Abstract
Tyrosine kinase receptors such as c-Met and its ligands are interesting therapeutic targets that have been reported to be involved in the progression of several types of cancers. Histone deacetylase inhibitor, valproic acid (VPA) is one such compound with promising anti-cancer properties. The current study was designed to evaluate the c-Met activity of VPA in thyroid carcinoma. A total 36 nu/nu mice with SW1736 cells-induced tumours were randomised into three treatment groups (5, 15, 30 mg/kg/day p.o. VPA; n = 9/group). Various cellular and enzymatic assays were performed to evaluate the dose-response relationship of VPA in c-Met inhibition. In vitro assays revealed that VPA (IC50, 5-26 nmol/l) shows c-Met phosphorylation and c-Met-dependent inhibition of cellproliferation. This causes inhibition of downstream signalling pathways in human thyroid cancer cell lines (SW1736, WRO). Additionally, VPA also showed anti-angiogenetic activity in HGF-stimulated endothelial cell. VPA showed significant reduction in tumour size in xenograft model (P = 0.023) with high levels of c-Met expression. The anticancer activity was found to be dose dependent and strongly correlated with c-Met expression. Thus, this novel finding paves way for investigation of new mechanism of action and its validation in clinical settings.
Keywords: Thyroid cancer, c-Met signalling, valproic acid, papillary thyroid carcinoma, RTK inhibitor
Introduction
Oncogenic receptor tyrosine kinases (RTK) targeted inhibitors have emerged as new prospective targets for cancer treatment. These receptors have beenreported to be mutated or exhibit impaired regulation in advanced cancers [1,2]. The latest evidence in favour of this interventional strategy has been highlighted by use of imatinib, a RTK inhibitor in gastrointestinal stromal tumors (GIST), erlotinib in non-small cell lung cancer (NSCLC), trastuzumab in HER-2 positive breast cancer and sutinib in renal cell carcinoma (RCC) [3]. Besidesthese, one commonly altered RTK in advanced tumours is c-Met making it an important in advance tumours [4].
The c-Met family of RTKs does not share structural similarity with other RTKs [5,6]. Additionally it has been reported that conjunction of HGF and c-Met extracellular domain results in phosphorylation and multimerisation of the intracellular region [5,6]. The catalytic activity of the C-Met is facilitated by phosphorylation at c-Met juxtamembrane. This enables docking of the regulatory substrates and also controls the internalisation process [7-9]. Further, the activation of C-Met causes the adaptor proteins such as members of growth factor receptor binding protein family, c-Cbl and Src homology and collagen (Shc) to bind and undergo phosphorylation. This in turn facilitates the activation of signal transducers such as STAT, ERK, Akt and phosphoinositide-3-kinase [10].
C-Met and HGF are expressed in numerous tissuesand for each of the cells their expression is of epithelial and mesenchymal origin [11,12]. C-Met and HGF have been reported to play vital role in several processes which include, but are not limited to, mesenchymal interactions, cell migration, cell proliferation and angiogenesis [13-16]. In recent decades the incidence of thyroid carcinoma has increased worldwide [17,18]. Among thyroid carcinoma phenotypes, the papillary thyroid carcinoma (PTC) accounts for around 90% of all thyroid cancer [19]. Genetic and epigenetic alterations such as inappropriateactivation of oncogeneor dysregulation of tumor suppressor genes are some of the factors that regulate progression of thyroid carcinoma [20]. However, the molecular mechanism and the progression of thyroid carcinoma are still poorly understood and there is a pressing for further systematic studies [20,21]. It is well established in literature that unlike normal tissues, c-Met is highly expressed thyroid carcinoma [22,23].
Valproic acid (VPA, 2-propyl main acid) as an anti-epileptic drug is a short-chain branched fatty acids [24]. Recently, VPA a histone deacetylase (HDAC) inhibitor has received considerable attention for its anti-cancer activity [24]. The anticancer activity of VPA has been shown in solid tumours such as prostate cancer [24]. It has been observed that VPA modulates multiple pathways and causes apoptosis or targets angiogenesis [25,26]. However; the association between the c-Met signalling and VPA has not been studied till date. The present study was therefore designed to investigate the c-Met inhibitory effect of valproic acid in thyroid cancer.
