Abstract
Cervical cancer is a leading cause of death by cancer among women worldwide. High-risk human papillomaviruses (HPVs) are the major etiological agents for cervical cancer, but other factors likely contribute to cervical cancer, because these cancers commonly arise decades after initial exposure to HPV. Estrogen is thought to be one such cofactor; however, its temporal requirements in human cervical cancer are not known. Here we evaluate the temporal requirements of estrogen in cervical carcinogenesis in a mouse model for HPV-associated cervical cancer. Tumors arising in HPV16 transgenic mice treated with estrogen for 9 months were greatly increased in their size compared with tumors developing after 6 months of estrogen treatment. HPV16 transgenic mice treated 6 months with estrogen followed by 3 months without exogenous estrogen had significantly fewer tumors and the tumors were smaller and less aggressive than those arising in mice treated the full 9 months. Importantly, cervical cancers that arose in the mice treated the first 6 of 9 months with estrogen must have regressed, based upon the reduced incidence of cancers in these mice compared with those treated for 6 months with estrogen, then immediately analyzed. We conclude that estrogen plays a critical role not only in the genesis of cervical cancer but also in its persistence and continued development in this mouse model. These findings raise the clinically relevant possibility that, if human cervical cancer has a similar dependence on estrogen for continued tumor growth, then antiestrogen therapy may be effective in the treatment of cervical cancer.
Cervical cancer remains a major worldwide health concern. This is despite the use of Pap smears, a highly successful means for early detection of cervical cancer precursors but limited in its use to countries with highly developed health care systems. Current approaches for treating cancer have limited success; consequently, 5-year survival rates for women with cervical cancer remain low. It is estimated that 500,000 women annually will develop cervical cancer, and 200,000 women die every year from cervical cancer.
Human papillomaviruses (HPVs) are associated with >99% of cervical cancers (1) and are considered to be the major etiologic factor in this and other anogenital cancers as well as a significant portion of head and neck cancers within the oral cavity (2). In HPV-associated cervical cancers, two HPV oncogenes, E6 and E7, are commonly up-regulated in their expression (3). E6 and E7, both multifunctional proteins, display transforming properties in tissue culture, including an ability to contribute to the immortalization process (4, 5). The in vivo properties of high-risk HPV E6 and E7 oncoproteins have been evaluated through the generation and characterization of HPV transgenic mouse strains (6–8). In these K14E6 and K14E7 mice, respectively, expression of the E6 and E7 genes of the high-risk HPV type 16 (HPV16) was directed to stratified epithelium, including the cervical epithelium, by the human keratin 14 (K14) promoter. Although K14E6 and K14E7 mice develop spontaneous tumors of the skin epithelium, no spontaneous reproductive malignancies arise (6, 7). A role of E6 and E7 in cervical cancer, however, was elucidated when these transgenic mice were treated with exogenous estrogen (9). When treated chronically for 6 months with 17β-estradiol, the K14E7, but not the K14E6 or nontransgenic mice, developed cervical cancer. The E6 oncoprotein contributed to increased tumor size in estrogen-treated K14E6/K14E7 doubly transgenic mice. Thus estrogen synergizes with high-risk HPV oncogenes to cause cancer in this mouse model for human cervical cancer. In the current study, the role of estrogen in cervical cancer is further evaluated.
Cervical cancers develop only in a minority of women who have been infected with high-risk HPVs, take on average decades to arise, and follow a progressive histopathological disease pattern that involves acquisition of multiple genetic changes to the cancer cell. These facts indicate that development of cervical cancer is a multifactorial process and likely involves other contributing factors in addition to HPVs, such as environmental (10–12), genetic (13, 14), biological (15), and hormonal factors. A role of estrogen in human cervical cancer has been hypothesized on the basis of two observations. First, extended use of oral contraceptives, which contain synthetic estrogens and/or progesterone, increases cervical cancer risk 2- to 4-fold, depending upon the length of use (16). The synthetic estrogens found in oral contraceptive formulations have increased estrogenic activity compared with endogenous estrogen in some tissues (17–19), as well as enhanced bioavailability (20). Second, parity increases cervical cancer risk up to 3.8-fold for seven or more pregnancies (21). During pregnancy, women are exposed to continuously elevated levels of estrogen (22). Likewise, in our transgenic mouse model for cervical cancer, the mice are exposed continuously to exogenous estrogen, with serum estrogen slightly elevated above the level seen normally in diestrus, producing a continuous estrus state (9). Thus, both in the HPV transgenic mouse model for human cervical cancer and in women, a role of estrogen in the genesis of cervical cancer is evident.
It is unknown at which stage(s) in the development of cervical cancer estrogen plays a role. Specifically, does estrogen play a role only in the onset of tumorigenesis, or does it also play a role in the persistence and continued malignant progression of cervical cancer, once it arises? In this study, we evaluate the temporal requirements of estrogen in cervical carcinogenesis in our HPV transgenic mouse model. We learn from these studies that estrogen plays a critical role not only in the genesis of cervical cancer but also its persistence and continued development. This finding has potentially important implications in the clinical management of human cervical cancer.
