribution of peak CS in the various layers exhibited a similar trend as that in controls [28]. Leeters et al reported the assessment of LV regional contraction abnormalities in combined right bundle with left anterior fascicular block (RBBB+LAFB) by echocardiographic, and demonstrated wall motion abnormalities between inferior and anterior LV walls as well as between septal and lateral walls in patients with left bundle branch block [29]. All these studies supported our findings that abnormal motion was associated with anterior and septal walls in VA patients without structural heart disease. Anterior, anteroseptal and septal walls are close to ventricular outflow track which was the major origin of ectopic pacemaker in VA without structural heart diseases. The reduction of peak strain values in these walls can be explained by the contraction pattern altered in patients with arrhythmia. Moreover, as compared with non-sustained VT, the strain peaks of anterior and septal segments decreased dramatically in patients with frequent PVCs, which indicate frequent PVC might originate from right ventricular outflow tract and is consistent with previous reports. Besides, we also found abnormality involved in anterolateral segment. Considering that ectopic pacemaker can cause the alteration in electrophysiological activation patterns and electrical wave conducts throughout the myocardium, our result might indicate deregulated ventricular function from other region, which deserves to be explore in the future.
Moreover, our study demonstrated that the segmental peak strain values were lower in patients with frequent PVCs than in those with non-sustained VT, so was the LVEF. Previous studies revealed a correlation between PVC frequency and LV dysfunction. Yao et al reported that patients with frequent PVCs had more significantly greater asynchronous segments compared was controls and a positive correlation existed between PVC frequency with asynchronous segments [28]. Markowitz et al demonstrated that patients with frequent PVC had more advanced LV remodeling than those with monomorphic VT [8]. To our knowledge, the arrhythmia can cause deregulated electrophysiological activation, but the mechanism of the relationship between contraction dyssynchorny and PVC frequency remains to be elucidated.
Besides, several limitations in this study should be acknowledged. First, the sample size in the study is small. Only 42 VA patients were enrolled and only 20 of them (47.6%) received radiofrequency catheter ablation to prove the origin. Therefore, we didn’t have enough power to compare CMR-FT with catheter ablation in detecting the origin of ectopic rhythm. A large-scale study with expanded patient cohort is still undergoing to systematically to evaluate the feasibility of CMR-FT in clinic. Second, VT also occurs in some patients with early-phase arrhythmogenic right ventricular cardiomyopathy (ARVC). In present study, we excluded the ARVC simply based on the absence of myocardial scar and fat infiltration. In our further study, we will take RV function into consideration in accordance with 2010 Task Force Criteria for ARVC. Third, all CMR examinations in the study were performed on a 1.5T and 3.0T scanner. No significant difference was identified between these two scanners, which is consistent with previous reports [30]. However, it is still possible that the independence of cardiac strain on CMR field strengths might be caused by small study population, and an in-depth study with large cohort is still undergoing. Fourth, the influence of age on myocardial strain hasn’t been considered in the current study mainly due the small range of age in control group and the small sample size. And the influence of age will be adjusted in the following large-scale study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grand No. 81360216 and 81660284), the Major Program of Natural Science Foundation of Jiangxi, China (Grand No. 20161ACB20013) and the Key Program of Bureau of Science & Technology of Jiangxi Province (Grand No. 20121BBG70040).
Disclosure of conflict of interest
None.