Materials and methods
Chemicals and compounds
Valproic acid and other chemicals were of reagent grade and purchased from Sigma Chemical Co. (St. Louis, Missouri, USA) unless otherwise mentioned. Anti-total human c-Met was obtained from ebiosciences (San Diego, CA, USA. Anti-CD31 and anti-phospho-tyrosine (PY-20) were purchased from Abcam (Cambridge, UK). Mouse specific antibody to c-Met was obtained from Thermofischer scientific (Waltham, Massachusetts, United States). All other antibodies were obtained from R&D systems (Minneapolis, MN, USA). For immunohistochemistry studies anti-phospho-c-Met and anti-Ki67 were obtained from Cell Signaling Technology (Danvers, MA, USA).
Cells
The human thyroid cancers cell lines SW1736, WRO, MDCK and rat cell line FRTL were purchased from X-Zell Biotec Co. Ltd (Bangkok, Thailand). MDAMB231 cells (negative for c-Met; ATCC® HTB 26TM) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) The cells were cultured in RPMI 1640 complete medium supplemented with 10% fetal bovine serum and maintained at 37°C with supply of 10% CO2. All other culture reagents were purchased from Life Technologies, Inc (Carlsbad, California, United States).
Xenograft models in nude mice
Five to eight week old nu/nu mice (n = 36) were usedfor the study. The animals were provided by Beijing Institute of Organism Inspection (Beijing, China). All animals were maintained in asepticcages with free access to food and water. The procedures were performed as per the guidelines of the Care and Use of Laboratory Animals. The study was approved by the Animal Ethical Care and Use Committee of the China-Japan Union Hospital of Jilin University, China.
Apoptosis assay
The apoptosis inducing potential of valproic acid in SW1736 and WRO cells was determined by using commercially available Annexin V-FITC Apoptosis Detection Kit (BD Pharmingen) and analysed by flow cytometry as per manufacturer’s specified protocol. Percentage of annexin positive cells, post treatment with VPA (0.1, 1.0, 10 μM) for 6 h and 24 h respectively were considered as apoptosis positive.
Kinase inhibition and catalytic activity assays
The catalytic activity of c-Met was evaluated by continuous-coupled spectrophotometric assay based on the principle of time-dependent consumption of NADH by ADP. Spectrophotometry readings were obtained at 340 nm. Various concentrations of VPA were added to the test wells for 10 min. Thereafter, the Ki values were obtained by initiating the reaction by the addition of c-Met enzyme. The Half-maximal inhibitory concentration (IC50) was evaluated using poly (Glu, Tyr) peptide as substrate at ATP concentrations or below the Km for each respective kinase.
Cellular kinase phosphorylation assay
Owing to the high expression of c-Met and its role in tumour survival, SW1736 tumor model was used to evaluate the effect of valproic acid on inhibition of tumor growth in vivo. VPA at concentration of 5, 15, 30 mg/kg/day (n = 12 per group) was administered p.o. to nude mice. Thereafter, the SW1736 tumours were harvested at 1, 3, 6, 12 and 24 h and c-Met phosphorylation was carried out using ELISA Kit (R&D systems, Minneapolis, MN, USA) as per manufacturer’s instructions.
Evaluation of cell proliferation/survival
A concentration of 105 SW1736 cells per well were plated in a 96-well plates and subjected to appropriate VPA treatment for 24 to 72 h. The antiproliferative activity was evaluated by a commercially available MTT assay kit (Sigma Aldrich, St. Louis, Missouri, USA) as per manufacturer’s protocol. In an independent experiment, Human umbilical vascular endothelial cells (HUVEC) were seeded in 96-well plates overnight and already identified concentration of valproic acid were added to each well. This was followed by the addition of HGF (100 ng/mL) after 1 h. Finally MTT assay kit (Sigma Aldrich, USA) was to evaluate the antiproliferative effect of VPA.
