Abstract
Food restriction and weight loss result in reduced plasma leptin, which is associated with a pleiotropic biologic response. However, because weight loss itself is also associated with changes in numerous other humoral and metabolic signals, it can be difficult to determine the precise features of the biologic response to acute leptin deficiency. To study this response in the absence of changes in nutritional state, we have developed a protocol that allows such analysis in normal, non-food-restricted animals. Wild-type mice are treated with high-dose leptin until fat mass is depleted and, as a consequence, endogenous leptin production is reduced. At this point, exogenous leptin is abruptly withdrawn, thus inducing a state of leptin deficiency in otherwise normal mice. Leptin deficiency is sustained by feeding the animals only as much as they consumed voluntarily before leptin withdrawal. The biologic response to leptin deficiency induced in this manner includes altered neuropeptide levels, decreased energy expenditure, and impaired reproductive and immune function. Replacement of leptin at physiological concentrations after withdrawal of high-dosage leptin blunts, but does not completely block, the hyperphagia and weight regain caused by acute leptin deficiency, nor does it correct the resulting reproductive and immune dysfunction. This suggests that high-dosage leptin treatment induces a state of partial leptin resistance. In aggregate, these studies establish the role of acute hypoleptinemia in regulating energy balance, the immune system, and reproductive function, and further suggest that high-dosage leptin treatment can induce a state of acquired leptin resistance.
Keywords: corticosterone, metabolism
The adipocyte-derived hormone leptin functions as an afferent signal in a negative-feedback loop that maintains constancy of adipose mass (1) and regulates the adaptive neuroendocrine and metabolic response to alterations in nutritional state (2). In animals and humans, mutations in leptin or its receptor are associated with profound metabolic and physiologic abnormalities, including hyperphagia, neuroendocrine dysfunction, and alterations of the immune system (3–7). Many of these abnormalities are also evident with leptin deficiency arising from other etiologies, including food restriction (8) and lipodystrophy (9, 10), the congenital absence of adipose tissue. However, in each of these syndromes, the contribution of leptin deficiency relative to other associated features of these conditions is unclear. In patients with leptin mutations, the profound obesity, hyperphagia, and the absence of leptin during development are also likely to contribute to the syndrome. Similarly, lipodystrophy is associated with a constellation of other abnormalities, resulting from the absence of adipose tissue as an energy depot and the lack of other plasma proteins normally produced by adipocytes (11). Starvation causes the loss of both lean and fat mass (12), and in addition to a rapid drop in the levels of circulating leptin, results in marked changes in the levels of a number of other hormonal and metabolic signals. Thus, whereas leptin treatment largely corrects starvation-induced immune dysfunction (13) and partially blunts the neuroendocrine response to starvation, it does not correct starvation-induced hypoglycemia, hypoinsulinemia, hyperphagia, or fully block weight regain in animals allowed to feed ad libitum (2).
For these reasons, it has been difficult to precisely delineate the direct physiologic response to leptin deficiency in otherwise normal adult animals. To characterize the specific biological response to reduced plasma leptin, we have developed a leptin withdrawal protocol that results in a physiologic reduction in the levels of this hormone in the absence of the possibly confounding alterations associated with congenital leptin deficiency, lipodystrophy, or food restriction. Here, we report the use of this protocol to study the effects of a physiologic decrease in leptin on energy balance and neuroendocrine and immune function.
Methods
Animal Maintenance. Female C57BL6/J and C57BL6/J ob/ob mice (8–10 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in individual cages in temperature-controlled rooms. All animals were subjected to a 12-h light:dark cycle, and unless otherwise noted, all procedures and measurements were conducted between 0900–1200 hours. Animal care and experiments were conducted in accordance with approved institutional guidelines of The Rockefeller University.
Leptin Administration. Alzet miniosmotic pumps with an exchange rate of 0.5 μl/h (Alza) were filled aseptically with either sterile PBS solution or leptin (Amgen, Thousand Oaks, CA) at a concentration of 500 ng/μl for ob/ob mice and 5 μg/μl for lean mice. The pumps were incubated in a sterile 0.9% NaCl solution at 37°C for a minimum of 6 h before surgery. Mice were anesthetized with methoxyflurane, and the pump was implanted s.c. Leptin was withdrawn or replaced by surgically removing or replacing the pumps after 8 days of leptin treatment.
Calorimetry. Energy expenditure was measured by using the Columbus Instruments Oxymax system (Columbus Instruments, Columbus, OH). Groups of four mice were housed individually in plastic chambers with unlimited access to food and water, except for leptin-normocaloric (leptin-NC) mice, which were fed a defined amount of food per 24-h period. Mice were acclimated to the chambers for 48 h before measurements began and for 2 h any time the cage was opened for replenishing food. Measurements were recorded every 15 min for a duration of 1 min and included O2 consumption, CO2 output, and linear and vertical movement by means of light beam-break recordings in three planes.
Serum Assays. Mice were anesthetized with methoxyflurane, and blood was collected from the retroorbital venous plexus by using heparinized microcapillary tubes. Plasma was separated by centrifugation for 10 min at 5,000 × g and used for all subsequent assays. Plasma leptin was measured by using the Quantikine murine leptin ELISA (R & D Systems). Insulin and corticosterone were measured by using the Mercodia ultrasensitive mouse insulin ELISA and OCTEIA corticosterone ELISA kits, respectively (ALPCO Diagnostics, Windham, NH). Leutinizing hormone (LH) was measured by using two LH RIA kits (ICN Biomedicals, Costa Mesa, CA, and ALPCO Diagnostics). Glucose and triglycerides were analyzed by using a trinder enzymatic assay and Infinity Triglycerides Reagent, respectively (Sigma Diagnostics, St. Louis). Free fatty acids (FFAs) were measured by using the NEFA C FFA kit (Wako Chemicals, Neuss, Germany).