Materials and Methods
Transgenic Mice and Hormone Treatments. We used nontransgenic, K14E7 transgenic [line 2304 heterozygotes (7)], and K14E6/K14E7 double transgenic virgin female mice [both transgenes in the heterozygous condition (6)] maintained on the FVB/n inbred genetic background. Starting at 4–6 weeks of age, these mice were anesthetized with 5% isofluorane, and continuous-release estrogen pellets delivering 0.05 mg of estrogen per 60-day time period (Innovative Research of America, Sarasota, FL) were s.c. placed in the dorsal skin of these animals, in the shoulder fat pads. Two separate groups were treated: the first group received 9 months of estrogen treatment with five estrogen tablets given every 60 days; the second group received three pellet insertions over 6 months with no further treatment for an additional 3 months. Control mice received no pellet insertions. Mice were housed in the American Association of Laboratory Animal Care-approved McArdle Laboratory for Cancer Research Animal Facility, and all mouse procedures were performed according to a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Tissue Procurement. One hour before euthanasia, mice were injected i.p. with 100 mg/kg 5-bromodeoxyuridine. Tissues were harvested and histological sections were prepared as described in ref. 9. Every 10th section was stained with hematoxylin and eosin (H&E) and evaluated for pathology.
Cancer Size Analysis. To determine the area of invasion of each cancer, all H&E sections from a given mouse were evaluated, and for each cancer the slide in which the cancer encompassed the greatest area was selected. The cancer areas were measured by using the Zeiss axiovision (version 3.1) software outline tool on images acquired on a Zeiss Axioskop imaging microscope equipped with an Axiocam MRc camera. Pixel conversion to micrometers was performed by calibrating each objective with a stage micrometer, using the 1,300 × 1,030 pixel resolution. Cancer size was compared statistically between genotypes and among estrogen treatments by using the Wilcoxon rank sum test.
Results
In our prior collaborative study, HPV-transgenic mice treated for 6 months with exogenous estrogen developed cervical cancer (9). In the current study, we examined the consequences of longer treatment with estrogen, using a 9-month treatment interval with the same dose of estrogen (0.05 mg/60 days) used previously (9). This dose is sufficient to induce persistent estrus in treated mice (23). Reproductive tracts from estrogen-treated cohorts of nontransgenic, K14E7 transgenic, and K14E6/K14E7 doubly transgenic female mice were harvested at the end of the 9-month treatment (average age at killing: 10.3 months) and subjected to histological analysis. Reproductive tracts from cohorts of untreated mice were also harvested and analyzed (nontransgenic mice, n = 14, all at 13 months of age; K14E7 mice, n = 13, average age of 12.5 months, range 11.5–13 months; K14E6K14E7 mice, n = 10, average age of 10.7 months, range 9–12 months). Every 10th 5-μm section was stained with H&E and subjected to careful histopathological analysis, to identify and quantify the presence and extent of preneoplastic and neoplastic lesions within the reproductive tract.
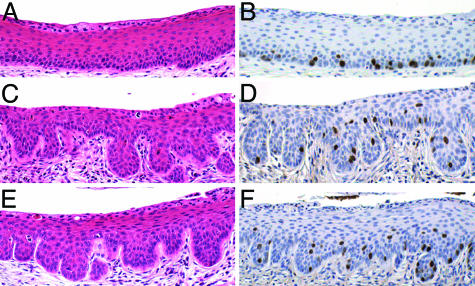
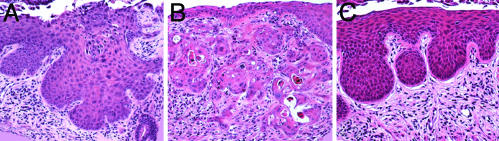
Papillomatosis and Suprabasal DNA Synthesis in Untreated, HPV-Transgenic Mice. None of the untreated mice developed epithelial cancers of the reproductive tract. However, among these untreated cohorts, the K14E7 and K14E6/K14E7 transgenic mice were easily distinguished from their nontransgenic counterparts by papillomatosis, evidenced by frequent invagination of the epithelium into the underlying stroma. This papillomatosis developed in the squamous regions of the vagina and the vaginal fornices, penetrating variably into the lower cervix (Fig. 1 C and E vs. A). The K14E7 and K14E6/K14E7 mice also showed a disruption of normal differentiation compared with nontransgenic mice in the same stage of estrus. Specifically, the suprabasal compartment of the stratified epithelium in both the vagina and cervix of HPV transgenic mice contained many rounded basal-like cells with a high nuclear-to-cytoplasmic ratio, suggestive of a delay in differentiation. This phenotype was absent in nontransgenic mice. Furthermore, double- and multinucleated cells were more prevalent in the K14E7 and K14E6/K14E7 mice compared with nontransgenic mice, consistent with the ability of E7 to induce endoreduplication (24, 25). There was no obvious difference in the severity of these phenotypes between the K14E7 and K14E6/K14E7 mice. The frequency and location of cells within the stratified epithelia of the reproductive tract that support DNA synthesis was evaluated by immunohistochemical staining for 5-bromodeoxyuridine. Positive nuclear staining, indicating cells supporting DNA synthesis, was restricted to the basal and parabasal layers in untreated nontransgenic animals (Fig. 1B), but expanded to include cells in the more superficial layers of the cervical epithelium in HPV transgenic mice (Fig. 1 D and F). A similar difference was observed in the stratified epithelium of the vagina (data not shown). These results indicate that the HPV oncogenes can reprogram suprabasal epithelial cells within the reproductive tract to support DNA synthesis, as has been previously reported for the skin (6, 7).