Cell migration assay
The migration and invasion capability of SW1736 was determined using the commercially available Matrigel invasion kit (Corning Inc, Tewksbury, MA, USA) as per the manufacturer’s specification. Briefly, 4 × 105 cell/ml of trypsinized cells were added to the plate inserts (either migration or invasion chamber) for 24 h. In the lower compartment 0.75 mL of HGF (25 ng/mL) was added to facilitate migration. The migrated cell were treated with paraformaldehyde and counter stained with 4’,6-diamidino-2-phenylindole for 15 min. The cells were washed and number of invading cells was quantified using Image J software (National Institute of Health, USA).
Human dermal microvascular endothelial cells vascular sprouting assay
Endothelial cells vascular sprouting assay was performed using the commercially available human dermal micro vascular endothelial cells (HMVEC-D) (Lonza, Basel, Switzerland), low concentration (500 cells/well) of HMVEC-D were added to the 96-well plates and incubated overnight in EGM-2 medium. The Spheroids obtained were subjected to valproic acid (0.02 µmol/L, 0.2 µmol/L and 2 µmol/L) treatment at standard culture condition on 7th day the cells were observed under an inverted microscope (Eclipse TS100, Nikon, Japan).
Madin-darby canine kidney (MDCK) cell scattering assay
MDCK cells at concentration of 25 cells/ml were seeded in a 96 well culture plates in minimum essential medium (MEM) and incubated. 50 ng/ml of HGF was added to stimulate the cells in presence of various concentration of valproic acid. At the end of the treatment the cells were fixed with paraformaldehyde and stained using crystal violet (0.2%) and analysed using Image J software (National Institute of Health, USA).
Evaluation of c-Met signal transduction and pharmacodynamic
The tumour xenograft model was developed using s.c. injection of 2-5 × 106 of SW1736 cells in the hind-flank region of each mouse. To supply the bioactive human HGF, MRC-5 cells were mixed with SW1736 cells in 2:1 ratio. After 30 days the mice were randomised into four groups (n = 9 per group) and size of tumour was evaluated (every alternate day). The three treatment groups (n = 9 in each) were administered 5 mg/kg/day, 15 mg/kg/day and 30 mg/kg/day VPA respectively, and the control group did not receive any therapeutic intervention. Nude mice with xenograft tumour volume in the range of 100-500 mm3 were included in the pharmacodynamics study.
The tumour size and volume were recorded using Vernier calipers. Total protein was isolated using TPETM kit (GE Biosciences, Sunnyvale, California, USA) from tumour lysate to carry out the immunoblotting and immunoassay procedures.
Immunohistochemistry
The resurrected tumour tissue from nude mice were fixed in paraformaldehyde for 24 h and then fixed in paraffin. Four micron thick sections of the paraffin embedded tissue were obtained on a poly-Lysine coated slide. After antigen retrieval, primary Ki-67 antibody (anti-c-Met (Abcam, Cambridge, USA; Cat# ab15580) was added for overnight followed by secondary antibody (anti-rabbit). The sections were counterstained with haematoxylin and observed under light microscope (Nikon, Japan), the image were quantified using Image J software (National Institute of Health, USA).
Statistical analysis
Statistical analysis was performed using SigmaStat® software version 3.5 (Systat Software Inc, San Jose, CA, USA). Statistical treatment was performed by using one-way ANOVA, Mann-Whitney U-test, and Duncan’s multiple range tests. Results were expressed as mean ± SEM, and significance was defined as P≤0.05.
Results
Valproic acid showed inhibition of catalytic activity of c-Met
Valproic acid showed a mean Ki of 3.8 nmol/L in inhibiting human c-Met kinase suggesting a potent c-Met inhibitory activity. In FRTL cells, VPA showed IC50 of 4 nmol/L.
