Abstract
Background: Investigations in factor VII activating protease (FSAP)-/- mice suggest a role for FSAP in stroke, thrombosis and neointima formation. Here, we analyzed the role of FSAP in vascular remodeling processes related to arteriogenesis and angiogenesis in the mouse hind limb ischemia model. Methods and results: Femoral artery ligation was performed in mice and exogenous FSAP was injected locally to examine its effect on arteriogenesis in the adductor and angiogenesis in the gastrocnemius muscle over 21 days. Perfusion was decreased by FSAP, which was reflected in a lower arterial diameter and was associated with reduced monocyte infiltration in the adductor muscle. There was increased angiogenesis in the gastrocnemius muscle triggered indirectly by less blood supply to the lower limb. Comparison of wild-type (WT) and FSAP-/- mice showed that perfusion was not different between the genotypes but there were 2.5-fold more collateral arteries in the adductor muscle of FSAP-/- mice at day 21. This was associated with a higher infiltration of monocytes at day 3. Capillary density in the gastrocnemius muscle was not altered. Activity of the two major proteolytic pathways associated with vascular remodeling; matrix metalloprotease (MMP)-9 and urokinase-type plasminogen activator (uPA) was elevated in the gastrocnemius but not in the adductor muscle in FSAP-/- mice. Conclusions: Arteriogenesis is enhanced, and this is associated with a higher infiltration of monocytes, in the absence of endogenous FSAP but angiogenesis is unchanged. Exogenous FSAP had the opposite effect on arteriogenesis indicating a possible therapeutic potential of modulating endogenous FSAP.
Keywords: Arteriogenesis, angiogenesis, serine protease, metalloprotease, inflammation
Introduction
In vascular occlusive diseases new vessels form either via capillary sprouting, termed angiogenesis, or via remodeling of pre-existing arteriolar connections into collateral arteries, a process named arteriogenesis. Tissue ischemia can lead to spontaneous angiogenesis and arteriogenesis to overcome the local loss of perfusion. When an artery develops a hemodynamically relevant stenosis, circulating mononuclear blood cells are attracted and migrate into the vessel wall, giving rise to the production of multiple cytokines and growth factors [1]. This local inflammatory reaction results in proliferation and growth of the collateral vasculature through a complex interplay between endothelial cells, vascular smooth muscle cells (VSMC) and monocytes [2]. Mediators that are involved in this process include chemokine (C-C motif) ligand-2 (CCL-2), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF-2), and platelet derived growth factor (PDGF)-AB are shown to be up-regulated in arteriogenesis and drive the large expansion in SMC [3].
Factor VII activating protease (FSAP) is encoded by a gene named hyaluronic acid binding protein 2 (HABP2). FSAP is a circulating plasma serine protease with multifunctional properties [4]. Genetic epidemiological studies have shown that the G534E single nucleotide polymorphism (SNP) in the FSAP gene, also called the Marburg I (MI) polymorphism (rs7080536), is found in about 5% of Caucasians and is associated with late complications of carotid stenosis [5] and stroke [6]. MI-FSAP has about a 5-fold lower proteolytic activity towards all substrates tested and thus represents a loss-of-function polymorphism [7]. We have previously shown that the local application of exogenous wild type (WT)-FSAP but not MI-FSAP following wire-induced injury of the femoral artery was able to inhibit neointimal lesion formation and SMC proliferation [8] whereas FSAP-/- mice showed enhanced neointima formation [9]. These data indicate that the cellular regulatory effects of FSAP could be important in the context of vascular remodeling processes. FSAP was also shown to reduce endothelial cell permeability via protease activated receptors (PARs) [10].
There are two reports to date that demonstrated that FSAP inhibits neovascularisation. In the chicken chorioallantoic membrane model recombinant FSAP was shown to be anti-angiogenic and it was proposed that the kringle domain was responsible, since a FSAP mutant without the kringle domain was not effective [11]. However, no evidence was provided that the mutant was correctly folded or if it had any enzymatic activity. Another report indicated that FGF-2-mediated endothelial cell proliferation is inhibited by FSAP due to binding, sequestering and/or degradation of the growth factor. This inhibition of cell proliferation was dependent on the proteolytic activity of FSAP [12]. The same authors also demonstrated that FSAP releases FGF-2 and bradykinin from endothelial cells [13].
Considering that MI-SNP is associated with a higher risk of vascular diseases as well as the known effects of FSAP on vascular remodeling processes we hypothesized that FSAP influences the responses to hind limb ischemia. After ligation of the femoral artery in FSAP-/- mice, collateral arterial growth was increased that was associated with enhanced monocyte accumulation. This was further supported by opposite effects of exogenously applied FSAP indicating that FSAP is a novel factor in vascular remodeling process in vivo.
Methods
FSAP-/- mice
FSAP-/- mice (official name: B6129S5-Habp2tm1Lex) were generated as recently described [14] and were backcrossed with C57BL/6J mice for 2 generations and heterozygous crosses were set up to produce FSAP-/- mice and littermate controls. Sex and age matched littermates were compared in all experiments and all animals were maintained in the same room under SPF conditions. All procedures involving experimental animals were approved by the local government animal care committee and complied with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1985).
Mouse femoral artery ligation model
10-12 weeks old mice were anaesthetized with 100 mg body weight ketamine hydrochloride (Ketanest®, Pfizer, Berlin, Germany) and 2 mg body weight xylazine hydrochloride (Rompun®, Bayer, Leverkusen, Germany). The femoral artery was occluded by a double ligation with 6-0 silk sutures (Ethicon, Norderstedt, Germany) distal to the origin of the deep femoral artery and proximal to the origin of any other distal branch [15].