Fig. 1.
Cervical squamous epithelium of untreated mice. (A, C, and E) H&E-stained cross sections of untreated nontransgenic (A), K14E7 transgenic (C), and K14E6/K14E7 transgenic (E) cervical squamous epithelium. Transgenic mice show extensive papillomatosis, more cells with basal-like appearance, and more double nuclei than nontransgenic mice. (B, D, and F) Immunohistochemical staining for 5-bromodeoxyuridine in control nontransgenic (B), K14E7 transgenic (D), or K14E6/K14E7 transgenic (F) sections. Bromodeoxyuridine-positive cells (indicated by brown nuclear staining) are restricted to the basal and parabasal layers of the epithelium in nontransgenic mice, whereas they extend into the suprabasal layers in transgenic mice.
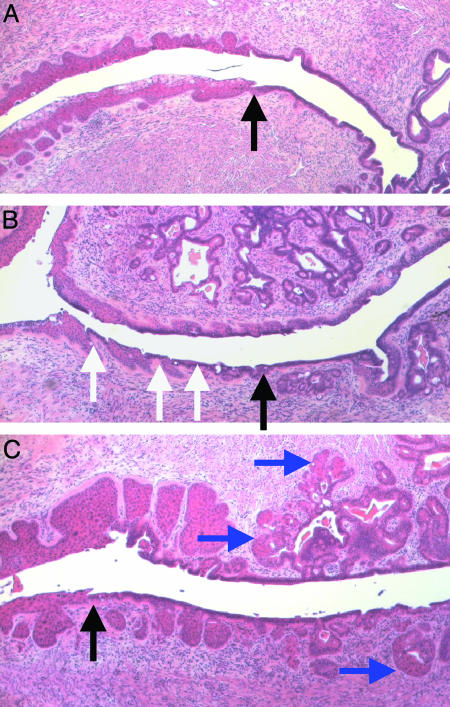
Reproductive Tract Phenotypes in Mice Treated 9 Months with Estrogen. One-hundred percent of K14E7 and K14E6/K14E7 mice treated 9 months with exogenous estrogen developed frank cancer with a frequency of 6.6 and 7.0 tumors per mouse, respectively (Table 1). In comparison, tumor penetrance was 6.7% in the 9-month estrogen-treated nontransgenic mice (one small, microinvasive cancer detected in 1 of 15 treated nontransgenic mice; frequency = 0.07 tumor per mouse; Table 1). The treated nontransgenic mice showed extensive glandular metaplasia with squamous/columnar cell junctions, a feature normally found only in the transformation zone of the cervix of untreated nontransgenic mice (Fig. 2A), evident throughout the entire cervical canal (Fig. 2B, see white arrows). Additionally, hyperplastic lower uterine glands penetrated the cervical septum in the treated nontransgenic mice, with squamous areas exhibiting hyperproliferation but without persistent nuclear atypia (Fig. 2B). In contrast with the treated nontransgenic mice, K14E7 and K14E6/K14E7 mice receiving 9 months of estrogen treatment developed primarily squamous metaplasia (i.e., extension of squamous epithelia into anterior portions of the cervix normally lined by columnar epithelium, see blue arrows) as well as severe dysplasia of all squamous epithelia (Fig. 2C). Thus the HPV16 E7 oncogene can alter the metaplastic properties of the cervical epithelium in estrogen-treated mice, shifting the propensity of cells to be squamous in nature, in addition to increasing the dysplastic nature of the tissue.
Table 1. Tumor incidence and tumor invasion area within reproductive tracts.
| Treated 9 months with estrogen
|
Treated first 6 of 9 months with estrogen
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | No. of mice with cancer* (%) | Avg. no. of cancers per mouse | Total area of tumor invasion per mouse, mm2 | Largest area of tumor invasion per mouse, mm2 | No. of mice with cancer* (%) | Avg. no. of cancers per mouse | Total area of tumor invasion per mouse, mm2 | Largest area of tumor invasion per mouse, mm2 |
| Nontransgenic | 1/15 (6) | 0.067 ± 0.26 | 0.029 ± 0.11 | 0.029 ± 0.11 | 0/12 (0) | — | — | — |
| K14E7 | 11/11 (100) | 6.6 ± 3.6 | 18 ± 13 | 13 ± 11 | 5/18 (38) | 0.54 ± 0.78 | 2.1 ± 3.6 | 2.0 ± 3.4 |
| K14E6/K14E7 | 6/6 (100) | 7.0 ± 2.9 | 49 ± 26 | 37 ± 21 | 9/11 (82) | 1.3 ± 0.79 | 6.3 ± 7.9 | 5.0 ± 6.4 |
Fig. 2.