Quantitative Real-Time PCR. Mice were killed, and the hypothalamus was removed and immediately immersed in liquid nitrogen before storage at -80 C. RNA was extracted from individual hypothalami by using TRIzol Reagent (Invitrogen). Single-stranded cDNA used as template for real-time PCR was generated by using TaqMan reverse transcription reagents (Applied Biosystems). Fluorescent probes containing a 5′ 6-carboxytetramethyl-rhodamine fluorescein fluorophore and 3′ tetramethylrhodamine quencher were made by Applied Biosystems, and oligonucleotides flanking the fluorescent probe binding sites were generated by Sigma Genosys (The Woodlands, TX). Sequences are shown in Table 2, which is published as supporting information on the PNAS web site. Quantitative real-time PCR was carried out in duplicate by using TaqMan universal PCR master mix (Applied Biosystems) on an Applied Biosystems ABI Prism 7700 sequence detector following the manufacturer's protocol.
FACS Analysis. The thymus and spleen were excised, and single-cell suspensions of splenocytes or thymocytes were generated by teasing out tissue with a sterile needle and passing it through nylon gauze. Red blood cells were lysed with ammonium chloride, and cell numbers determined by averaging the count in three separate areas of a hemocytometer. Flow cytometry followed routine procedures by using 1 × 105 cells per sample. To measure expression of CD4 and CD8, cells were labeled with either a FITC- or a phycoerythrin-conjugated antibody (Caltag, Burlingame, CA). Flow cytometric analysis was conducted on a FACSCalibur and analyzed by using the cellquest program (Becton Dickinson, Mountain View, CA). For quantitation of apoptosis, cells were stained with FITC-labeled Annexin V and propidium iodide and evaluated by flow cytometry.
Statistical Analysis. Data are expressed as the means ± SE. Statistical significance was assessed by Student's paired t tests with a significance cutoff of P ≤ 0.05 or less.
Results
Leptin Withdrawal Induces Acute Leptin Deficiency in Wild-Type Mice.
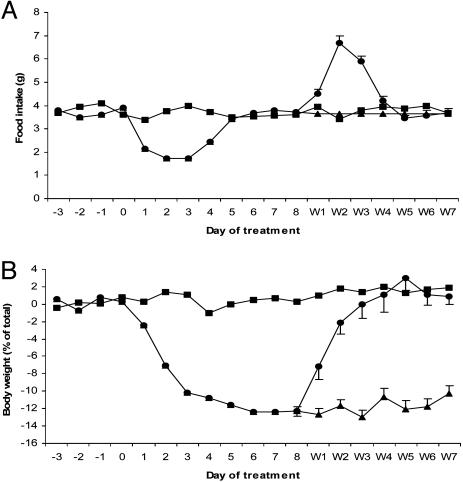
Wild-type mice were treated with a high dose of leptin (2.5 μg/h) for 8 days until all measurable fat mass was depleted, followed by the abrupt cessation of treatment (Fig. 1A). At this point, half of the mice were allowed to feed freely [designated leptin-free feeding (FF)], and the other half were given the same amount of food they consumed voluntarily before leptin withdrawal (leptin-NC). By essentially pair feeding the leptin-NC mice to their own individual prewithdrawal intake, we can study the effects of leptin deficiency in wild-type animals whose caloric intake has not changed before and after leptin withdrawal. Thus, unlike food restriction, the principle variable being altered in leptin-NC mice is circulating plasma leptin, thus allowing a direct analysis of the effects of acute leptin deficiency.
Fig. 1.
Food intake and body weight in response to leptin treatment and subsequent withdrawal. (A) Daily food intake in leptin-FF mice (•), leptin-NC mice (▴), and PBS control mice (▪). (B) Body mass of leptin-FF mice, leptin-NC mice, and PBS control mice. Leptin treatment begins on day 0 and withdrawal begins on day 8. [n = 50 per group and decreases by 6–10 per day (days 1–4 and 7), beginning on day 8 as animals were killed for analysis.]
After leptin withdrawal, leptin-FF mice became markedly hyperphagic (Fig. 1 A) and rapidly recovered fat mass over the course of 4 days (Fig. 1B). At 24 h after leptin withdrawal, plasma leptin was markedly reduced in both leptin-FF and -NC mice relative to controls (Table 1). As leptin-FF mice regained body mass, plasma leptin also increased, returning to baseline levels within 7 days. In contrast, leptin-NC mice did not regain any weight and maintained reduced plasma leptin for the 7 days after leptin withdrawal (Table 1). The amount of leptin mRNA in white adipose tissue paralleled the plasma levels of the hormone (Fig. 6, which is published as supporting information on the PNAS web site).