FSAP isolation and intra-muscular application
FSAP was isolated as described previously [16]. The buffer for FSAP was diluted in 0.2 M arginine, 0.2 M lysine, 5 mM citrate, pH 4.5 and this buffer was also used at the appropriate dilution to exclude any influence of the vehicle in all experiments. 7.5 μg of FSAP was injected into the upper hind limb at 5 defined points in a final volume of 30 ul in C57BL6/J mice at 1 h, 24 h and 3 days after the femoral artery ligation.
Laser Doppler perfusion imaging (LDPI)
Laser Doppler perfusion imaging was used to record serial blood flow measurements before and after the ligation of the left femoral artery and furthermore over a period of 3 weeks, postoperatively. The animals were placed for 5 min on a 38°C heating plate (Föhr Instruments, Seeheim-Ober, Germany) to avoid vasoconstriction by anaesthetic heat loss. The LDPI system (PeriScan System, Perimed, Järfälla, Sweden) scans the skin of the mouse foot up to a depth of 500 µm and the mean signal intensity is represented by false colours. For calculation of the hind limb perfusion the mean pixel intensity of both legs was determined and a “perfusion index” (ligated/unligated side) was used for quantification [17,18].
Immune histochemistry and morphometrical analysis
1, 3 and 7 days after ligation, tissue samples of adductor and gastrocnemius muscle from both hind limb muscles were snap-frozen in methyl butane and stored at -80°C until use [19]. Cryosections of 5-7 µm thickness were prepared with a Leica CM 1900 Cryomicrotome (Leica, Wetzlar, Germany), fixed and permeabilized in ice cold acetone, blocked with serum free protein block (Dako, Hamburg, Germany) for 20 minutes and incubation with rabbit anti-CD31 (Abcam, Cambridge, UK) for 1 h at room temperature, followed by appropriate cross-absorbed secondary antibody conjugated to Cy3 fluorochrome (Jackson ImmunoResearch Suffolk, UK). An FITC-conjugated mouse anti mouse-SMC-α-actin antibody (Sigma Aldrich, Hamburg, Germany) was included as a counterstain, followed by nuclear counterstain with DAPI. Only vessels with media-to-lumen-ratio typical for arteries were counted and their diameter determined. Sections were analyzed on an AxioverS100 (Carl Zeiss, Jena, Germany) equipped with appropriate filters sets and a RT/SE camera system (Diagnostic Instruments, Sterling Heights, MI USA). A total of 10 sections per animal were stained and vessel density was quantified by a single blind observer, the SpotAdvanced system/ImagePro (Media Cybernetics Inc., Bethesda, MD USA) and furthermore the computer Imaging System (MetaMorph™, Molecular Devices Sunnyvale, U.S.A.).
For confocal microscopy, cryosections of 10 µm were fixed in 4% (wt/vol) paraformaldehyde and incubated with a rat antibody raised against the mouse CD68 antigen (Serotec, Puchheim, Germany). It was followed by biotinylated secondary donkey anti-rat antiserum (Dianova, Hamburg, Germany) and Cy2-Streptavidin (Rockland, USA). After repeated washes in PBS, sections were incubated with anti-SMC-α-actin directly labeled with Cy3 (Sigma). F-actin was visualized with phalloidin conjugated with Alexa 633 (Molecular Probes). Finally, nuclei were stained with DAPI. The sections were examined by laser scanning confocal microscopy LEICA TCS5 which allows 4 channel concomitant scanning. Ten confocal optical sections were taken through the depth of tissue samples at 0.5 μm intervals by using a Leica Planapo 63/1.4 objective. Three-dimensional reconstruction was performed using Imaris 7.2 the multichannel image processing software (Bitplane, Zurich, Switzerland).
Preparation of tissue extracts
Tissue pieces were homogenized in a glass homogenizer in TBS (50 mM Tris, pH 7.4, containing 100 mM NaCl) with 1% (w/v) SDS. After centrifugation the extracts were frozen at -80°C until further analysis.
qPCR
RNA was extracted using Total RNA Miniprep Kit (Sigma Aldrich). Reverse transcription was performed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). For real-time PCR, SensiMix SYBR Kit (Bioline, Luckenwalde, Germany) was used and analysis was performed on a Step One Plus Real-Time PCR System (Applied Biosystems). Melting curve was performed at 60°C for 1 min following 95°C for 15 sec. Amplification plot was monitored over 40 cycles and continuous fluorescence measurement indicated mRNA expression of analyzed genes. Fluorescent threshold cycles (ct) were set and normalized against ct of housekeeping gene GAPDH (Δct). Afterwards expression of target gene was calculated as 2-Δct.
Quantitative zymography and western blotting
Tissue extracts were boiled for Western blotting but not for zymography. After normal SDS-PAGE the gels were washed with Triton X-100 (1%) and overlayed with an agarose gel containing casein and plasminogen. Dark bands indicated proteolysis of casein and the activation of plasminogen. For gelatine zymography 0.2% (w/v) was directly incorporated into the gel and after extensive washing was incubated for 48 h at 37°C. Coomassie staining identified gelatinase activity as clear bands. Western blot with anti-COX IV was performed to adjust for differences in protein concentrations. Band density was measured using the image analysis system Quantity One system (Biorad, Munich, Germany).