Abnormalities resulting from 9 months of chronic estrogen treatment. Shown are histological cross sections of the cervical transformation zone of an untreated K14E6/K14E7 mouse (representative of all untreated mice; A), with black arrow indicating the junction between squamous and columnar epithelium. In nontransgenic mice treated for 9 months with estrogen, alternating squamous and columnar regions (B; indicated by white arrows) are found below the transformation zone (black arrow). Additionally, hyperplastic uterine glands extend into the entire cervical septum. Transgenic mice treated for 9 months with estrogen (C) show metaplastic squamous cells growing past the transformation zone (black arrow) and replacing columnar cells within the glands, indicated by blue arrows.
The prolonged treatment of HPV transgenic mice for 9 months with estrogen, compared with the 6-month treatment regimen used in our prior study (9), led to an increase in the size of tumors in the HPV transgenic mice. The average size of the largest tumors in each of the 9-month-treated K14E7 mice (≈13 mm2, Table 1) was 6 times larger than that seen in the 6-month-treated K14E7 mice (9), with 82% of the 9-month-treated K14E7 mice having cancers with sizes >2 mm2 (this study) as compared with 6% in the 6-month-treated K14E7 mice (9).
The HPV16 E6 oncogene increased significantly the total area of tumor invasion in the 9-month-treated K14E6/K14E7 mice compared with the 9-month-treated K14E7 mice (Table 1, P = 0.009). A significant effect of E6 on tumor size was also detected when we considered only the largest tumor arising in each mouse (P = 0.01). The increased size of tumors in the 9-month-treated K14E6/K14E7 mice produced palpable tumor masses that were clearly evident in reproductive tracts isolated from these doubly transgenic mice; palpable tumors were not observed in the reproductive tracts of 9-month-treated K14E7 mice. These findings parallel observations in the previously characterized 6-month-treated mice, where 26% of doubly transgenic mice developed large cancers (defined in that study as those >2 mm2 in area), as opposed to only 6% of K14E7 mice (9). Similar to K14E7 mice, the longer (9-month) treatment regimen increased tumor size in K14E6/K14E7 doubly transgenic mice compared with the 6-month treatment. Specifically, whereas 26% of the 6-month-treated K14E6/K14E7 mice had tumors >2 mm2 in area, 100% of 9-month-treated K14E6/K14E7 mice had one or more such tumor. Furthermore, the average area of cancer invasion for the largest cancers in each of the 9-month-treated K14E6/K14E7 mice (≈37 mm2) was ≈18 times larger than cancers arising in the 6-month-treated K14E6/K14E7 mice. These data together indicate that E6 significantly contributes to tumor growth.
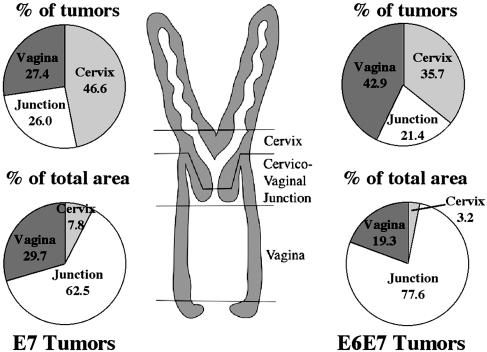
Location of Tumors Arising After 9 Months of Estrogen Treatment. Epithelial tumors arose throughout the K14-positive squamous regions of the reproductive tracts of the 9-month-treated HPV-transgenic mice. We monitored the frequency and size of tumors within three distinct locations of the reproductive tract: the cervix, the cervical/vaginal junction, and the vagina (Fig. 3). Similar data were obtained for both the 9-month-treated K14E7 and K14E6/K14E7 mice. The cervix (including both the cervical transformation zone and areas below it) developed a large percentage of the tumors, yet accounted for only a small fraction of the area of cancer invasion. This distribution was due to a high multiplicity of small tumors arising in this area; of these, the majority arose at the transformation zone. However, the cervicovaginal junction region, although representing a small number of tumors, accounted for the bulk of the area of cancer invasion in both K14E7 and K14E6/K14E7 animals. Tumors arising in the vagina accounted for the residual tumors seen in these mice.
Fig. 3.
Classification of tumors by location. Diagram in center shows a schematic of the mouse reproductive tract and demarcation of approximate boundaries used in classifying location of malignancies. Pie charts on the left (for K14E7) or right (for K14E6/K14E7) show, by location, the percentage of the total tumors arising in each region (upper) and the percentage of the total tumor area these tumors encompassed (lower).