Table 1. Physiological effects of leptin withdrawal.
| Plasma factor | Group | Day 8 | Day W1 | Day W2 | Day W3 | Day W4 | Day W7 |
|---|---|---|---|---|---|---|---|
| Leptin, ng/ml | FF | 161 ± 13.8† | 0.99 ± 0.08† | 2.11 ± 0.06† | 2.66 ± 0.15† | 4.2 ± 0.30 | 4.92 ± 0.21 |
| NC | 161 ± 13.8† | 0.67 ± 0.04† | 0.54 ± 0.01* | 1.02 ± 0.03* | 0.96 ± 0.05* | 1.03 ± 0.04* | |
| PBS | 5.72 ± 0.92 | 4.97 ± 0.55 | 4.94 ± 0.52 | 5.27 ± 0.74 | 5.60 ± 0.84 | 5.39 ± 0.41 | |
| Insulin, ng/ml | FF | 0.31 ± 0.04† | 1.49 ± 0.51 | 1.76 ± 0.51 | 1.61 ± 0.37 | 1.34 ± 0.15 | 1.20 ± 0.20 |
| NC | 0.31 ± 0.04† | 0.22 ± 0.02* | 0.23 ± 0.03* | 0.41 ± 0.05* | 0.24 ± 0.04* | 0.37 ± 0.04* | |
| PBS | 1.28 ± 0.49 | 1.12 ± 0.24 | 1.05 ± 0.35 | 0.94 ± 0.21 | 1.15 ± 0.22 | 1.24 ± 0.19 | |
| Corticosterone, ng/ml | FF | 91.2 ± 10.1 | 59.2 ± 6.30# | 59.2 ± 4.34# | 65.9 ± 9.76 | 72.8 ± 3.57# | 99.6 ± 6.24 |
| NC | 91.2 ± 10.1 | 117 ± 6.11 | 101.8 ± 4.31 | 128.1 ± 12.1 | 144.4 ± 18.2 | 110.8 ± 2.36 | |
| PBS | 104.2 ± 8.74 | 98.0 ± 3.99 | 94 ± 9.27 | 93.6 ± 10.9 | 106.2 ± 8.24 | 105.2 ± 8.74 | |
| Triglycerides, mg/dl | FF | 90 ± 6.30 | 127.4 ± 12.0# | 206 ± 19.0# | 119 ± 6.90# | 104 ± 6.80 | 92.0 ± 7.30 |
| NC | 90 ± 6.30 | 86.0 ± 10.5 | 89.0 ± 8.60 | 86.0 ± 5.40 | 91.0 ± 6.10 | 89.0 ± 8.90 | |
| PBS | 84.6 ± 6.12 | 91.0 ± 8.18 | 86.0 ± 10.5 | 88.0 ± 5.60 | 93.0 ± 6.30 | 90.5 ± 6.50 | |
| FFAs, μM | FF | 107 ± 11.8 | 740 ± 84.1 | 569 ± 48.1 | 589 ± 64.0 | 601 ± 53.9 | 567 ± 68.0 |
| NC | 107 ± 11.8 | 798 ± 92.7 | 805 ± 86.6 | 921 ± 120* | 905 ± 93.3* | 850 ± 105* | |
| PBS | 553 ± 64.6 | 605 ± 58.0 | 589 ± 84.0 | 609 ± 38.1 | 584 ± 67.0 | 554 ± 54.2 | |
| LH, ng/ml | FF | 1.81 ± 0.23 | 1.75 ± 0.18 | 1.95 ± 0.14 | 1.86 ± 0.16 | 1.76 ± 0.10 | 1.79 ± 0.24 |
| NC | 1.81 ± 0.23 | 1.68 ± 0.21 | 1.34 ± 0.20* | 1.21 ± 0.24* | 1.32 ± 0.23* | 1.42 ± 0.19 | |
| PBS | 1.76 ± 0.21 | 1.89 ± 0.19 | 2.12 ± 0.31 | 1.79 ± 0.19 | 1.85 ± 0.17 | 1.85 ± 0.27 |
n = 6 per group. Data are ± SEM. *, P < 0.05 vs. PBS, FF; †, P < 0.05 vs. PBS; #, P < 0.05 vs. PBS, NC. W, day of withdrawal.
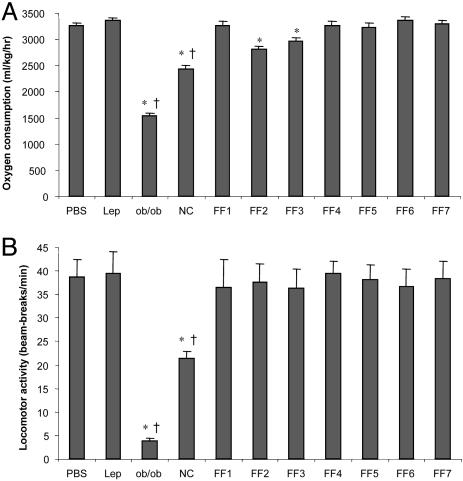
Metabolic Function. Energy expenditure was measured in both the leptin-FF and -NC groups by using indirect calorimetry. Whereas daily energy expenditure was reduced slightly in leptin-FF mice in the first 72 h after leptin withdrawal (Fig. 2A), leptin-NC mice exhibited a sustained 25% reduction for 7 days after leptin withdrawal. Locomotor activity was measured concurrently by light beam-break analysis, revealing a sustained 50% reduction in activity in leptin-NC mice relative to PBS controls (Fig. 2B). Locomotor activity was unaffected in leptin-FF mice. Additionally, core body temperature was measured daily with a YSI Precision 4600 thermometer with rectal probe (YSI Precision Temperature Group, Dayton, OH) and was unchanged in both leptin-FF and -NC mice relative to controls at all time points (data not shown).
Fig. 2.