FSAP levels in plasma
These were measured as described previously [20].
Statistics
Statistical analysis was carried out using SAS® software, version 9.2 (SAS Institute Inc., Cary, NC, USA) and SPSS 22 (IBM, Germany). To analyze these effects we performed a two factorial ANOVA with cross effects using the GLM procedure in SAS®. In case of a significant global test the Tukey test was used as post hoc test for pairwise group comparisons. Significance levels were associated with the adjusted F-Test by Huynh-Feldt. P-values are used to differ between the significance levels factors in ANOVA. All statistical decisions were made two-tailed with a critical probability of a=5% without a-adjustment except the Tukey test for pairwise group comparisons. For all biochemical data one way ANOVA followed by Bonferroni post hoc test was used. A p value of <0.05 was considered to be significant.
Results
Effect of exogenous FSAP on arteriogenesis and angiogenesis
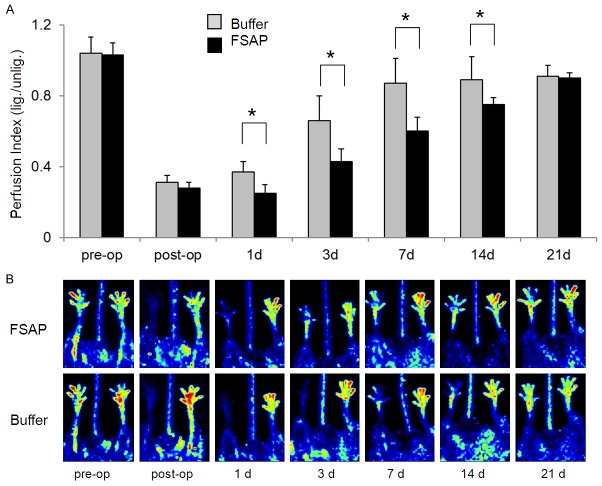
After femoral artery ligation, the effect of direct local application of FSAP protein and control buffer on blood flow, collateral vascular growth in adductor muscle (upper leg) and angiogenesis in gastrocnemius (lower leg) was determined over 21 days. Perfusion index was measured by laser Doppler perfusion imaging (LDPI) and calculated as mean pixel intensity of ligated/un-ligated side. Application of FSAP led to a delay in the recovery of perfusion index at day 1, 3, 7 and 14 after ligation but perfusion reached a plateau at day 21 and was identical thereafter (Figure 1). This was confirmed histologically since FSAP-treated animals showed a lesser increase of the vessel diameter compared to control buffer treated animals 7 days after ligation (P<0.05). However, there were no subsequent differences at later time points (Figure 2A). Capillary density in the adductor musculature, determined by the CD31 positive signals per defined area, showed no significant differences between the FSAP and control group (data not shown), while the capillary density in the gastrocnemius muscle was increased 2-fold (P<0.05) in the FSAP group (Figure 2B). Application of exogenous FSAP reduced the number of monocytes in the adductor muscle, but this was not the case in the gastrocnemius muscle (Figure 2C). Thus, application of exogenous FSAP inhibited arteriogenesis in the upper limb and this resulted in a reflex increase in angiogenesis in the lower limb due to sustained hypoxia.
Figure 1.
Perfusion in mice after application of exogenous FSAP. A. After femoral artery ligation in WT mice, the recovery of perfusion over 21 days was determined in FSAP-treated mice compared to buffer treatment using LPDI. The contralateral limb was used as an internal control for calculating left to right ratios to correct for inter-individual differences. Mean ± SD (n=8, *P<0.05). The 2-factor ANOVA for treatment groups (FSAP and buffer) as the main factor and the restoration of hind limb perfusion from the time point post-ligation up to 21 days as the repeated factor shows significance between these 2 factors and in cross effects of these factors at day 1, 3, 7 and 14 (P<0.05). Tukey’s post hoc test at day 1, 3, 7 and 14 indicates a difference between buffer and FSAP (P<0.05). Only the significant differences between the two treatment groups are indicated by *. B. Representative LPDI images of mice from each group are shown.
Figure 2.
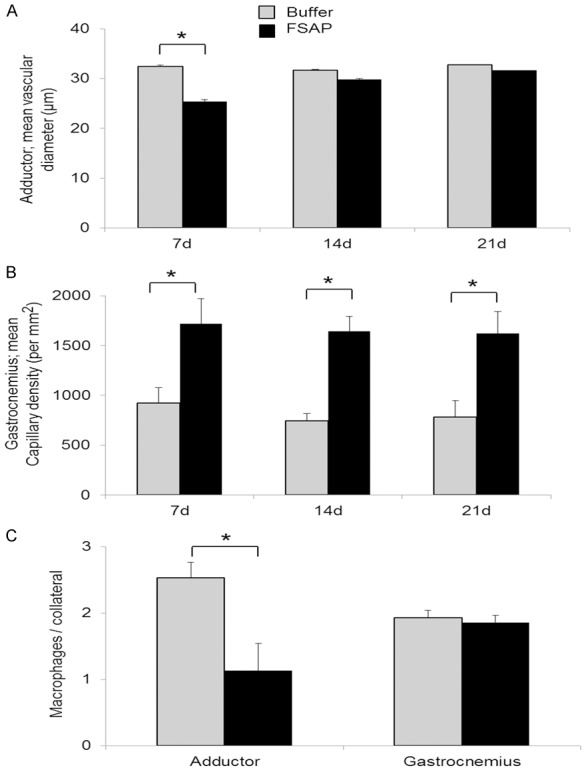
Arteriogenesis in mice after application of exogenous FSAP. After femoral artery ligation histological analysis of collateral arteries in adductor and gastrocnemius muscle sections of the ischemic hind limb at 21 days was performed. Staining of αSMC, CD31, F4/80 and nuclei was used to identify collateral arteries in the adductor muscle (A) (ANOVA, mean vessel diameter ± SD, n=6, *P<0.05, Tukey’s post hoc test). Capillary density in the gastrocnemius muscle (ANOVA, number/μm2 ± SD, n=6, *P<0.05 (day 7, 14 and 21, Tukey’s post hoc test) (B) and infiltrating macrophages (C) in both muscles (ANOVA, cell/collateral vessel, means ± SD, n=6, *P<0.05, Tukey’s post hoc test, significant only within the adductor muscle). Only the significant differences between the two treatment groups are indicated by *.