Valproic acid inhibited c-Met-dependent cancer cells
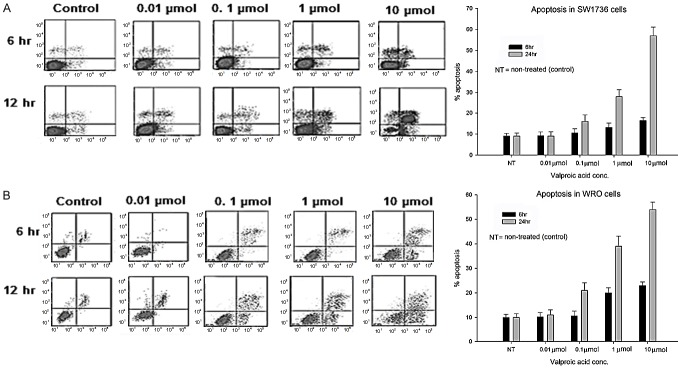
Treatment of SW1736 and WRO cell lines with 1 µmol and 10 µmol for 24 h showed significant increase in apoptosis (P = 0.029) as compared to non-treated (control) cells. However, no significant increase in apoptotic cells was observed for the same concentration of VPA but at 6 h of incubation (Figure 1A, 1B). The control cell (non-treated) cells showed apoptosis of 10%. At 10 µM the apoptosis in SW1736 and WRO cells was 17% and 24% respectively. There was no statistical increase in apoptosis on treatment with 0.01 and 0.1 µM of VPA in both SW1736 and WRO cell lines.
Figure 1.
Induction of apoptosis by different concentrations (0.1, 1.0, 10 μmol) of VA (A) SW1736 and (B) WRO cells were treated with indicated concentrations of VPA for 6 and 24 hrs. Results expressed as Mean ± SD. *P<0.05.
Valaproic acid showed an IC50 of 10 nmol/L and inhibited anaplastic thyroid carcinoma (SW1736) cell migration and invasion. In HUVEC studies, valproic acid inhibited phosphorylation of c-Met invigorated by HGF (IC50, 10.5 nmol/l), cell survival (IC50, 12.8 nmol/l), and matrigel invasion (IC50, 36 nmol/l) (Figure 2). On the basis of the inhibitory activity of c-Met phosphorylation and its correlation with IC50, we assumed that the pharmacological activity of valproic acid is mediated through c-Met inhibition.
Figure 2.
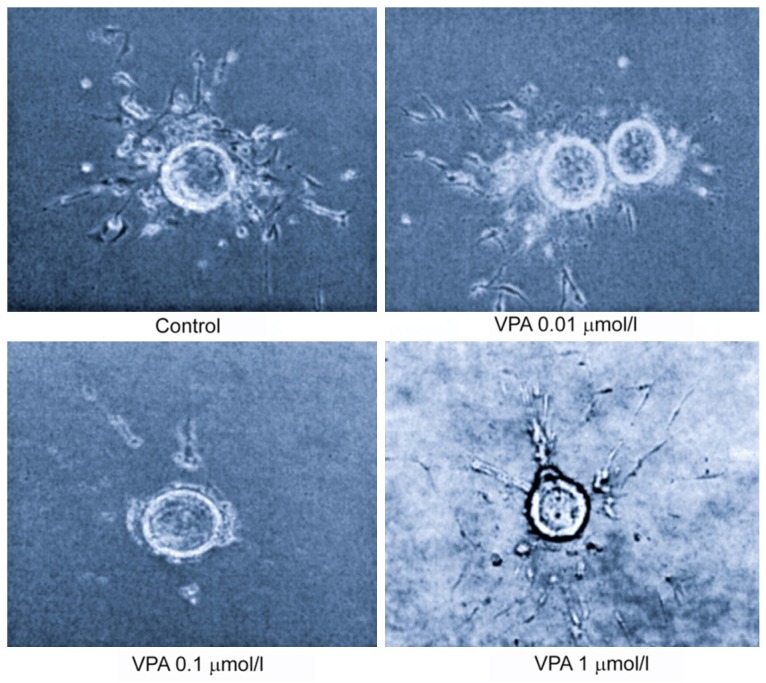
VPA inhibited tubulogenesis in HMVEC endothelial cell at indicated concentrations. Using a collagen-fibrin matrix, the HMVEC cells were plated and tubulogenesis was observed within 7 days after plating.
c-Met phosphorylation inhibition by valproic acid in xenograft model
Administration of 30 mg/kg of VPA resulted in 100% inhibition of c-Met activity after 24 h, which correlated with total inhibition of tumour growth (P = 0.023). Concentration of 15 mg/Kg showed only partial inhibition of both C-Met and tumour growth, further supporting the hypothesis that the inhibitory activity of valproic acid is dose dependent (Figure 3A). At 5 mg/kg/day the inhibition of tumour growth (P = 0.062) correlated with 25-50% inhibition of c-Met phosphorylation.