Effect of leptin withdrawal on 24-h energy expenditure. Energy expenditure and locomotor activity was monitored on days 6–8 in leptin-treated mice (Lep), days 1–7 after leptin withdrawal in leptin-FF (FF1–7) and leptin-NC (NC) mice, days 1–3 after leptin withdrawal in PBS controls, and for 72 consecutive hours in ob/ob mice (n = 4 per group for all groups). Because the PBS control mice, ob/ob mice, and leptin-NC mice showed little day-to-day variability in energy expenditure and activity, all data were averaged and reported as a single value. The data for leptin-FF mice vary over time and are thus reported at 24-h intervals. (A) Energy expenditure. (B) Locomotor activity (*, P < 0.01 vs. PBS; †, P < 0.05 vs. FF at every time point).
Plasma glucose was unchanged in both leptin-FF and -NC mice relative to PBS controls at all time points (data not shown). As reported previously (14, 15), sustained administration of leptin resulted in a significant drop in plasma insulin and FFA levels as measured on day 8 of leptin treatment (Table 1). Triglycerides were unaffected by leptin treatment. FFAs returned to control levels within 24 h after leptin withdrawal in leptin-FF mice. There was a marked increase in plasma insulin and triglycerides for 3–4 days after leptin withdrawal, corresponding to the period of hyperphagia (days W1–W4, Fig. 1 A). In contrast, plasma insulin levels in leptin-NC mice remained, on average, 50–80% lower than controls, while plasma FFAs were increased 30% for 48 h after leptin withdrawal (Table 1).
Leptin-NC mice did not show any significant difference in plasma triglycerides relative to controls.
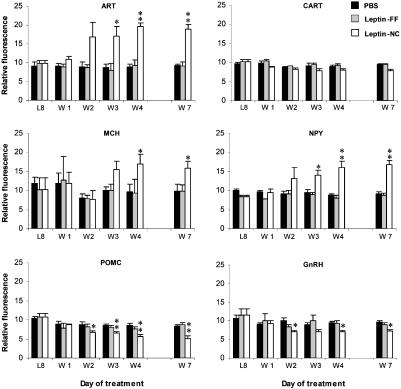
Neuroendocrine Function. Reproductive function of mice after leptin withdrawal was assessed by monitoring changes in estrus cycle. Mice at the end of estrus just before withdrawal of leptin were monitored with daily pap smears to determine the time until the onset of the next estrus phase. Leptin-FF mice exhibited a normal 4–5 day cycle after leptin withdrawal. In contrast, the estrus cycle of all leptin-NC mice was completely arrested for the full 7 days after leptin withdrawal. Leptin-NC mice that were pair-fed for 7 days after leptin withdrawal and then allowed to feed freely showed a restoration of estrus cycle with an average additional delay of ≈4.9 days (Fig. 7, which is published as supporting information on the PNAS web site). Leptin has been shown to affect reproductive function at the level of the hypothalamus, possibly by regulating the levels of gonadotropin-releasing hormone (GnRH) and its target, LH (16). As measured by TaqMan quantitative real-time RT-PCR, leptin-NC mice showed a slight, but significant decrease in hypothalamic GnRH mRNA. There was no change in GnRH mRNA or plasma LH observed in leptin-FF mice (Fig. 3 and Table 1). Leptin-NC mice showed a modest reduction in plasma LH levels, suggesting that this occurrence, together with decreased expression of GnRH in the hypothalamus, contribute to the suspension of estrus observed in these animals. ob/ob mice and food-restricted mice have elevated levels of plasma corticosterone (17). In contrast, leptin-FF mice showed a significant reduction in plasma corticosterone on days 1–4 after leptin withdrawal (Table 1). Leptin-NC mice had a slightly higher, but statistically insignificant increase in plasma corticosterone on days 1, 3, and 4 after leptin withdrawal.
Fig. 3.
Quantitative real-time PCR analysis of neuropeptide expression in the hypothalamus. Neuropeptide RNA expression levels in PBS control mice, leptin-FF mice, and leptin-NC mice were analyzed at the end of the dark cycle on day 8 of leptin treatment (L8) and days 1, 2, 3, 4, and 7 after leptin withdrawal (W1–W7, n = 6 per group). Data for TaqMan analysis are reported in relative fluorometric units (×10) normalized to cyclophilin expression. *, P < 0.5 vs. PBS, FF; **, P < 0.005 vs. PBS.
Congenital leptin deficiency results in increased expression of the hypothalamic neuropeptides agouti-related transcript (ART), melanin-concentrating hormone (MCH), and neuropeptide Y (NPY) (18–20) and decreased expression of cocaine- and amphetamine-regulated transcript (CART) and proopiomelanocortin (POMC) (21, 22). TaqMan analysis was used to determine the expression levels of these neuropeptides in the hypothalamus of mice with acute leptin deficiency (Fig. 3). Surprisingly, little or no change was observed in leptin-FF hypothalamus. In contrast, the expression of the orexigenic peptides ART, MCH, and NPY were all increased ≈2-fold in the hypothalamus of leptin-NC mice. Whereas the level of POMC RNA was decreased 30–50% in leptin-NC mice compared with PBS controls, there was only a slight, statistically insignificant change in CART mRNA. There was some latency in the hypothalamic response to decreased leptin in leptin-NC mice because the changes in ART, NPY, and POMC expression were only observed, beginning 2 days after leptin withdrawal, whereas MCH expression began to increase by day 3. This delay may contribute to the lack of any substantial changes observed in the leptin-FF animals.