Effect of FSAP deficiency on arteriogenesis and angiogenesis
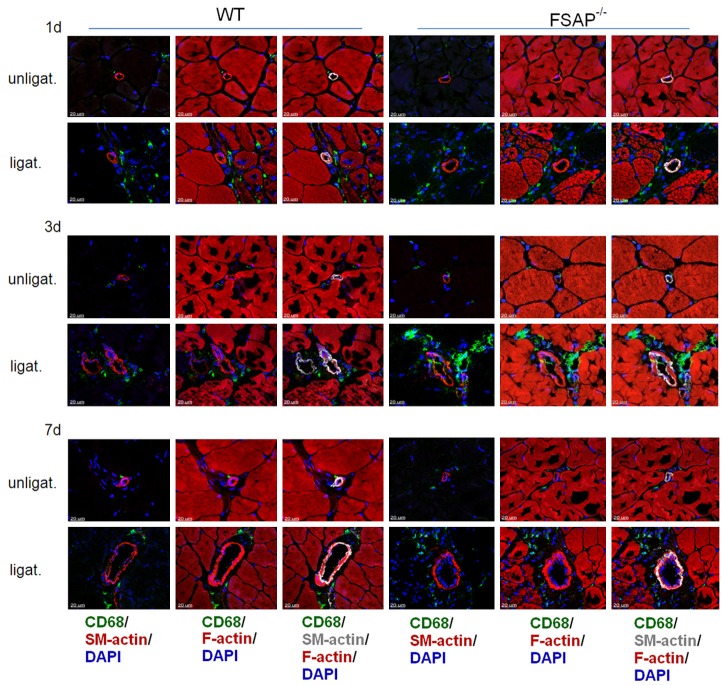
After ligation of the femoral artery in FSAP-/- mice and littermate WT mice, remodeling in the hind limbs was determined. The drop in perfusion and the subsequent recovery after 7, 14 and 21 days were similar in both genotypes (Figure 3A, 3B). At 21 days post ligation, histological evaluation demonstrated more collateral arteries with a larger vessel diameter in the upper limb of FSAP-/- mice (P<0.05) (Figure 3C, 3D). In contrast, capillary density in the gastrocnemius muscle did not show any significant differences between the genotypes (Figure 3E). Collateral growth and angiogenesis is dependent on the accumulation of monocytes/macrophages, so we also determined the number of monocytes/macrophages cells at time points 1, 3 and 7 days after ligation (Figure 4). Quantitative analysis showed that the number of monocytes/macrophages was statistically higher in FSAP-/- mice compared to WT mice at day 3 (P<0.05) but not on day 1 or day 7 (Figure 5A). The number of cells was very low in the unligated limbs and not different between the genotypes. Circulating FSAP levels were not altered at 1, 3 and 7 days after ligation in WT mice and no FSAP was detected in FSAP-/- mice (Figure 5B). The lack of endogenous FSAP led to an increase in arteriogenesis in the upper limb as determined histologically but recovery of perfusion was unaffected. This was associated with an increase in the number of monocytes. Angiogenesis in the lower limb was not affected by the lack of endogenous FSAP.
Figure 3.

Vascular remodeling in the hind limb after femoral artery ligation in WT and FSAP-/- mice. A. Perfusion was measured using LPDI over 21 days as indicated in the figure. The unligated/contralateral limb was used as an internal control for calculating left to right ratios to correct for inter-individual differences. Between these groups, no significant differences were noted (The results are presented as mean ± SD., n=8, *P<0.05 ANOVA. ANOVA indicates analysis of variance. And Tukey’s post hoc test. B. Representative LPDI images of mice from each group are shown. C. Histological analysis of collateral arteries in the adductor muscle sections of the ischemic hind limb at 21 days. Staining of αSMC and nuclei was used to identify collateral arteries (ANOVA, collateral arteries/muscle ± SD, n=6, *P<0.05, Tukey’s post hoc test). D. Diameter of the identified arteries (μm) was determined morphometrically (ANOVA, meanvessel diameter ± SD, Tukey’s post hoc test not significant, n=6). E. Histological analysis of angiogenesis in the gastrocnemius muscle sections of the ischemic hind limb at 21 days. Staining of CD31 and nuclei was used to identify capillaries (ANOVA, number/μm2 ± SD, n=6, Tukey’s post hoc test). Only the significant differences between the two genotypes are indicated by *.
Figure 4.