References
- 1.European Heart Rhythm Association; Heart Rhythm Society; Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL American Heart Association Task Forc and European Society of Cardiology Committee for Practice Guidelines. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force and the European society of cardiology committee for practice guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) J Am Coll Cardiol. 2006;48:e247–346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Prystowsky EN, Padanilam BJ, Joshi S, Fogel RI. Ventricular arrhythmias in the absence of structural heart disease. J Am Coll Cardiol. 2012;59:1733–1744. doi: 10.1016/j.jacc.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Hasdemir C. PVC-induced cardiomyopathy: the cut-off value for the premature ventricular contraction burden. Europace. 2013;15:1063. doi: 10.1093/europace/eut006. [DOI] [PubMed] [Google Scholar]
- 4.Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, De Conti G, Sarto P, Serratosa L, Patrizi G, De Maria E, Pelliccia A, Basso C, Schiavon M, Bauce B, Iliceto S, Thiene G, Corrado D. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol. 2016:9. doi: 10.1161/CIRCEP.116.004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, Armstrong W, Good E, Chugh A, Jongnarangsin K, Pelosi F Jr, Crawford T, Ebinger M, Oral H, Morady F, Bogun F. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Efremidis M, Letsas KP, Sideris A, Kardaras F. Reversal of premature ventricular complexinduced cardiomyopathy following successful radiofrequency catheter ablation. Europace. 2008;10:769–770. doi: 10.1093/europace/eun060. [DOI] [PubMed] [Google Scholar]
- 7.Hor KN, Baumann R, Pedrizzetti G, Tonti G, Gottliebson WM, Taylor M, Benson DW, Mazur W. Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp. 2011 doi: 10.3791/2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowitz SM, Weinsaft JW, Waldman L, Petashnick M, Liu CF, Cheung JW, Thomas G, Ip JE, Lerman BB. Reappraisal of cardiac magnetic resonance imaging in idiopathic outflow tract arrhythmias. J Cardiovasc Electrophysiol. 2014;25:1328–1335. doi: 10.1111/jce.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buss SJ, Krautz B, Hofmann N, Sander Y, Rust L, Giusca S, Galuschky C, Seitz S, Giannitsis E, Pleger S, Raake P, Most P, Katus HA, Korosoglou G. Prediction of functional recovery by cardiac magnetic resonance feature tracking imaging in first time ST-elevation myocardial infarction. Comparison to infarct size and transmurality by late gadolinium enhancement. Int J Cardiol. 2015;183:162–170. doi: 10.1016/j.ijcard.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Heermann P, Hedderich DM, Paul M, Schulke C, Kroeger JR, Baessler B, Wichter T, Maintz D, Waltenberger J, Heindel W, Bunck AC. Biventricular myocardial strain analysis in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) using cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2014;16:75. doi: 10.1186/s12968-014-0075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogarapu S, Puchalski MD, Everitt MD, Williams RV, Weng HY, Menon SC. Novel cardiac magnetic resonance feature tracking (CMR-FT) analysis for detection of myocardial fibrosis in pediatric hypertrophic cardiomyopathy. Pediatr Cardiol. 2016;37:663–673. doi: 10.1007/s00246-015-1329-8. [DOI] [PubMed] [Google Scholar]
- 12.Ohyama Y, Volpe GJ, Lima JA. Subclinical myocardial disease in heart failure detected by CMR. Curr Cardiovasc Imaging Rep. 2014;7:9269. doi: 10.1007/s12410-014-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prati G, Vitrella G, Allocca G, Muser D, Buttignoni SC, Piccoli G, Morocutti G, Delise P, Pinamonti B, Proclemer A, Sinagra G, Nucifora G. Right ventricular strain and dyssynchrony assessment in arrhythmogenic right ventricular cardiomyopathy: cardiac magnetic resonance feature-tracking study. Circ Cardiovasc Imaging. 2015;8:e003647. doi: 10.1161/CIRCIMAGING.115.003647. discussion e003647. [DOI] [PubMed] [Google Scholar]
- 14.Hamdan A, Thouet T, Kelle S, Wellnhofer E, Paetsch I, Gebker R, Schnackenburg B, Fahmy AS, Osman NF, Bornstedt A, Fleck E. Strainencoded MRI to evaluate normal left ventricular function and timing of contraction at 3.0 Tesla. J Magn Reson Imaging. 2009;29:799–808. doi: 10.1002/jmri.21684. [DOI] [PubMed] [Google Scholar]