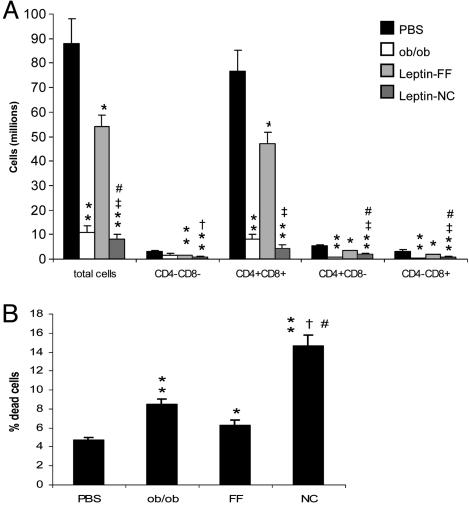
Immune Function. Leptin deficiency is closely associated with alterations in immune function (7). To determine the impact of acute leptin deficiency on the immune system, we examined thymic cellularity in mice post leptin withdrawal. Leptin-FF animals were analyzed 2 days after leptin withdrawal, the point at which a nadir in plasma leptin was observed, and also in leptin-NC mice 7 days after leptin withdrawal. Leptin-FF mice showed an ≈40% reduction in total thymocyte numbers (CD4+/CD8+, CD4-/CD8-, CD4+/CD8-, and CD4-/CD8+) compared with PBS controls (Fig. 4A). Similar to that previously reported in ob/ob mice, thymic cellularity of leptin-NC mice was 5-fold further reduced and was 10-fold lower than controls as measured 7 days after leptin withdrawal. The reduction in thymic cellularity observed in leptin-FF and -NC mice was associated with increased apoptotic activity (Fig. 4B). Flow cytometry of thymocytes stained with annexin-V and propidium iodide revealed a moderate increase in thymic apoptosis in leptin-FF mice relative to PBS controls, whereas leptin-NC mice exhibited an ≈2- and 3-fold increase compared with leptin-FF (and ob/ob) and PBS controls, respectively.
Fig. 4.
Effect of leptin withdrawal on the immune system. (A) Numbers of thymocytes as measured in PBS and leptin-NC mice on day 7 after leptin withdrawal, leptin-FF mice on day 2 after leptin withdrawal, and age-matched ob/ob mice. *, P < 0.05 vs. PBS; **, P < 0.01 vs. PBS; †, P < 0.05 vs. FF; ‡, P < 0.01 vs. FF; #, P < 0.05 vs. ob/ob. (B) Analysis of thymic apoptosis. *, P < 0.05 vs. PBS; **, P < 0.001 vs. PBS; †, P < 0.001 vs. FF; #, P < 0.001 vs. ob/ob. (n = 6 per group for both experiments.)
At 7 days after leptin withdrawal, leptin-NC mice also showed an ≈50% reduction in the number of splenocytes relative to PBS-treated mice, leptin-FF mice, and ob/ob mice (Fig. 8, which is published as supporting information on the PNAS web site).
Reduced splenic cellularity has also been reported in food-restricted mice (13).
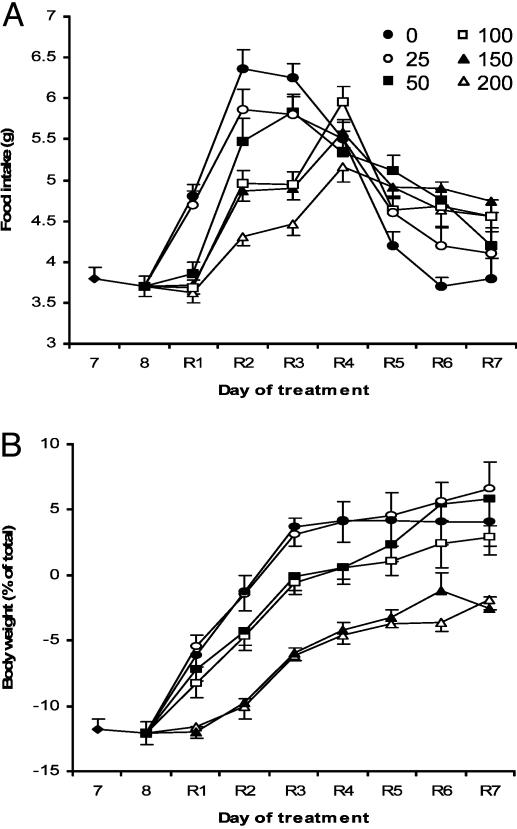
Leptin Replacement. We next sought to establish the concentration of leptin required to suppress the hyperphagia, weight gain, and defects in reproductive and immune function observed in mice after leptin withdrawal. In these studies, the leptin withdrawal protocol was modified such that, instead of leptin being withdrawn entirely, the high-dose leptin was replaced with lower dose infusions as indicated (Fig. 5A). Leptin replacement at doses of 25, 50, 100, 150, and 200 ng/h resulted in plasma concentrations of 1.07, 2.52, 5.41, 8.98, and 11.97 ng/ml, respectively, at 24 h after the dosage was changed. Animals in which high-dose leptin was replaced with PBS became hyperphagic, and rapidly regained fat mass as previously observed in leptin-FF mice (Fig. 5B). Replacement of high-dose leptin with a dose of 25 ng/h did not suppress hyperphagia or weight gain. In contrast, the 50- to 200-ng/h infusions blunted food intake and weight reaccumulation in a dose-dependant manner, with infusions of 200 ng/h achieving the most robust reduction. However, none of the doses of leptin were capable of completely preventing the increase in food intake associated with acute leptin deficiency, and all mice returned to their original, pre-leptin treatment weights within 7 days (Fig. 5B). Note that the inability of leptin to prevent weight gain in this paradigm is in contrast to the response of naïve animals to leptin treatment where a 200 ng/h dose is capable of significantly reducing fat mass (23).