Figure 3.
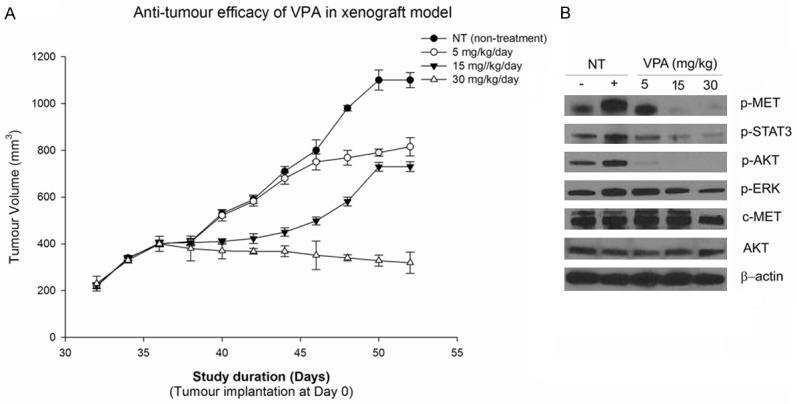
A. Valproic acid shows inhibition of tumour growth in SW1736 xenograft model. B. Valproic acid induced inhibition of c-Met phosphorylation and downstream signaling pathways in SW1736 tumors.
Similarly at 15 mg/kg/day 50% inhibition of tumour growth was observed, which correlated with 75-90% inhibition of c-Met phosphorylation at 1-6 h. The impediment of signalling events due to inhibition of downstream targets of c-Met were found to be pharmacologically relevant and correlated with anti-tumour efficacy of VPA.
In vivo effect of valproic acid on tumour related signal transduction pathways
The c-Met-dependent signalling effect of valproic acid was investigated through western blotting using xenograft tumour tissue. The study showed significant inhibition of phosphorylated c-Met and proteins such as p-AKT, STAT3, ERK and AKT in SW1736 tumors after treatment with valproic acid (Figure 3B). The inhibition of these signalling proteins was found to correlate with antitumor efficacy and inhibition of c-Met phosphorylation rendered by valproic acid (Figure 3B).
Evaluation of pharmacological activity of valproic acid in c-Met-dependent models
The effect of VPA on caspase-3 activation (apoptosis) and tumor mitotic index (Ki67) was evaluated through immunohistochemistry. There was a three times decrease in the Ki67 expression on day 18 at concentration of 30 mg/kg/day of VPA in SW1736 cell lines which correlated with tumour efficacy (Figure 4).
Figure 4.
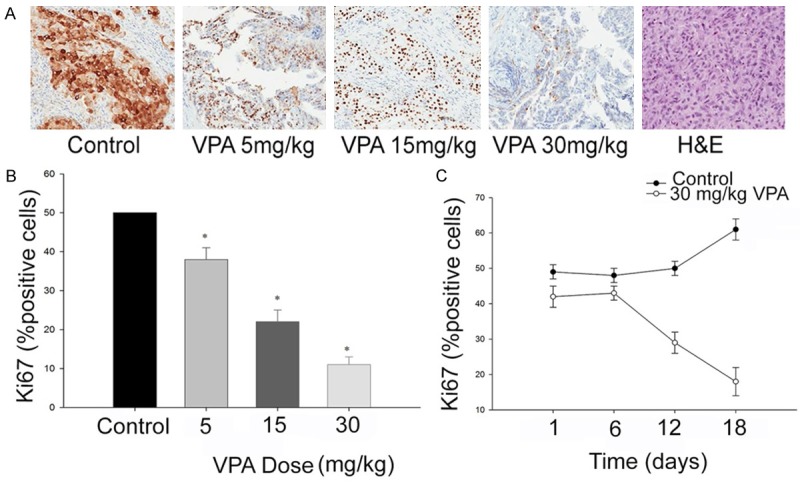
A. Immunohistochemical evaluation of Ki67 expression after administration of valproic acid. B. Dose dependent inhibition of c-Met phosphorylation in xenograft model. C. Inhibition of tumour cell proliferation. *, P≤0.01, shows values are statistically significant.