Effect of Withdrawal from Exogenous Estrogen. In comparison with the HPV transgenic mice treated with exogenous estrogen for 6 months, at which time 80% of K14E7 and 95% of K14E6/K14E7 mice had developed reproductive tract tumors (9), we found that 100% of the same mouse genotypes, given 9 months of exogenous estrogen treatment, developed much larger squamous carcinomas of the reproductive tract (Table 1 and text above). This finding could be a consequence of the continued exposure to exogenous estrogen, the presence of the viral oncogenes, or both. Importantly, our mouse model provided us a unique opportunity to assess the temporal role of estrogen in cancer. We therefore treated nontransgenic, K14E7 transgenic, and K14E6/K14E7 transgenic mice with estrogen for 6 months to induce tumor development (9), then let them age for an additional three months without continued exposure to exogenous estrogen. These mice displayed a remarkable reduction in the incidence and size of reproductive tract tumors compared with mice of matched genotypes treated for the full 9 months with exogenous estrogen (Table 1). Whereas the K14E7 and K14E6/K14E7 mice treated with estrogen for 9 months developed on average 6.6 and 7.0 tumors per mouse, respectively, the same genotypes developed only 0.5 and 1.3 tumors per mouse when treated for only the first 6 of 9 months with estrogen. These represent significant decreases in tumor multiplicity (P < 0.001 for both K14E7 and K14E6/K14E7 mice). Likewise, when we compared the cumulative tumor area within the reproductive tracts of these cohorts of mice we saw statistically significant differences between the 9-month-treated versus the 6-of-9-month-treated cohorts both for the K14E7 (18 mm2 vs. 2 mm2, P = 0.0003) and K14E6/K14E7 (49 mm2 vs. 6 mm2, P = 0.002) mice (Table 1).
Of further note, none of the tumors developing in the K14E7 and K14E6/K14E7 mice treated for 6 of 9 months with estrogen arose within the cervix. In comparison, 47% and 36% of tumors arising in the 9-month-treated K14E7 and K14E6/K14E7 mice, respectively, arose within the cervix (Fig. 3). The tumors that arose in the vagina and cervicovaginal junction of the K14E7 and K14E6/K14E7 mice treated the first 6 of 9 months with estrogen showed a less aggressive tumorigenic phenotype than those cancers arising in the mice given 9 months of continuous estrogen (Fig. 4). Specifically, the epithelial cells within the lesions had a more cohesive endophytic growth pattern, with those cells at the stromal interface reestablishing polarity and a more orderly growth pattern, akin to that seen in cervical intraepithelial neoplasia. These results indicate that the high cervical tumor incidence, tumor growth, and invasive character of the tumors seen after 9 months of treatment with estrogen are dependent upon the continued exposure of the HPV transgenic animals to exogenous estrogen.
Fig. 4.
Cancer phenotype alterations in mice withdrawn from exogenous estrogen treatment. Shown are H&E-stained histological cross sections of tumors arising in the K14E6E7 mice after differing estrogen treatments. Withdrawal of exogenous estrogen led to a much more organized phenotype in the tumor tissue (A) than in mice given 9 months of continuous estrogen treatment (B). Notice the more organized structure in A, where most masses are confined by a basement membrane. Additionally, B shows more fibrous stromal reaction that is indicative of malignancy. At higher magnification, mice withdrawn from exogenous estrogen treatment (C) show less nuclear atypia and a lower number of individually invasive cells than mice given 9 months of continuous estrogen treatment (D).
We also compared the K14E7 mice to the K14E6/K14E7 within the 6-of-9-month treatment groups. The K14E6/K14E7 mice had significantly more tumors than the K14E7 mice (P = 0.03). This “withdrawn” group of mice was the first to show a difference in cancer incidence (number of cancers per mouse) between the two genotypes (K14E7 and K14E6/K14E7), implying that E6 has a role in tumor persistence. When we compared the area of tumor invasion between the K14E7 and K14E6/K14E7, the differences almost reached statistical significance, whether we compared the total tumor area per mouse (P = 0.067) or only the largest tumor area per mouse (P = 0.067).
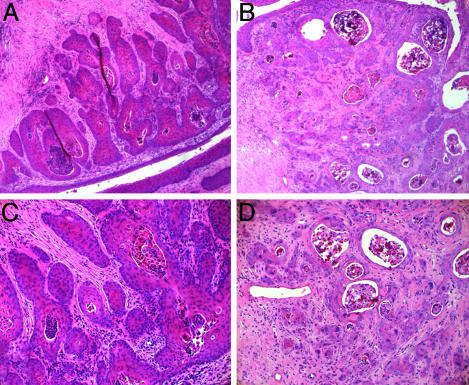
When we compared the histopathology of mice treated for only 6 months that were described previously (9) to those treated the first 6 of 9 months, we found further evidence for a role of continued exposure to estrogen for the growth and progression of cervical cancer. First, in the K14E7 and K14E6/K14E7 mice treated for 6 months, then directly analyzed, a large percentage (25% and 40%, respectively) of the tumors arose in the transformation zone of the cervix (9). In contrast, in these same strains of mice treated the first 6 of 9 months with estrogen, none had cancers of the transformation zone, or any other location in the cervix proper. Instead the tumors were exclusively found in the cervicovaginal junction or the vagina itself. Additionally, whereas, after an initial 6 months of treatment, severe dysplasia at the minimum was seen in 100% of the K14E7 and K14E6/K14E7 mice, only 38% of the same genotypes displayed severe dysplasia at minimum when treated the first 6 of 9 months with estrogen (Fig. 5). This difference was highly significant (P < 0.0001). These differences strongly suggest that withdrawal of exogenous estrogen not only arrests further development of tumors but also leads to a partial regression of cervical disease in these HPV transgenic mice.
Fig. 5.