Fig. 5.
Leptin replacement in adipose-depleted mice. (A) After 8 days of leptin treatment, high-dosage leptin was withdrawn and replaced with PBS (0 ng/h) or increasing amounts of leptin as indicated (ng/h). Mice were then allowed to feed freely for 7 days (R1–R7) (n = 6 per group). (B) Daily body weight of mice receiving leptin replacement.
To test the ability of leptin replacement to ameliorate the deficits in reproductive and immune function observed in leptin-NC mice, leptin replacement was performed as above, but in this case, mice were fed a normocaloric diet for 10 days. None of the leptin doses, including those that resulted in physiological (100 ng/ml) or supraphysiological plasma levels, were able to restore estrus cycle during the 10 days of leptin replacement (data not shown). Similarly, no improvement was observed in thymic cellularity at any dose as assessed by FACS analysis; the severe degree of thymic atrophy in mice receiving leptin replacement was indistinguishable from that measured in mice receiving PBS (Fig. 9, which is published as supporting information on the PNAS web site). These data show that the biologic response to exogenous leptin is markedly attenuated in animals that have been previously exposed to a high level of the hormone.
Discussion
Here, we report the use of an experimental approach to induce a state of transient (leptin-FF animals) or protracted (leptin-NC animals) leptin deficiency in wild-type mice. This outcome is accomplished by first administering exogenous leptin, followed by abruptly withdrawing the hormone once adipose stores and endogenous leptin have been depleted. Hypoleptinemic leptin-NC mice manifest a unique biologic response that includes markedly reduced energy expenditure and locomotor activity, decreased reproductive capacity, and immune defects. All of these abnormalities are also evident in ob/ob mice (3, 6, 7), suggesting that in ob/ob mice these abnormalities are largely a direct consequence of leptin deficiency, and not a result of the associated obesity, hyperphagia, or leptin deficiency during development. A notable difference between congenital and acute leptin deficiency is that ob/ob mice have high corticosterone levels, whereas corticosterone levels are not significantly altered in leptin-NC mice. Although this result would suggest that corticosterone is not required for weight gain with leptin deficiency, it has also been shown that adrenalectomy blocks or attenuates the development of obesity in ob/ob mice, and that corticosterone replacement restores it (24). Similarly, starvation causes a rapid rise in corticosterone (2). The basis for the discrepancy in corticosterone levels between ob/ob mice, fasted mice, and leptin-NC mice is unclear; however, the difference allowed us to assess the effects of leptin deficiency on immune function independent of the possibly confounding effects of increased corticosterone.
Acute leptin deficiency has a potent and detrimental effect on the immune system, which is even more pronounced in leptin-NC mice compared with ob/ob mice. Leptin-NC mice have fewer numbers of total thymocytes (Fig. 6A), 2-fold more apoptotic cells than ob/ob mice (Fig. 6B), and also show splenic hypocellularity, a feature that ob/ob mice do not show, despite a reduction in their spleen size (13). Starvation does result in reduced splenic cellularity, suggesting the possibility that additional adipocyte derived factors are involved in this aspect of immune dysfunction. Given the extreme susceptibility of thymocytes to the proapoptotic effects of glucocorticoids, a possible role of increased glucocorticoids in inducing thymic atrophy in ob/ob mice and in starved mice has been proposed. However, the present results indicate that a profound thymic atrophy develops in leptin-NC mice under conditions where corticosterone levels are not significantly altered.
Whereas many known features of leptin deficiency are observed in leptin-NC mice, these abnormalities do not develop in hyperinsulinemic, hyperphagic, leptin-FF mice. The suppression of the leptin deficiency syndrome in leptin-FF mice is unlikely to be entirely a result of hyperphagia, because mice treated with high-dose leptin do not have adipose tissue and are not hyperphagic before leptin withdrawal (Fig. 1 A), yet fail to manifest any of the neuroendocrine, metabolic, and immune abnormalities reported here. It is possible that in leptin-FF mice, the rapid normalization of fat mass and leptin concentration blunts the response. It is also possible that the response to leptin deficiency is suppressed as a result of hyperinsulinemia combined with enough hyperphagia to prevent hypoglycemia.
Insulin has been proposed to be an afferent signal of energy balance, with modes of action similar to leptin. Serum insulin levels positively correlate with adipose tissue mass (25), and insulin administration results in a decrease in food intake and body weight (26), in part by blunting the increase in both NPY and ART in the arcuate nucleus of the hypothalamus, where insulin receptors are highly localized (27). Targeted deletion of the insulin receptor specifically in neurons results in increased food intake, diet-induced obesity, and many other features characteristic of leptin deficiency (28). Furthermore, insulin administration hyperpolarizes the same glucose-responsive neurons in the hypothalamus as leptin (29), suggesting that insulin and leptin signaling pathways may overlap, or otherwise share signal transduction components. In the context of normal physiology, insulin levels fall when leptin levels fall. In leptin-FF mice, insulin levels actually rise with leptin deficiency, suggesting the possibility that an increase in the activity of insulin could counteract the effects of reduced leptin.