Accumulation of monocytes in arteriogenesis. Adductor muscle from WT and FSAP-/- mice days 1, 3 and 7 after femoral artery ligation were subjected to staining with CD68 (monocytes/macrophages, green), F-actin (muscle, red), α-smooth muscle actin (VSMC, grey) and nuclei (DAPI, blue) as indicated in the figure.
Figure 5.
Quantitative analysis of monocytes in arteriogenesis and circulating FSAP levels. A. Adductor muscle from WT and FSAP-/- mice 1, 3 and 7 days after femoral artery ligation were stained for CD68 as described in legend to Figure 4 and quantified (means ± SEM, n=5, *P<0.05, ANOVA followed by Bonferroni post test. B. Plasma FSAP activity was measured using a combination of immune capture and activity assay. No FSAP was detected in the plasma of FSAP-/- mice. Values were quantified with human plasma as a standard reference (mU/ml ± SEM, n=5, *P<0.05, ANOVA followed by Bonferroni post test). Only the significant differences between the two genotypes are indicated by *.
Levels of MMP-2, MMP-9 and uPA during remodeling in FSAP-/- mice
Since FSAP is a circulating protease, we hypothesized that difference in remodeling between the genotypes are related to an altered proteolytic balance. We quantified gelatinase activity in tissue extracts at day 3 in relation to the expression of the house keeping enzyme cytochrome oxidase IV. In the adductor muscle there was no induction of either MMP-2 or MMP-9 mRNA, protein levels or activity after ligation in both strains of mice (Figure 6). In the gastrocnemius muscle there was a tendency towards increased MMP-2 and MMP-9 mRNA but this was not significantly different between both genotypes. MMP-9 was increased in the gastrocnemius of FSAP-/- mice at the activity and protein level (Figure 6).
Figure 6.
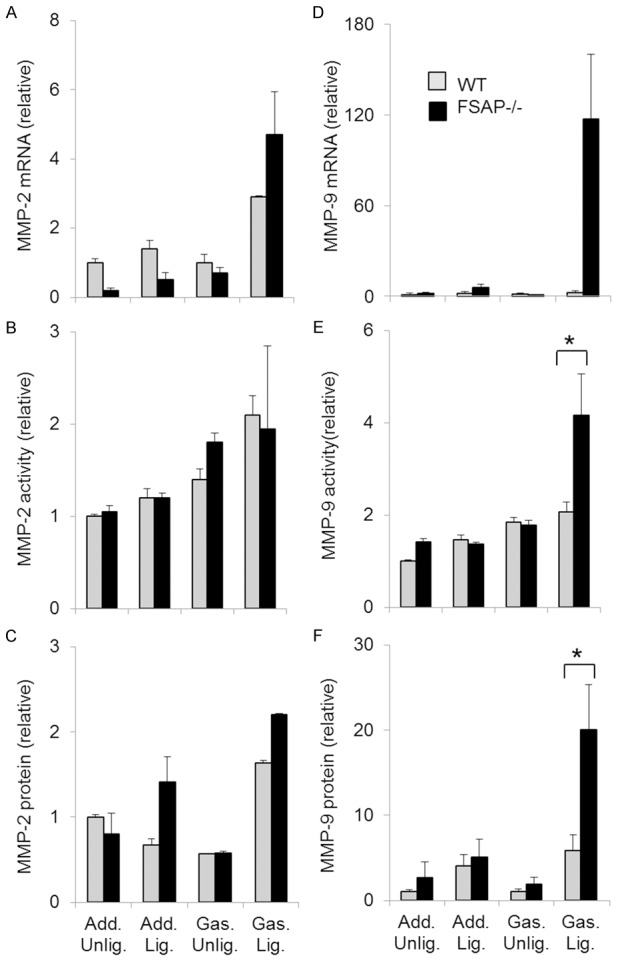
Expression of MMP-2 and MMP-9 in arteriogenesis. In the excised gastrocnemius and adductor muscles of WT- and FSAP-/- mice MMP-2, MMP-9 mRNA (A, D), activity (B, E) and protein (C, F) were measured 3 days after injury using qPCR; protease activity was related to the expression of cytochrome C Oxidase (COXIV) as measured by Western blotting. Antigen levels were also measured by Western blotting. Densitometry was performed to quantify activity and antigen levels (ANOVA followed by Bonferroni post test, means ± SEM, n=4, *P<0.05); only the significant differences between the two genotypes are indicated by *.
Since it has been reported that FSAP can activate pro-urokinase (uPA) we also measured the mRNA levels of uPA in tissue undergoing vascular remodeling. Adductor muscles showed no significant changes in uPA (Figure 7). Increased uPA mRNA, protein and activity was observed in the gastrocnemius muscle of FSAP-/- mice (Figure 7). Thus, angiogenesis in the gastrocnemius muscle, but not collateral formation in the adductor muscle, was associated with differences in the proteolytic pathways between the two genotypes.
Figure 7.
