Abstract
The current study examined the neurochemical mechanisms and neuroanatomical changes underlying coexisting behavioral effects associated with chronic-stress-induced alterations in serotonin (5HT) neurons. Chronic unpredictable stress (CUS) to adult male rats produced depression-like changes with cognitive dysfunction and selective cell death in the interfascicular nucleus of the dorsal raphe (DRif), resulting in decreased 5HTergic innervation of medial prefrontal cortex (mPFC). Twenty-one days of CUS decreased basal plasma levels of corticosterone and produced a shorter latency to immobility and longer durations of immobility in the force-swim test that persisted for 1 month after CUS. Deficits in acquisition, recall, perseveration, and reversal learning were evident 1 month after CUS. MK801 treatment during CUS blocked the changes in the forced-swim test and deficits in memory recall. These behavioral changes were associated with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive soma and the eventual loss of 5HT neurons in the DRif and its projections to the mPFC as evidenced by fewer labeled cells in the DRif after retrograde tracer injections into the mPFC of stressed rats. Similar to the effects of MK801 on behavior, MK801 pretreatment during stress blocked the CUS-induced decreases in 5HT soma within the DRif and its projections to the mPFC. Finally, the depression-like behaviors were blocked by acute injection of the 5HT2A/C agonist (−)-2,5-dimethoxy-4-iodoamphetamine hydrochloride into the mPFC before forced-swim testing. These results identify a cause and mechanism of 5HTergic dysfunction of the mPFC and associated mood and cognitive behaviors.
SIGNIFICANCE STATEMENT Chronic stress causes persistent mood and cognitive changes typically associated with dysregulated serotonin (5HT) transmission in the medial prefrontal cortex (mPFC), but the cause of this dysregulation is unknown. Prior studies have focused on 5HTergic terminals in this region, but this study shows that chronic stress causes NMDA-receptor-dependent and subregion-specific cell death of 5HT neurons in the dorsal raphe. The consequent decreased 5HT innervation of the mPFC was associated with mood and cognitive changes that persisted long after the termination of stress. These findings identify a mechanism of subregion-selective death of 5HT neurons in the dorsal raphe, a defined neuroanatomical pathway, and a behavioral phenotype that mirror stress-associated diseases such as major depressive disorder.
Keywords: chronic stress, frontal cortex, raphe, serotonin
Introduction
Stress increases risk for development of diseases in which lasting mood and cognitive deficits are observed (Marshall et al., 1996). An animal model used to study stress induced behavioral changes is the chronic unpredictable stress (CUS) paradigm, which approximates the psychological aspects of the chronicity, unpredictability, and uncontrollability of everyday stressors experienced by humans. This paradigm has been used in an attempt to model stress-related disorders such as depression and cognitive dysfunction (Demitrack et al., 1991).
CUS-induced neuropsychopathologies have been attributed typically to dysfunctions of the brain serotonin (5HT) system characterized by 5HT-induced dysregulation of the hypothalamus–pituitary–adrenal stress axis, desensitized 5HT receptors, and decreased neuronal firing (Demitrack et al., 1991; van Riel et al., 2003; Bondi et al., 2008; Bambico et al., 2009). 5HT innervations and terminals within the medial prefrontal cortex (mPFC) in particular have received the most attention because a key feature of patients with major depressive disorder is decreased 5HT transporter binding and 5HT turnover in the mPFC (Mann, 1999; Mann et al., 2000). However, few studies have examined the source of 5HT dysfunction in these behaviors.
A major source of 5HT innervations of the mPFC and throughout the forebrain is derived from the dorsal raphe nucleus (DRN) (Hale and Lowry, 2011). Stressors can activate distinct subregions of the DRN differentially (Grahn et al., 1999) (Hale and Lowry, 2011), such as the interfascicular DRN (DRif), which projects to mPFC (Littrell, 2012; Paul and Lowry, 2013). Interestingly, 5HT soma in the DRif have unique morphological, electrophysiological, and receptor profiles that could render it selectively sensitive to stress (Ahima and Harlan, 1990; Valentino et al., 2010; Hale and Lowry, 2011; Lukkes et al., 2011; Soiza-Reilly and Commons, 2011). Furthermore, no studies have investigated whether chronic stress exposure is linked to a loss of 5HT soma within the DRN or the DRif specifically that is associated with long-term changes in 5HT- and mPFC-dependent mood and cognitive function.