Cervical squamous epithelial phenotypes differ among estrogen treatment regimens. Shown are cross sections of cervical squamous epithelium from K14E6/K14E7 mice stained with H&E from mice treated with estrogen for 6 months (A), 9 months (B), or 6 months followed by 3 months of aging (C). One-hundred percent of mice given 6 months of estrogen treatment showed severe dysplasias (A) or microinvasive cancers, a phenotype that became more aggressive after 9 months of treatment with estrogen (B). In contrast, mice exposed to estrogen for 6 months and then withdrawn from exogenous estrogen treatment showed a lower degree of dysplasia than either of the continuous estrogen treatments (C), with only 38% of these mice diagnosed with severe dysplasia or beyond.
Discussion
Here we present evidence that estrogen contributes not only to the onset but also to the persistence and malignant progression of cervical cancer in an HPV-transgenic mouse model. These findings are of potential clinical significance, because there has been no evaluation of the estrogen dependence of human cervical cancers. Tumors arising in K14E7 and K14E6/K14E7 mice after 9 months of estrogen treatment were greatly increased in size compared with those reported previously to arise after 6 months of treatment (9). Similar to the 6-month treatment, E6 made a significant contribution to tumor size. When these HPV transgenic mice were treated for only the first 6 of 9 months with estrogen, the differences were striking in comparison with the 9-month-treated as well as the 6-month-treated mice. These 6-of-9-month-treated HPV transgenic mice had significantly fewer tumors than their 9-month-treated as well as their 6-month-treated counterparts. Additionally, these tumors were significantly smaller than those arising in their 9-month-treated counterparts and showed a less aggressive phenotype. Therefore, we conclude that the continued growth and persistence of tumors that have arisen in the reproductive tract of mice expressing E7 alone, or E6 and E7, remain estrogen-dependent after their initial development. This finding raises the possibility that estrogen dependence in human cervical cancer might make antiestrogen therapy valuable.
The reduction in tumor incidence in animals withdrawn from exogenous estrogen the last 3 of 9 months was most evident in the cervix proper, as opposed to the vagina and cervicovaginal junction. The cervix contains the transformation zone, the physical junction of the squamous and columnar cells, which in women is the location of the majority of HPV-induced cervical tumors (26). The transformation zone is hypothesized to contain multipotent stem cells that can give rise to both endocervical and exocervical epithelial cell types. Infection of these stem cells by HPVs is hypothesized to contribute to long-term viral persistence, a requisite for cervical carcinogenesis, which takes decades to develop in most women. Because tumors regressed particularly in the cervix in our mice withdrawn from exogenous estrogen, this finding reinforces the potential role of estrogen in the persistence and growth of the type of tumors arising in the cervix of women.
It remains unclear why the tumors in the vagina and cervicovaginal region were more likely to persist than those in the cervix proper after removal of exogenous estrogen, although the continued growth and invasive characteristics of tumors in these sites were significantly reduced after removal of exogenous estrogen. The tumors arising in the cervicovaginal junction, and to lesser a degree the vagina itself, on average grew much larger than those arising in the cervix. Thus, it is possible that the epithelial surfaces within the vaginal cavity, and/or the environment in which they lie, are more permissive for tumor growth, and thereby less dependent upon exogenous estrogen.
E6 was able to contribute significantly to tumor size in the 9-month-treatment regimen, as was shown previously for mice given 6 months of treatment. This trend persisted in the mice withdrawn from exogenous estrogen treatment, although the difference was not as great (P = 0.067) as that seen in the 9-month-treated group (P = 0.008). Interestingly, there were no significant differences in tumor number between the K14E7 and K14E6/K14E7 mice in any treatment except for the mice withdrawn from exogenous estrogen treatment, where the tumor number was significantly larger in the doubly transgenic mice. This observation implies that E6, in addition to influencing cancer size, may play a role in tumor persistence.
The increased risk for cancer in the mice maintained on exogenous estrogen for the full 9 months is consistent with data from human epidemiological studies concerning parity and oral contraceptive use. The risk of squamous-cell cancer of the cervix increases with increasing number of pregnancies and with number of years of oral contraceptive use. The odds ratio for seven or more full-term pregnancies was determined to be 3.8 in comparison with nulliparous women and 2.3 in comparison with women with one or two full-term pregnancies (21). For oral contraceptive users in comparison with nonusers, the odds ratios of developing cervical cancer was 2.82 for 5–9 years of use and 4.03 for 10 or more years of use (16). Interestingly, these effects may be multiplicative, because five pregnancies and >5 years of oral contraceptive use increased the risk of cervical squamous carcinoma 12-fold (21). Because these studies were restricted to HPV-positive women in both the patients and the controls, they controlled for the strong effect of HPV on the development of cervical cancer. Thus, hormonal changes associated with pregnancy and oral contraceptive use likely influence the risk of cervical cancer, similar to how estrogen influenced markedly the development of cervical cancer in our mouse model. These human epidemiological studies could not attribute these effects to estrogen alone, because additional hormones such as progesterone are also greatly increased during pregnancy and included in the formulation of most oral contraceptives. Further study is needed, therefore, to learn whether estrogen levels per se are what are contributing to the increased risk of cervical cancer in these women.