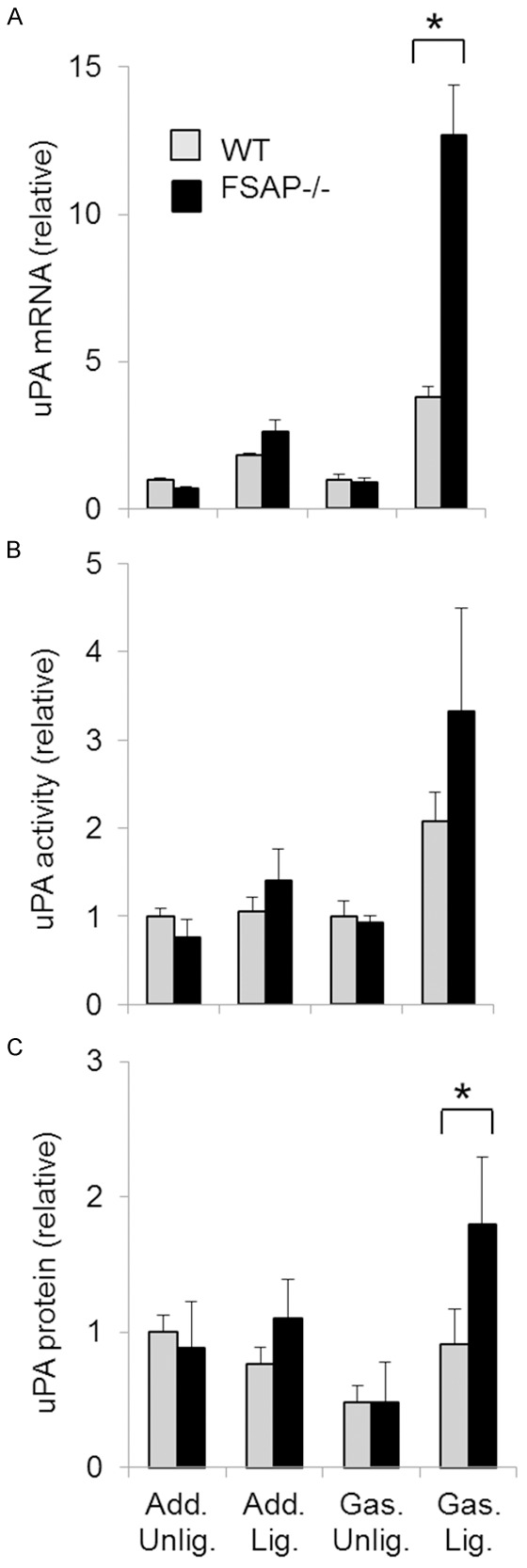
Expression of urokinase in arteriogenesis. In the excised gastrocnemius and adductor muscles of WT- and FSAP-/- mice uPA mRNA (A), uPA activity (B) and uPA antigen (C) were measured at day 3. Quantification was performed as described in legend to Figure 6. (ANOVA followed by Bonferroni post test, means ± SEM, n=4, *P<0.05). Only the significant differences between the two genotypes are indicated by *.
Discussion
FSAP-/- mice display no obvious developmental defects and the histology of the adult liver, lungs and heart showed no abnormalities, indicating that endogenous FSAP has no major role in vascular remodeling processes during development. However, when adult FSAP-/- mice were subjected to femoral artery ligation, which leads to hind limb ischemia and vascular remodeling in the limb, the lack of endogenous FSAP caused distinct changes in the remodeling process. Increased arteriogenesis in the adductor muscle could be measured in terms of an increase in the number of arteries as well as their diameter in FSAP-/- mice. This increased arteriogenesis in FSAP-/- mice compared with WT mice was not measurable using the LPDI method probably because this method is not sensitive enough to determine small changes or that the changes are in the deeper tissue levels that are not readily quantified by LPDI [21]. Upon exogenous FSAP application, we observed a significantly delayed increase in vessel diameter associated with a slower recovery of hind limb perfusion as measured by LPDI. The difference in LPDI results between mice that lack endogenous FSAP and exogenous FSAP application would suggest that the latter has a more robust effect on arteriogenesis. These data provide comparable results to those obtained in the injury induced neointima formation; application of exogenous FSAP inhibited this process [8] whereas it was enhanced in FSAP-/- mice [9]. Both these results confirm a role for FSAP in vascular remodeling in vivo in adult mice. Contrary to the mechanism of collateral vessel growth, in which fluid shear stress is one of the most potent triggers; angiogenesis is mostly triggered by ischemia. After ligation of the femoral artery, ischemia typically occurs in the lower hind limb (gastrocnemius) leading to sprout capillary growth [22]. There was no significant difference in angiogenesis between the two strains of mice. However, capillary density was increased in mice with exogenous FSAP application. We conclude that local intramuscular administration of FSAP in the upper limb resulted in a delay of arteriogenesis after ligation of the femoral artery in mice. This resulted in an increase of ischemia in the calf region and induces angiogenesis in this area. Thus, at least in vivo a lack of endogenous FSAP does not seem to have any direct consequences for angiogenesis which refutes earlier reports obtained using in vitro methods [11-13]. One of the key drivers of vascular remodeling processes is monocytes/macrophages and we found evidence for increased number of macrophages as sites of arteriogenesis at the initial stages of remodeling. We have previously reported that there were no generalized changes in the number of leukocytes in the circulation of FSAP-/- mice [9]. The difference in monocyte accumulation between WT and FSAP-/- mice was only transient at day 3 but this may be sufficient to account for the increased number of arteries measured. The enhanced leukocyte accumulation complements our observations in the mouse stroke [23] model, neointimal lesion formation [9] as well as the liver fibrosis [14] model that we have investigated in these mice. An increased sensitivity of FSAP-/- leukocytes to chemokine CCL-2 in the cremaster vessels using intravital microscopy underscores this repeated observation across various model systems. It is likely that the increased inflammatory response also induces an enhanced secretion of various growth factors, which in turn exaggerate proliferation of smooth muscle cells and endothelial cells [24].