The dose dependent anti-angiogenic activity of VPA in vitro was evaluated through change in the microvessel density (MVD) through immune staining for CD31. VPA showed dose dependent inhibition of CD31-positive microvessel at doses of 5, 15 and 30 mg/kg in SW1736 tumour models (Figure 5). However, the MVD model was only evaluated for short period. Administration of valproic acid showed reduction in plasma VEGF and IL-8, observed due to the anti-angiogenic effects on the tumour vessels or may be an indirect effect. There was a dose-dependent decrease in the plasma levels of VEGF and IL-8 in the SW1736 model (P<0.05). The reduction in the cytokines (VEGF and IL-8) correlated well with the reduction in tumour size in the xenograft model.
Figure 5.
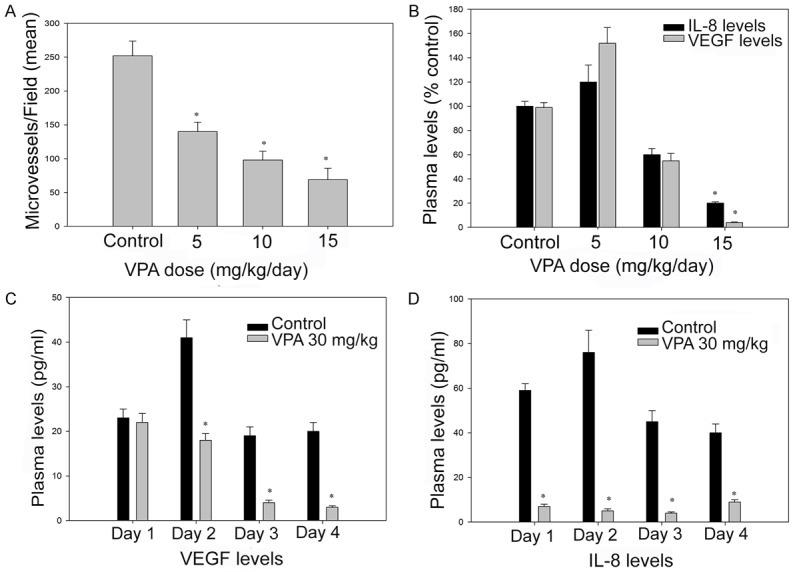
Efficacy of valproic acid on tumour MVD (A and B) and secretion of proangiogenic factors (C and D) in SW1736 induced xenograft model. Athymic mice bearing established SW1736 xenografts were administered Valproic acid p.o. at the indicated dose levels or DMSO (control). *, P≤0.05, shows that the values are statistically significant.
Discussion
Our study highlights the c-Met signalling activity of VPA and its effect on the inhibition RTK targets in thyroid cancer. Valproic acid was found to exhibit dynamic effect on inhibition of c-Met phosphorylation in human thyroid cancer cell lines and xenograft model. Moreover, the c-Met inhibitory activity of VPA correlated to antiangiogenic activity in nude mice model of thyroid carcinoma. Our results are well supported by previous studies investigating VPA in thyroid cancer [27,28]. While as some studies have shown that VPA promotes the Erk1/2 activation [28-30], still others have reported Erk1/2 inhibitory activity of VPA [31]. However, the present study for first times reports the c-Met signalling activity of VPA.