In normal animals, infusion of leptin at physiologic or greater doses significantly suppresses food intake and reduces body weight (23). In contrast, animals pretreated with a high dose of leptin remain hyperphagic and gain weight, even when plasma leptin levels that are 2-fold higher than the physiological levels are achieved by means of leptin replacement. This dose also fails to normalize neuroendocrine and immune function in adipose-depleted leptin-NC mice after leptin withdrawal. This finding suggests that the prior treatment with leptin may have induced a state of leptin resistance. Although it is possible that other adipose tissue-derived factors are required for leptin to normalize these functions, leptin is fully capable of suppressing food intake and other metabolic abnormalities in lipodystrophic animals that also lack adipose tissue (30, 31). Furthermore, lipodystrophic mice also exhibit impaired immune function and leptin treatment restores thymic cellularity and reduces thymic apoptosis in these mice (J. A. Sennello, E. Asilmaz, J.M.F., and G. Fantuzzi, unpublished observations). It is possible, however, that fat cells that have been depleted of their cellular lipid secrete factors that antagonize leptin's actions. For example, the secretion of adiponectin by adipose tissue is increased after weight loss (32).
An alternative possibility for the difference in leptin's potency in naïve vs. pretreated animals is that prior exposure to high leptin levels leads to a down-regulation of its response and a relative leptin resistance. This possibility is consistent with data from other studies in which an attenuation of the response to leptin has been reported over the course of several weeks (33). The data reported here amplify this conclusion by showing that reduced leptin sensitivity can be observed within 8 days of high-dose leptin treatment and extends the analysis to effects of leptin not previously studied, such as on immune function and reproduction.
These data also suggest that leptin deficiency modulates the different physiologic systems at different thresholds. For example, after leptin withdrawal, doses of exogenous hormone capable of partially suppressing appetite and weight are incapable of restoring normal immune and neuroendocrine functions. Differences in the threshold of leptin's effects have been previously observed in animals expressing a leptin transgene (34). Further studies of the neural response to various doses of leptin replacement in leptin-NC mice could clarify the role of various neural pathways in regulating diverse aspects of the response to leptin deficiency.
Overall, these data suggest that leptin-treated animals develop a partial insensitivity to many, and perhaps all, of its effects. Because human obesity is frequently associated with leptin resistance (35), the elucidation of the cellular mechanism responsible for this form of acquired leptin resistance could have important implications for our understanding of the molecular mechanisms that regulate body weight.
Supplementary Material
Acknowledgments
We thank Drs. P. Cohen and S. C. Rohani for thoughtful discussions and critical reading of the manuscript, Dr. W. Liedtke for assistance with immunological experiment, and S. Korres for assistance in preparing this manuscript. This work was supported by National Institutes of Health Grant DK41096 (to J.M.F.).
Author contributions: J.M.M., A.S., G. Fantuzzi, and J.M.F. designed research; J.M.M., A.S., E.A., G. Fayzikhodjaeva, G. Fantuzzi, and J.M.F. performed research; J.M.M., A.S., E.A., G. Fayzikhodjaeva, G. Fantuzzi, and J.M.F. analyzed data; and J.M.M., A.S., E.A., G. Fantuzzi, and J.M.F. wrote the paper.
Abbreviations: LH, leutinizing hormone; NC, normocaloric; FF, free feeding; FFA, free fatty acid; GnRH, gonadotropin-releasing hormone; ART, agouti-related transcript; MCH, melanin-concentrating hormone; NPY, neuropeptide Y.
References
- 1.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. & Friedman, J.M. (1994) Nature 372, 425-432. [DOI] [PubMed] [Google Scholar]
- 2.Ahima, R. S., Prabakaran, D., Mantzoros, C., Qu, D., Lowell, B., Maratos-Flier, E. & Flier, J.S. (1996) Nature 382, 250-252. [DOI] [PubMed] [Google Scholar]
- 3.Coleman, D. L. (1978) Diabetologia 14, 141-148. [DOI] [PubMed] [Google Scholar]
- 4.Montague, C. T., Farooqi, I. S., Whitehead, J. P., Soos, M. A., Rau, H., Wareham, N. J., Sewter, C. P., Digby, J. E., Mohammed, S. N., Hurst, J. A., et al. (1997) Nature 387, 903-908. [DOI] [PubMed] [Google Scholar]
- 5.Clement, K., Vaisse, C., Lahlou, N., Cabrol, S., Pelloux, V., Cassuto, D., Gourmelen, M., Dina, C., Chambaz, J., Lacorte, J. M., et al. (1998) Nature 392, 398-401. [DOI] [PubMed] [Google Scholar]
- 6.Mantzoros, C. S. (2000) Ann. N.Y. Acad. Sci. 900, 174-183. [DOI] [PubMed] [Google Scholar]