The mPFC contains multiple neurotransmitters in addition to its 5HTergic innervation, including glutamate and GABA, and alterations in their functions affect mPFC-dependent behaviors (Drevets et al., 2008). Few studies, however, have examined the role of the DRN and its projections to the mPFC in mood and cognitive behavior and the role of the NMDA receptor in modulating changes in these projections.
Based on the afferents and efferents of the DRif and the involvement of 5HT in mediating stress-related psychopathologies, we tested the hypothesis that exposure of rats to CUS is associated with persistent depression-like changes and cognitive deficits. Furthermore, we investigated the possibility that these changes are associated with cell death of 5HT soma specifically in the DRif, which is produced by activation of the NMDA receptor to cause a decreased 5HTergic innervation of the mPFC.
Materials and Methods
Animals.
All experiments were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and were approved by the Indiana University Institutional Animal Care and Use Committee. Adult male Sprague Dawley rats (175–200 g, n = 360; Harlan Laboratories) were initially housed two per cage under a 12 h light/dark (6:00 A.M. lights on/6:00 P.M. lights off) cycle in a temperature- and humidity-controlled environment. Rats had ad libitum access to food and water and were allowed to acclimate to the housing facility for 4 d before experimentation began.
CUS paradigm.
Rats were either handled daily (NoStress) or exposed to the CUS paradigm (Stress) consisting of 21 d of twice daily exposure to mild stressors. The CUS paradigm was used to prevent habituation and adaptation to stress and attempted to model unanticipated stressful experiences that occur in humans (Katz et al., 1981; Willner et al., 1992). Other than the days indicated below, all rats were on a 12 h light/dark cycle. The stress schedule was as follows: day 1: 60 min 4°C cold room (10:00 h) and 60 min restraint (14:00 h); day 2: 3 h lights off (11:00 h) and lights on overnight (18:00 h); day 3: 3 min swim (11:30 h) and food and water deprivation overnight (17:00–8:00 h); day 4: 60 min wet bedding (9:30 h) and 60 min social stress where cage partners were switched (15:00 h); day 5: 60 min restraint (11:00 h) and 90 min cage agitation in an orbital shaker (14:30 h); day 6: 3 h lights off (10:00 h) and isolation overnight (17:00–8: 00 h); day 7: 3 min swim (10:00 h) and 60 min 4°C cold room (13:00 h); day 8: 60 min wet bedding (9:30 h) and 60 min restraint (16:00 h); day 9: 30 min cage agitation (11:30 h) and 60 min social stress (14:30 h); day 10: 60 min 4°C cold room (9:30 h) and 60 min restraint (15:30 h); day 11: 3 h lights off (10:30 h) and lights on overnight (18:00 h); day 12: 3 min swim (10:00 h) and food and water deprivation overnight (17:30–8:00 h); day 13: 90 min wet bed (10:00 h) and 30 min social stress (14:00 h); day 14: 60 min restraint (11:30 h) and 60 min cage agitation (15:30 h); day 15: 3 h lights off (9:30 h) and isolation overnight (17:00–8:00 h); day 16: 3 min swim (10:30 h) and 60 min 4°C cold room (13:30 h); day 17: 30 min wet bedding (11:30 h) and food and water deprivation overnight (18:00–8:00 h); day 18: 90 min social stress (11:00 h) and 30 min cage agitation (15:00 h); day 19: 3 h lights off (9:00 h) and 60 min restraint (14:00 h); day 20: 3 min swim (11:00 h) and lights on overnight (17:30 h); and day 21: 30 min wet bedding (10:00 h) and 60 min social stress (13:30 h).
Corticosterone assay.
Rats were decapitated and trunk blood was collected in the morning (08:00 h) after the last stressor. Blood was centrifuged for 15 min at 800 relative centrifugal force (RCF) at 4°C. The supernatant was transferred into a 1 ml tube and centrifuged for 7 min at 800 RCF at 4°C. The serum was then assayed for corticosterone (CORT) levels (Corticosterone EIA kit, K014-H1; Arbor Assays). Briefly, 5 μl of the serum sample and dissociation reagent in 490 μl of assay buffer was vortexed. This sample mixture (50 μl) or standards were added to the wells of the plate in triplicate, along with 75 μl of assay buffer, 25 μl of CORT conjugate, and antibody, and incubated at room temperature for 1 h on a shaker. The wells of the plate were then aspirated and washed in 300 μl of wash buffer. 3,3′,5,5′-tetramethylbenzidine substrate (100 μl) was added to the wells and incubated for 30 min at room temperature, followed by 50 μl of the stop solution. The plate was then transferred to a spectrophotometer and the optical density at 450 nm of each well was recorded.