The evidence for partial tumor regression in the 6-of-9-month-treated HPV transgenic mice was surprising to us. These mice, while deprived of exogenous estrogen, retain the ability to cycle through estrus, and thus are subject to endogenous estrogen stimulation. Therefore, we expected continued growth/progression of the tumors, but to a lesser extent than in the 9-month animals. Instead, we observed a reduced frequency of tumors, particularly in the cervix proper. These data indicate that the constant administration of estrogen creates a favorable environment for tumor growth, whereas the cycling estrogen levels that arise normally in female mice are not as favorable. Compared with humans, however, circulating estrogen levels in adult female rodents are roughly 1/10th (27). The higher range of estrogen concentration in women might be sufficient to contribute to cervical cancer development in concert with high-risk HPV infection, although not as efficiently as seen when these levels are elevated through oral contraceptive use or pregnancy. The high frequency of spontaneous regression of high-grade cervical dysplasia in women is consistent with the likelihood that the natural range in concentrations of estrogen in women is suboptimal for carcinogenesis.
The mechanism by which estrogen contributes to cancer is unclear. Estrogen has been shown to be a direct carcinogen, and therefore could contribute to initiation of lesions (28). Estrogen also is a recognized mitogen, and therefore it could contribute to tumor promotion (28). In the context of HPVs, a potential role of estrogens has been made in the context of altering the efficiency of viral gene expression (29, 30). However, this possible activity clearly is not contributing to estrogen's influence in our transgenic mouse model, because transgene expression from the K14 promoter is not altered by estrogen (31).
The results of our study indicate that estrogen and estrogen-responsive pathways may be important therapeutic targets in human cervical cancer, provided that human genital mucosa behaves similarly to its murine counterpart. Unfortunately, there is a paucity of information at this time as to whether antiestrogenic compounds influence cervical disease in women. Some limited clinical trials have been carried out monitoring the influence of tamoxifen on cervical disease, with variable results (32–34). Variation in dosing between these studies and their short treatment time (the longest treatment was 10 days) likely contribute to the variability in findings. Importantly, it remains unclear whether tamoxifen acts as an estrogen receptor agonist or antagonist in the context of cervical epithelium (35, 36). Clearly, additional studies are needed to assess the biological relevance of the findings regarding estrogen's role in cervical cancer described here, using a mouse model for cervical cancer, to cervical disease in women.
Acknowledgments
We thank Drs. Christopher Crum, Henry Pitot, and Ruth Sullivan for pathology consultations, Harlene Edwards and Jane Weeks for expert histological support, Amy Liem for assistance in mouse breeding, Scott Balsitis for helpful discussions, and Rebecca Riley for consultation on data from the prior study of 6-month-treated mice. This study was supported by grants from the National Institutes of Health (CA98428, CA22443, CA14520, CA84227, and CA09135) and the American Cancer Society (RSG-96-043-06-MBC).
Author contributions: T.B. and P.F.L. designed research; T.B. performed research; T.B. and P.F.L. analyzed data; and T.B. and P.F.L. wrote the paper.
Abbreviations: HPV, human papillomavirus; H&E, hematoxylin and eosin.
References
- 1.Walboomers, J. M., Jacobs, M. V., Manos, M. M., Bosch, F. X., Kummer, J. A., Shah, K. V., Snijders, P. J., Peto, J., Meijer, C. J. & Munoz, N. (1999) J. Pathol. 189, 12-19. [DOI] [PubMed] [Google Scholar]
- 2.Gillison, M. L. & Shah, K. V. (2001) Curr. Opin. Oncol. 13, 183-188. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen, H. (2002) Nat. Rev. Cancer 2, 342-350. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani, F. & Banks, L. (2001) Oncogene 20, 7874-7887. [DOI] [PubMed] [Google Scholar]
- 5.Munger, K., Basile, J., Duensing, S., Eichten, A., Gonzalez, S. L., Grace, M. & Zacny, V. L. (2001) Oncogene 20, 7888-7898. [DOI] [PubMed] [Google Scholar]