Vascular remodeling is associated with changes in the expression pattern of matrix components, their degradation and reorganization [25]. Proteases play an important role in this and it has been shown that uPA-/- mice [26] as well as MMP-9-/- in bone marrow [27], show altered arteriogenesis in this model. uPA and MMP’s are produced by all vascular cells and in particular by infiltrating leukocytes [28]. We have previously reported that in the wire-induced injury of the femoral artery, the subsequent remodeling is associated with higher activity of MMP-2 and MMP-9 but not uPA [9]. Since changes in proteolytic activity are a consequence of alterations in their expression, activation of the zymogen forms as well as the relative concentration of their inhibitors we have measured these at the mRNA, protein and activity level. In the adductor muscle there were no significant changes in the mRNA, protein levels activity as for MMP-2, MMP-9 or uPA between the genotypes. The changes upon ligation within each genotype were also not significant. Thus, the differences in arteriogenesis in FSAP-/- mice compared to WT mice are not related to changes in these proteases.
On the other hand, gastrocnemius muscle showed robust changes in proteolytic activity associated with angiogenesis. The changes upon ligation within each genotype were significant and confirm similar results from other reports [29]. MMP-9 and uPA activity was increased at day 3 in FSAP-/- mice, compared to WT mice, which was mirrored by increased protein and mRNA. MMP-2 did not follow this pattern. However, the increased MMP-9 and uPA activity in the gastrocnemius muscle of FSAP-/- mice may not be directly related to the process of angiogenesis, since capillary density was not different in this genotype. The use of whole tissue extracts may have confounded the results of these experiments and in situ zymography to measure localized changes in proteolytic activity may have provided more relevant information that better mirror the remodeling changes.
The Marburg-I, loss of function, SNP in the FSAP encoding gene is found in about 5% of Caucasians and is linked to higher odds ratio for carotid stenosis [5] or stroke [6]. We show that FSAP is capable of regulating vascular remodeling processes in a mouse model of under-expression and addition of exogenous FSAP. These findings consolidate the notion that FSAP is a vascular regulatory factor in vivo and that its regulation may be of therapeutic value.
Acknowledgements
We thank Martin Wagner, Christian Deffge, Susanne Tannert-Otto and Thomas Schmidt-Woell for their excellent technical assistance. This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (Bonn, Germany) to SMK (Ka 1468/4-1), Behring Roentgen Stiftung (Marburg, Germany), Excellence Cluster in Cardiopulmonary Sciences (Giessen, Germany) and Helse Sør-Øst (Oslo, Norway) to SMK.
Disclosure of conflict of interest
None.
References
- 1.Simons M, Eichmann A. Molecular controls of arterial morphogenesis. Circ Res. 2015;116:1712–1724. doi: 10.1161/CIRCRESAHA.116.302953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvestre JS, Mallat Z, Tedgui A, Levy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78:242–249. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- 3.Degen A, Millenaar D, Schirmer SH. Therapeutic approaches in coronary collateral circulation. Curr Cardiol Rev. 2014;10:65–72. doi: 10.2174/1573403X113099990027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanse SM, Parahuleva M, Muhl L, Kemkes-Matthes B, Sedding D, Preissner KT. Factor VII-activating protease (FSAP): vascular functions and role in atherosclerosis. Thromb Haemost. 2008;99:286–289. doi: 10.1160/TH07-10-0640. [DOI] [PubMed] [Google Scholar]
- 5.Willeit J, Kiechl S, Weimer T, Mair A, Santer P, Wiedermann CJ, Roemisch J. Marburg I polymorphism of factor VII--activating protease: a prominent risk predictor of carotid stenosis. Circulation. 2003;107:667–670. doi: 10.1161/01.cir.0000055189.18831.b1. [DOI] [PubMed] [Google Scholar]
- 6.Trompet S, Pons D, Kanse SM, de Craen AJ, Ikram MA, Verschuren JJ, Zwinderman AH, Doevendans PA, Tio RA, de Winter RJ, Slagboom PE, Westendorp RG, Jukema JW. Factor VII activating protease polymorphism (G534E) is associated with increased risk for stroke and mortality. Stroke Res Treat. 2011;2011:424759. doi: 10.4061/2011/424759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etscheid M, Muhl L, Pons D, Jukema JW, Koenig H, Kanse SM. The Marburg I polymorphism of factor VII activating protease is associated with low proteolytic and low pro-coagulant activity. Thromb Res. 2012;130:935–941. doi: 10.1016/j.thromres.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Sedding D, Daniel JM, Muhl L, Hersemeyer K, Brunsch H, Kemkes-Matthes B, Braun-Dullaeus RC, Tillmanns H, Weimer T, Preissner KT, Kanse SM. The G534E polymorphism of the gene encoding the factor VII-activating protease is associated with cardiovascular risk due to increased neointima formation. J Exp Med. 2006;203:2801–2807. doi: 10.1084/jem.20052546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel JM, Reichel CA, Schmidt-Woell T, Dutzmann J, Zuchtriegel G, Krombach F, Herold J, Bauersachs J, Sedding DG, Kanse SM. Factor VII-activating protease deficiency promotes neointima formation by enhancing leukocyte accumulation. J Thromb Haemost. 2016;14:2058–2067. doi: 10.1111/jth.13417. [DOI] [PubMed] [Google Scholar]