Drug treatments.
MK801 (0.3 mg/kg, i.p.; catalog #0924; R&D Systems) was dissolved in 0.9% NaCl (saline) and injected intraperitoneally 15 min before each stressor. This dose of MK801 is not neurotoxic, does not affect locomotor activity (Ikonomidou et al., 2000; Popke et al., 2002), and has been shown to reverse stress-induced anhedonia (Papp and Moryl, 1994).
Forced-swim test.
The forced-swim test was conducted as described previously (Porsolt et al., 1977) with minor modifications. Rats underwent a forced swim pretest 3 d after CUS and again at 1 month after CUS. The forced-swim tests were performed for a duration of 15 min rather than the 3 min swim during the CUS exposure procedure to measure multiple aspects of depressive-like behavior such as latency to immobility and duration of immobility. The longer duration of testing provides more accurate measure of changes in these behaviors that may not be apparent in a shorter 3 min test. The rats were placed individually in a transparent chamber (11” w × 8” l × 22” h) and allowed to swim for 15 min. Forced-swim testing was conducted the following day. The rats were once again placed in the swim chamber and swim behavior was video recorded for 10 min. The latency to immobile behavior and the duration of time spent exhibiting immobile behavior was analyzed by a trained observer who was blinded to the treatment conditions.
Barnes maze.
A separate group of rats were trained and tested on the Barnes maze (Barnes, 1979) 1 month after termination of CUS exposure. The Barnes maze is composed of a white circular disc that has 18 holes along the perimeter. The hole at the 2:00 position contained a dark goal box underneath it that the rat can enter to escape the brightly lit, exposed surface of the maze. Proximal and distal visual cues were provided around the maze to help the rat orient spatially and enable it to locate the goal box. Rats underwent Barnes maze acquisition training once daily for 5 d, followed by a 2 d break during which they remained in the home cage. They were then tested the next day for memory recall function. For the following 5 d, rats were overtrained to reach the goal box in <15 s by using an aversive auditory cue. The next day, a probe trial was conducted without any goal box placed in the maze and the time spent in the quadrant containing the goal box was noted as an indicator of perseverative behavior. The next day, the goal box was placed back in the 2:00 position and the rats were once again tested in the maze. The following day, the goal box was moved to the 5:00 position and the latency to find the goal box in the new location was noted to indicate cognitive flexibility and reversal learning.
Immunofluorescence.
One week after the last stressor, rats were anesthetized and 100 ml of 0.1 m PBS followed by 400 ml of 4% paraformaldehyde (PFA) was perfused intracardially. Brains were removed and immersed in 4% PFA overnight and cryoprotected by serial overnight immersion in 10% and 20% glycerol, after which they were flash-frozen in cooled isopentane solution. Brains were then sectioned using a cryostat. Serial slices (25 μm thick) were taken through the DRN between −7.8 and −8.2 from bregma and 3 slices, each 125 μm apart, per rat were immunolabeled and imaged by confocal microscopy for analysis.
Slices were mounted onto subbed slides and stored at −20°C. To visualize 5HT cells in the DRN, sections were double labeled for NeuN, a neuronal marker and tryptophan hydroxylase (TPH), the rate-limiting enzyme in the synthesis of 5HT. Slides were submerged in 10 mm citrate buffer and heated to 80°C for 15 min for antigen retrieval, washed in 0.1 m PBS, blocked with 10% normal goat serum, and incubated overnight with Ms × NeuN antibodies (1:1000, catalog #MAB377, RRID: AB_2298772; Millipore) and Rb X TPH (1:1000, catalog #NB100–74555, RRID: AB_1049988; Novus Biologicals). The following day, appropriate Alexa Fluor-conjugated secondary antibodies were applied for 1 h and the sections were washed and then coverslipped with Fluoromount-G (catalog #17984; Fisher Scientific). Confocal microscopy and ImageJ software were used to visualize cell bodies with colocalized NeuN and TPH staining in the DRN. All cell counts were conducted by an observer blinded to the treatment conditions. Cell counts were taken from each of 3 slices over the range of the DRN between −7.8 and −8.2 from bregma. The cell counts were averaged across the three slices and the average was recorded for each rat.