- 6.Song, S., Pitot, H. C. & Lambert, P. F. (1999) J. Virol. 73, 5887-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herber, R., Liem, A., Pitot, H. & Lambert, P. F. (1996) J. Virol. 70, 1873-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song, S., Liem, A., Miller, J. A. & Lambert, P. F. (2000) Virology 267, 141-150. [DOI] [PubMed] [Google Scholar]
- 9.Riley, R. R., Duensing, S., Brake, T., Munger, K., Lambert, P. F. & Arbeit, J. M. (2003) Cancer Res. 63, 4862-4871. [PubMed] [Google Scholar]
- 10.Castellsague, X. & Munoz, N. (2003) J. Natl. Cancer Inst. Monogr., 20-28. [PubMed]
- 11.Brinton, L. A. & Fraumeni, J. F. J. (1986) J. Chronic Dis. 39, 1051-1065. [DOI] [PubMed] [Google Scholar]
- 12.Daling, J. R., Sherman, K. J. & Weiss, N. S. (1986) Sex. Transm. Dis. 13, 16-18. [DOI] [PubMed] [Google Scholar]
- 13.de Araujo Souza, P. S. & Villa, L. L. (2003) Mutat. Res. 544, 375-383. [DOI] [PubMed] [Google Scholar]
- 14.Lin, P., Koutsky, L. A., Critchlow, C. W., Apple, R. J., Hawes, S. E., Hughes, J. P., Toure, P., Dembele, A. & Kiviat, N. B. (2001) Cancer Epidemiol. Biomarkers Prev. 10, 1037-1045. [PubMed] [Google Scholar]
- 15.Koskela, P., Anttila, T., Bjorge, T., Brunsvig, A., Dillner, J., Hakama, M., Hakulinen, T., Jellum, E., Lehtinen, M., Lenner, P., et al. (2000) Int. J. Cancer 85, 35-39. [DOI] [PubMed] [Google Scholar]
- 16.Moreno, V., Bosch, F. X., Munoz, N., Meijer, C. J. L. M., Shah, K. V., Walboomers, J. M., Herrero, R. & Franceschi, S. (2002) Lancet 359, 1085-1092. [DOI] [PubMed] [Google Scholar]
- 17.Aten, R. F. & Eisenfeld, A. J. (1982) Endocrinology 111, 1292-1298. [DOI] [PubMed] [Google Scholar]
- 18.Marr, W., Elder, M. G. & Lim, L. (1980) Biochem. J. 190, 563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marr, W., White, J. O., Elder, M. G. & Lim, L. (1980) Biochem. J. 190, 17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolt, H. M. (1979) Pharmacol. Ther. 4, 155-181. [DOI] [PubMed] [Google Scholar]
- 21.Munoz, N., Franceschi, S., Bosetti, C., Moreno, V., Herrero, R., Smith, J. S., Shah, K. V., Meijer, C. J. L. M. & Bosch, F. X. (2002) Lancet 359, 1093-1101. [DOI] [PubMed] [Google Scholar]
- 22.Haggerty, C. L., Ness, R. B., Kelsey, S. & Waterer, G. W. (2003) Ann. Allergy Asthma Immunol. 90, 284-291. [DOI] [PubMed] [Google Scholar]
- 23.Elson, D. A., Riley, R. R., Lacey, A., Thordarson, G., Talamantes, F. & Arbeit, J. M. (2000) Cancer Res. 60, 1267-1275. [PubMed] [Google Scholar]
- 24.Duensing, S., Duensing, A., Flores, E. R., Do, A., Lambert, P. F. & Munger, K. (2001) J. Virol. 75, 7712-7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas, J. T. & Laimins, L. A. (1998) J. Virol. 72, 1131-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paavonen, J., Koutsky, L. A. & Kaviat, N. (1990) in Sexually Transmitted Diseases, ed. Wiegner, P. J. (McGraw Hill, New York), 2nd Ed., pp. 561-592.
- 27.Clarke, R. B., Howell, A. & Anderson, E. (1997) Breast Cancer Res. Treat. 45, 121-133. [DOI] [PubMed] [Google Scholar]
- 28.Liehr, J. G. (2000) Endocr. Rev. 21, 40-54. [DOI] [PubMed] [Google Scholar]
- 29.Mitrani-Rosenbaum, S., Tsvieli, R. & Tur-Kaspa, R. (1989) J. Gen. Virol. 70, 2227-2232. [DOI] [PubMed] [Google Scholar]
- 30.Kim, C. J., Um, S. J., Kim, T. Y., Kim, E. J., Park, T. C., Kim, S. J., Namkoong, S. E. & Park, J. S. (2000) Int. J. Gynecol. Cancer 10, 157-164. [DOI] [PubMed] [Google Scholar]
- 31.Arbeit, J. M., Howley, P. M. & Hanahan, D. (1996) Proc. Natl. Acad. Sci. USA 93, 2930-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrandina, G., Ranelletti, F. O., Larocca, L. M., Maggiano, N., Fruscella, E., Legge, F., Santeusanio, G., Bombonati, A., Mancuso, S. & Scambia, G. (2001) Clin. Cancer Res. 7, 2656-2661. [PubMed] [Google Scholar]
- 33.Vargas Roig, L. M., Lotfi, H., Olcese, J. E., Lo Castro, G. & Ciocca, D. R. (1993) Anticancer Res. 13, 2457-2464. [PubMed] [Google Scholar]
- 34.Scambia, G., Benedetti Panici, P., Baiocchi, G., Battaglia, F., Ferrandina, G., Greggi, S. & Mancuso, S. (1990) Gynecol. Oncol. 37, 323-326. [DOI] [PubMed] [Google Scholar]
- 35.Grenman, S., Shapira, A. & Carey, T. E. (1988) Gynecol. Oncol. 30, 228-238. [DOI] [PubMed] [Google Scholar]
- 36.Hwang, J.-Y., Lin, B.-Y., Tang, F.-M. & Yu, W. C. Y. (1992) Cancer Res. 52, 6848-6852. [PubMed] [Google Scholar]
- 37.Tindle, R. W., Herd, K., Doan, T., Bryson, G., Leggatt, G. R., Lambert, P., Frazer, I. H. & Street, M. (2001) J. Virol. 75, 5985-5997. [DOI] [PMC free article] [PubMed] [Google Scholar]