- 10.Mambetsariev N, Mirzapoiazova T, Mambetsariev B, Sammani S, Lennon FE, Garcia JG, Singleton PA. Hyaluronic Acid binding protein 2 is a novel regulator of vascular integrity. Arterioscler Thromb Vasc Biol. 2010;30:483–490. doi: 10.1161/ATVBAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon JW, Song HS, Moon EJ, Park SY, Son MJ, Jung SY, Kim JT, Nam DH, Choi-Miura NH, Kim KW, Kim YJ. Anti-angiogenic action of plasma hyaluronan binding protein in human umbilical vein endothelial cells. Int J Oncol. 2006;29:209–215. [PubMed] [Google Scholar]
- 12.Etscheid M, Beer N, Kress JA, Seitz R, Dodt J. Inhibition of bFGF/EGF-dependent endothelial cell proliferation by the hyaluronan-binding protease from human plasma. Eur J Cell Biol. 2004;82:597–604. doi: 10.1078/0171-9335-00349. [DOI] [PubMed] [Google Scholar]
- 13.Kress JA, Seitz R, Dodt J, Etscheid M. Induction of intracellular signalling in human endothelial cells by the hyaluronan-binding protease involves two distinct pathways. Biol Chem. 2006;387:1275–1283. doi: 10.1515/BC.2006.158. [DOI] [PubMed] [Google Scholar]
- 14.Borkham-Kamphorst E, Zimmermann HW, Gassler N, Bissels U, Bosio A, Tacke F, Weiskirchen R, Kanse SM. Factor VII activating protease (FSAP) exerts anti-inflammatory and anti-fibrotic effects in liver fibrosis in mice and men. J Hepatol. 2013;58:104–111. doi: 10.1016/j.jhep.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Francke A, Weinert S, Strasser RH, Braun-Dullaeus RC, Herold J. Transplantation of bone marrow derived monocytes: a novel approach for augmentation of arteriogenesis in a murine model of femoral artery ligation. Am J Transl Res. 2013;5:155–169. [PMC free article] [PubMed] [Google Scholar]
- 16.Kannemeier C, Feussner A, Stohr HA, Weisse J, Preissner KT, Romisch J. Factor VII and single-chain plasminogen activator-activating protease: activation and autoactivation of the proenzyme. Eur J Biochem. 2001;268:3789–3796. doi: 10.1046/j.1432-1327.2001.02285.x. [DOI] [PubMed] [Google Scholar]
- 17.Herold J, Francke A, Weinert S, Schmeisser A, Hebel K, Schraven B, Roehl FW, Strasser RH, Braun-Dullaeus RC. Tetanus toxoid-pulsed monocyte vaccination for augmentation of collateral vessel growth. J Am Heart Assoc. 2014;3:e000611. doi: 10.1161/JAHA.113.000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wuestenfeld JC, Herold J, Niese U, Kappert U, Schmeisser A, Strasser RH, Braun-Dullaeus RC. Indocyanine green angiography: a new method to quantify collateral flow in mice. J Vasc Surg. 2008;48:1315–1321. doi: 10.1016/j.jvs.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 19.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hindlimb collateral artery growth (arteriogenesis) Virchows Arch. 2000;436:257–270. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- 20.Subramaniam S, Thielmann I, Morowski M, Pragst I, Sandset PM, Nieswandt B, Etscheid M, Kanse SM. Defective thrombus formation in mice lacking endogenous factor VII activating protease (FSAP) Thromb Haemost. 2015;113:870–880. doi: 10.1160/TH14-06-0519. [DOI] [PubMed] [Google Scholar]
- 21.Kang Y, Choi M, Lee J, Koh GY, Kwon K, Choi C. Quantitative analysis of peripheral tissue perfusion using spatiotemporal molecular dynamics. PLoS One. 2009;4:e4275. doi: 10.1371/journal.pone.0004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 23.Joshi AU, Orset C, Engelhardt B, Baumgart-Vogt E, Gerriets T, Vivien D, Kanse SM. Deficiency of Factor VII activating protease alters the outcome of ischemic stroke in mice. Eur J Neurosci. 2015;41:965–975. doi: 10.1111/ejn.12830. [DOI] [PubMed] [Google Scholar]
- 24.Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]
- 25.Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21:736–744. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Deindl E, Ziegelhoffer T, Kanse SM, Fernandez B, Neubauer E, Carmeliet P, Preissner KT, Schaper W. Receptor-independent role of the urokinase-type plasminogen activator during arteriogenesis. FASEB J. 2003;17:1174–1176. doi: 10.1096/fj.02-0800fje. [DOI] [PubMed] [Google Scholar]
- 27.Meisner JK, Annex BH, Price RJ. Despite normal arteriogenic and angiogenic responses, hind limb perfusion recovery and necrotic and fibroadipose tissue clearance are impaired in matrix metalloproteinase 9-deficient mice. J Vasc Surg. 2015;61:1583–94. e1–10. doi: 10.1016/j.jvs.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 29.Muhs BE, Plitas G, Delgado Y, Ianus I, Shaw JP, Adelman MA, Lamparello P, Shamamian P, Gagne P. Temporal expression and activation of matrix metalloproteinases-2, -9, and membrane type 1-matrix metalloproteinase following acute hindlimb ischemia. J Surg Res. 2003;111:8–15. doi: 10.1016/s0022-4804(02)00034-3. [DOI] [PubMed] [Google Scholar]