The disease model utilized in this study for studying the pharmacology of c-Met dependent kinase do not express ALK indicating that the pharmacological activity of VPA is due to the inhibition of c-Met. However, the fact that some wild-type ALK is expressed in endothelial cells and therefore its attribution towards pharmacological activity of VPA cannot be neglected [32]. This study shows dose dependent inhibition of thyroid cancer cell lines and induction of apoptosis in experimental model (mice xenograft). Therefore, the c-Met inhibitory mechanism may directly account for inhibitory effect of VPA on tumour cells. Shen et al. showed that down-regulation of bcl-2 and bcl-xl in thyroid cancer using VPA [33]. Therefore investigation into regulation of pro-apoptotic genes in thyroid cancer in responseto VPA may enable exploration of additional mechanisms.
VPA also showed inhibition of migration and survival of HGF-stimulated endothelial cells and inhibition of tubulogenesisas well. This suggests an anti-angiogeneic effect of VPA in cancer cells. However, its relation with VEGF pathway needs to be further investigated [34]. Of note, antineoplastic activity of VPA has been reported to sustain upto seven days, in our study we investigated it only for 24 h and may form one of the limitations of the current study [35,36].
The study confirms the pharmacologic effects of valproic acid on human thyroid carcinoma. Its established safety profile in patients with seizures and its strong antineoplastic and antiangiogenic activity support the use of this drug in a clinical setting. In addition, the role of c-Met in thyroid cancer has already been established. However, a proof of concept clinical trial will further validate its efficacy in patients with thyroid carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 2.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 3.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312:1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 4.Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 5.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 6.Naldini L, Vigna E, Narsimhan RP, Gaudino G, Zarnegar R, Michalopoulos GK, Comoglio PM. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- 7.Fournier TM, Lamorte L, Maroun CR, Lupher M, Band H, Langdon W, Park M. Cbltransforming variants trigger a cascade of molecular alterations that lead to epithelial mesenchymal conversion. Mol Biol Cell. 2000;11:3397–3410. doi: 10.1091/mbc.11.10.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla ZP, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues GA, Park M. Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene. 1994;9:2019–2027. [PubMed] [Google Scholar]
- 10.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 11.Di Renzo MF, Narsimhan RP, Olivero M, Bretti S, Giordano S, Medico E, Gaglia P, Zara P, Comoglio PM. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991;6:1997–2003. [PubMed] [Google Scholar]
- 12.Sonnenberg E, Weidner KM, Birchmeier C. Expression of the met-receptor and its ligand, HGF-SF during mouse embryogenesis. EXS. 1993;65:381–394. [PubMed] [Google Scholar]
- 13.Comoglio PM, Trusolino L. Invasive growth: from development to metastasis. J Clin Invest. 2002;109:857–862. doi: 10.1172/JCI15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 16.Tsarfaty I, Rong S, Resau JH, Rulong S, da Silva PP, Vande Woude GF. The Met proto-oncogene mesenchymal to epithelial cell conversion. Science. 1994;263:98–101. doi: 10.1126/science.7505952. [DOI] [PubMed] [Google Scholar]
- 17.Di Renzo MF, Olivero M, Martone T, Maffe A, Maggiora P, Stefani AD, Valente G, Giordano S, Cortesina G, Comoglio PM. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19:1547–1555. doi: 10.1038/sj.onc.1203455. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Han SU, Cho H, Jennings B, Gerrard B, Dean M, Schmidt L, Zbar B, Vande Woude GF. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947–4953. doi: 10.1038/sj.onc.1203874. [DOI] [PubMed] [Google Scholar]
- 19.Kuniyasu H, Yasui W, Kitadai Y, Yokozaki H, Ito H, Tahara E. Frequent amplification of the c-met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun. 1992;189:227–232. doi: 10.1016/0006-291x(92)91548-5. [DOI] [PubMed] [Google Scholar]