- 15.Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol. 1990;259:H300–308. doi: 10.1152/ajpheart.1990.259.2.H300. [DOI] [PubMed] [Google Scholar]
- 16.Moore CC, Lugo-Olivieri CH, McVeigh ER, Zerhouni EA. Three-dimensional systolic strain patterns in the normal human left ventricle: characterization with tagged MR imaging. Radiology. 2000;214:453–466. doi: 10.1148/radiology.214.2.r00fe17453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 18.Andre F, Steen H, Matheis P, Westkott M, Breuninger K, Sander Y, Kammerer R, Galuschky C, Giannitsis E, Korosoglou G, Katus HA, Buss SJ. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2015;17:25. doi: 10.1186/s12968-015-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor RJ, Moody WE, Umar F, Edwards NC, Taylor TJ, Stegemann B, Townend JN, Hor KN, Steeds RP, Mazur W, Leyva F. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging. 2015;16:871–881. doi: 10.1093/ehjci/jev006. [DOI] [PubMed] [Google Scholar]
- 20.Augustine D, Lewandowski AJ, Lazdam M, Rai A, Francis J, Myerson S, Noble A, Becher H, Neubauer S, Petersen SE, Leeson P. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson. 2013;15:8. doi: 10.1186/1532-429X-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster A, Stahnke VC, Unterberg-Buchwald C, Kowallick JT, Lamata P, Steinmetz M, Kutty S, Fasshauer M, Staab W, Sohns JM, Bigalke B, Ritter C, Hasenfuss G, Beerbaum P, Lotz J. Cardiovascular magnetic resonance featuretracking assessment of myocardial mechanics: Intervendor agreement and considerations regarding reproducibility. Clin Radiol. 2015;70:989–998. doi: 10.1016/j.crad.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maciver DH. The relative impact of circumferential and longitudinal shortening on left ventricular ejection fraction and stroke volume. Exp Clin Cardiol. 2012;17:5–11. [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bansal M, Jeffriess L, Leano R, Mundy J, Marwick TH. Assessment of myocardial viability at dobutamine echocardiography by deformation analysis using tissue velocity and speckle-tracking. JACC Cardiovasc Imaging. 2010;3:121–131. doi: 10.1016/j.jcmg.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Marwick TH, Neubauer S, Petersen SE. Use of cardiac magnetic resonance and echocardiography in population-based studies: why, where, and when? Circ Cardiovasc Imaging. 2013;6:590–596. doi: 10.1161/CIRCIMAGING.113.000498. [DOI] [PubMed] [Google Scholar]
- 26.Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D’Andrea A, Bruder O, Cosyns B, Davin L, Donal E, Freitas A, Habib G, Kitsiou A, Petersen SE, Schroeder S, Lancellotti P, Camici P, Dulgheru R, Hagendorff A, Lombardi M, Muraru D, Sicari R. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European association of cardiovascular imaging endorsed by the saudi heart association. Eur Heart J Cardiovasc Imaging. 2015;16:280. doi: 10.1093/ehjci/jeu291. [DOI] [PubMed] [Google Scholar]
- 27.Gardner BI, Bingham SE, Allen MR, Blatter DD, Anderson JL. Cardiac magnetic resonance versus transthoracic echocardiography for the assessment of cardiac volumes and regional function after myocardial infarction: an intrasubject comparison using simultaneous intrasubject recordings. Cardiovasc Ultrasound. 2009;7:38. doi: 10.1186/1476-7120-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao J, Yang R, Xu D, Zhuang Y, Yong Y, Cao K. Circumferential myocardial contraction patterns in patients with idiopathic frequent premature ventricular complexes from the right ventricular outflow tract. Int J Cardiol. 2013;166:166–172. doi: 10.1016/j.ijcard.2011.10.105. [DOI] [PubMed] [Google Scholar]
- 29.Leeters IP, Davis A, Zusterzeel R, Atwater B, Risum N, Sogaard P, Klem I, Nijveldt R, Wagner GS, Gorgels AP, Kisslo J. Left ventricular regional contraction abnormalities by echocardiographic speckle tracking in combined right bundle branch with left anterior fascicular block compared to left bundle branch block. J Electrocardiol. 2016;49:353–361. doi: 10.1016/j.jelectrocard.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Schuster A, Morton G, Hussain ST, Jogiya R, Kutty S, Asrress KN, Makowski MR, Bigalke B, Perera D, Beerbaum P, Nagel E. The intraobserver reproducibility of cardiovascular magnetic resonance myocardial feature tracking strain assessment is independent of field strength. Eur J Radiol. 2013;82:296–301. doi: 10.1016/j.ejrad.2012.11.012. [DOI] [PubMed] [Google Scholar]