- 7.Fantuzzi, G. & Faggioni, R. (2000) J. Leukocyte Biol. 68, 437-446. [PubMed] [Google Scholar]
- 8.Schwartz, M. W., Dallman, M. F. & Woods, S. C. (1995) Am. J. Physiol. 269, R949-R957. [DOI] [PubMed] [Google Scholar]
- 9.Moitra, J., Mason, M. M., Olive, M., Krylov, D., Gavrilova, O., Marcus-Samuels, B., Feigenbaum, L., Lee, E., Aoyama, T., Eckhaus, M., et al. (1998) Genes Dev. 12, 3168-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimomura, I., Hammer, R. E., Richardson, J. A., Ikemoto, S., Bashmakov, Y., Goldstein, J. L. & Brown, M. S. (1998) Genes Dev. 12, 3182-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trayhurn, P. & Beattie, J. H. (2001) Proc. Nutr. Soc. 60, 329-339. [DOI] [PubMed] [Google Scholar]
- 12.Roubenoff, R. & Kehayias, J. J. (1991) Nutr. Rev. 49, 163-175. [DOI] [PubMed] [Google Scholar]
- 13.Howard, J. K., Lord, G. M., Matarese, G., Vendetti, S., Ghatei, M. A., Ritter, M. A., Lechler, R. I. & Bloom, S. R. (1999) J. Clin. Invest. 104, 1051-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivitz, W. I., Walsh, S. A., Morgan, D. A., Thomas, M. J. & Haynes, W. G. (1997) Endocrinology 138, 3395-3401. [DOI] [PubMed] [Google Scholar]
- 15.Chen, Y. & Heiman, M. L. (2000) Regul. Pept. 92, 113-119. [DOI] [PubMed] [Google Scholar]
- 16.Nagatani, S., Guthikonda, P., Thompson, R. C., Tsukamura, H., Maeda, K. I. & Foster, D. L. (1998) Neuroendocrinology 67, 370-376. [DOI] [PubMed] [Google Scholar]
- 17.Edwardson, J. A. & Hough, C. A. (1975) J. Endocrinol. 65, 99-107. [DOI] [PubMed] [Google Scholar]
- 18.Shutter, J. R., Graham, M., Kinsey, A. C., Scully, S., Luthy, R. & Stark, K. L. (1997) Genes Dev. 11, 593-602. [DOI] [PubMed] [Google Scholar]
- 19.Qu, D., Ludwig, D. S., Gammeltoft, S., Piper, M., Pelleymounter, M. A., Cullen, M. J., Mathes, W. F., Przypek, R., Kanarek, R. & Maratos-Flier E. (1996) Nature 380, 243-247. [DOI] [PubMed] [Google Scholar]
- 20.Wilding, J. P., Gilbey, S. G., Bailey, C. J., Batt, R. A., Williams, G., Ghatei, M. A. & Bloom S. R. (1993) Endocrinology 132, 1939-1944. [DOI] [PubMed] [Google Scholar]
- 21.Kristensen, P., Judge, M. E., Thim, L., Ribel, U., Christjansen, K. N., Wulff, B. S., Clausen, J. T., Jensen, P. B., Madsen, O. D., Vrang, N., et al. (1998) Nature 393, 72-76. [DOI] [PubMed] [Google Scholar]
- 22.Thornton, J. E., Cheung, C. C., Clifton, D. K. & Steiner, R. A. (1997) Endocrinology 138, 5063-5066. [DOI] [PubMed] [Google Scholar]
- 23.Halaas, J. L., Boozer, C., Blair-West, J., Fidahusein, N., Denton, D. A. & Friedman, J. M. (1997) Proc. Natl. Acad. Sci. USA 94, 8878-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makimura, H., Mizuno, T. M., Roberts, J., Silverstein, J., Beasley, J. & Mobbs, C. V. (2000) Diabetes 49, 1917-1923. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox, B. J., Corp, E. S., Dorsa, D. M., Figlewicz, D. P., Greenwood, M. R., Woods, S. C. & Baskin, D. G. (1989) Peptides (Tarrytown, NY) 10, 1159-1164. [DOI] [PubMed] [Google Scholar]
- 26.Woods, S. C., Lotter, E. C., McKay, L. D. & Porte, D., Jr. (1979) Nature 282, 503-505. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz, M. W., Marks, J. L., Sipols, A. J., Baskin, D. G., Woods, S. C., Kahn, S. E. & Porte, D., Jr. (1991) Endocrinology 128, 2645-2647. [DOI] [PubMed] [Google Scholar]
- 28.Bruning, J. C., Gautam, D., Burks, D. J., Gillette, J., Schubert, M., Orban, P. C., Klein, R., Krone, W., Muller-Wieland, D. & Kahn, C. R. (2000) Science 289, 2122-2125. [DOI] [PubMed] [Google Scholar]
- 29.Spanswick, D., Smith, M. A., Mirshamsi, S., Routh, V. H. & Ashford, M. L. (2000) Nat. Neurosci. 3, 757-758. [DOI] [PubMed] [Google Scholar]
- 30.Shimomura, I., Hammer, R. E., Ikemoto, S., Brown, M. S. & Goldstein, J. L. (1999) Nature 401, 73-76. [DOI] [PubMed] [Google Scholar]
- 31.Asilmaz, E., Cohen, P., Miyazaki, M., Dobrzyn, P., Ueki, K., Fayzikhodjaeva, G., Soukas, A. A., Kahn, C. R., Ntambi, J. M., Socci, N. D. & Friedman J. M. (2004) J. Clin. Invest. 113, 414-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang, W. S., Lee, W. J., Funahashi, T., Tanaka, S., Matsuzawa, Y., Chao, C. L., Chen, C. L., Tai, T. Y. & Chuang, L. M. (2001) J. Clin. Endocrinol. Metab. 86, 3815-3819. [DOI] [PubMed] [Google Scholar]
- 33.Scarpace, P. J., Matheny, M., Zhang, Y., Tumer, N., Frase, C. D., Shek, E. W., Hong, B., Prima, V. & Zolotukhin, S. (2002) Neuropharmacology 42, 548-561. [DOI] [PubMed] [Google Scholar]
- 34.Ioffe, E., Moon, B., Connolly, E. & Friedman, J. M. (1998) Proc. Natl. Acad. Sci. USA 95, 11852-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maffei, M., Halaas, J., Ravussin, E., Pratley, R. E., Lee, G. H., Zhang, Y., Fei, H., Kim, S., Lallone, R., Ranganathan, S., et al. (1995) Nat. Med. 1, 1155-1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.