- 20.Bellusci S, Moens G, Gaudino G, Comoglio P, Nakamura T, Thiery JP, Jouanneau J. Creation of an hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene. 1994;9:1091–1099. [PubMed] [Google Scholar]
- 21.Jeffers M, Rong S, Anver M, Vande Woude GF. Autocrine hepatocyte growth factor/scatter factor-Met signaling induces transformation and the invasive/metastastic phenotype in C127 cells. Oncogene. 1996;13:853–856. [PubMed] [Google Scholar]
- 22.Jeffers M, Schmidt L, Nakaigawa N, Webb CP, Weirich G, Kishida T, Zbar B, Vande Woude GF. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci U S A. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin DT, Wu W, Li M, Wang QE, Li H, Wang Y, Tang Y, Xing M. DKK3 is a potential tumor suppressor gene in papillary thyroid carcinoma. Endocr Relat Cancer. 2013;20:507–514. doi: 10.1530/ERC-13-0053. [DOI] [PubMed] [Google Scholar]
- 24.Rahbari R, Kitano M, Zhang L, Bommareddi S, Kebebew E. RTN4IP1 is down-regulated in thyroid cancer and has tumor-suppressive function. J Clin Endocrinol Metab. 2013;98:E446–E454. doi: 10.1210/jc.2012-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulikova S, Chmelarova M, Petera J, Palicka V, Paulik A. Hypermethylation of RAD51L3 and XRCC2 genes to predict late toxicity in chemoradiotherapy-treated cervical cancer patients. Folia Biol (Praha) 2013;59:240–245. doi: 10.14712/fb2013059060240. [DOI] [PubMed] [Google Scholar]
- 26.Mineo R, Costantino A, Frasca F, Sciacca L, Russo S, Vigneri R, Belfiore A. Activation of the hepatocyte growth factor (HGF)-Met system in papillary thyroid cancer: biological effects of HGF in thyroid cancer cells depend on Met expression levels. Endocrinology. 2004;145:4355–4365. doi: 10.1210/en.2003-1762. [DOI] [PubMed] [Google Scholar]
- 27.Bergstrom JD, Hermansson A, Diaz de ST, Heldin NE. Non-autocrine, constitutive activation of Met in human anaplastic thyroid carcinoma cells in culture. Br J Cancer. 1999;80:650–656. doi: 10.1038/sj.bjc.6690406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuniyasu H, Yasui W, Kitadai Y, Yokozaki H, Ito H, Tahara E. Frequent amplification of the c-met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun. 1992;189:227–232. doi: 10.1016/0006-291x(92)91548-5. [DOI] [PubMed] [Google Scholar]
- 29.Kuniyasu H, Yasui W, Kitadai Y, Yokozaki H, Ito H, Tahara E. Frequent amplification of the c-met gene in scirrhous type stomach cancer. Biochem Biophys Res Commun. 1992;189:227–232. doi: 10.1016/0006-291x(92)91548-5. [DOI] [PubMed] [Google Scholar]
- 30.Bellusci S, Moens G, Gaudino G, Comoglio P, Nakamura T, Thiery JP, Jouanneau J. Creation of an hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene. 1994;9:1091–1099. [PubMed] [Google Scholar]
- 31.Fortunati N, Catalano MG, Arena K, Brignardello E, Piovesan A, Boccuzzi G. Valproic acid induces the expression of the Na+/I- symporter and iodine uptake in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2004;89:1006–1009. doi: 10.1210/jc.2003-031407. [DOI] [PubMed] [Google Scholar]
- 32.Catalano MG, Fortunati N, Pugliese M, Costantino L, Poli R, Bosco O, Boccuzzi G. Valproic acid induces apoptosis and cell cycle arrest in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2005;90:1383–1389. doi: 10.1210/jc.2004-1355. [DOI] [PubMed] [Google Scholar]
- 33.Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vulcano F, Ciccarelli C, Mattia G, Marampon F, Giampiero M, Milazzo L, Pascuccio M, Zani BM, Giampaolo A, Hassan HJ. HDAC inhibition is associated to valproic acid induction of early megakaryocytic markers. Exp Cell Res. 2006;312:1590–1597. doi: 10.1016/j.yexcr.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Shen WT, Wong TS, Chung WY, Wong MG, Kebebew E, Duh QY, Clark OH. Valproic acid inhibits growth, induces apoptosis, and modulates apoptosis-regulatory and differentiation gene expression in human thyroid cancer cells. Surgery. 2005;138:979–984. doi: 10.1016/j.surg.2005.09.019. discussion 984-5. [DOI] [PubMed] [Google Scholar]
- 36.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]