TUNEL assay.
Sections were washed in 0.1 m PBS and the assay performed per directions provided by the In Situ Cell Death Detection Fluorescein Kit from Roche (catalog #11684795910). Briefly, 50 μl of the enzyme solution was added to 450 μl of label solution. This mixture was applied to each of 3 tissue sections (25 μm) that were taken every 125 μm between −7.8 and −8.2 bregma through the DRN. Sections were incubated in TUNEL reaction mixture for 1 h at 37°C in a humidified chamber. Slides were washed in 0.1 m PBS 3 times for 5 min and incubated with Rb × TPH and treated as described in the “Immunofluorescence” section above. Sections (25 μm) were imaged using confocal microscopy. The intensity of TUNEL was dependent on the extent of active apoptosis and the stage of apoptosis within the cell. Cells of the DRif that were positive for TPH and TUNEL were counted from each of 3 different slices over the range of −7.8 to −8.2 from bregma. The ratio of TUNEL to TPH was obtained for each section and the average of the three sections was recorded for each rat by an observer blinded to the treatment condition.
Retrograde tracer surgery.
Five days after the last stressor, rats were anesthetized with ketamine (75 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.) and prepared for stereotaxic surgery. The retrograde tracer TrueBlue (0.6 μl of 2.5%) (1,2 bis [5-Amidino-2benzofuranyl ethylene] Diaceturate salt, catalog #T5891; Sigma-Aldrich) was pressure injected through a glass pipette stereotaxically positioned bilaterally into the mPFC (2.7 mm bregma, 0.5 mm lateral, 4 mm ventral). Two days after TrueBlue injection, 0.1 m PBS and 4% PFA were perfused as described above. Brains were removed, cryoprotected, and sectioned through the rostrocaudal extent of the mPFC to verify tracer localization and diffusion from the site of injection. Each of 3 sections (25 μm) collected every 125 μm were representative of the rostral, middle, and caudal DRN between −7.8 and −8.2 from bregma. TrueBlue-positive cells of each section were imaged using confocal microscopy and visualized using ImageJ software. Cells containing the tracer in the DRif examined over three sections within the extent of the DRN were counted by an observer blinded to the treatment condition.
Bilateral cannula implantation surgery.
On day 19 of CUS, rats were anesthetized with ketamine (75 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.) and secured into a stereotaxic apparatus. Bilateral guide cannulae (26 gauge stainless steel) were implanted above the mPFC (2.7 mm bregma, 0.5 mm lateral, 3 mm ventral; Paxinos and Watson, 2004). Four days after the end of CUS, rats were temporarily anesthetized using isoflurane, the stylet from the outer guide cannulae removed, and an injection cannulae made from silica tubing was filled with either artificial CSF (aCSF) or the 5HT2A/C agonist (−)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI). The injection cannula was inserted 1 mm past the guide cannula so that the tip was positioned in the mPFC (2.7 mm bregma, 0.5 mm lateral, 4 mm ventral; Paxinos and Watson, 2004). Either aCSF or 10 nm DOI (0.25 μl) was injected into the mPOA. This dose of DOI has been shown to elicit a cardiovascular and hyperthermic response when injected into the hypothalamus (Lin et al., 1998; Bell et al., 1999).
Experimental design and statistical analysis.
Data analyses were conducted using SigmaPlot 11.0 software. Comparisons between the NoStress and Stress groups were made using unpaired t test to analyze basal CORT (see Fig. 1), latency and duration of immobility in the 1 month forced-swim tests (see Fig. 3a,b), and DRif TUNEL-positive cells (see Fig. 6b). Barnes maze acquisition data were analyzed using three-way ANOVA to compare stress × treatment × day and was followed by post hoc Tukey's multiple-comparisons tests. All other data were analyzed using a two-way ANOVA to compare stress × treatment groups followed by post hoc Tukey's multiple-comparisons tests. Histological sections through the DRN that had tears in the area of interest and rats having placement of tracer/DOI injection outside of the targeted area were excluded from histological analysis. All data are presented as mean ± SEM and significance was determined at p < 0.05.
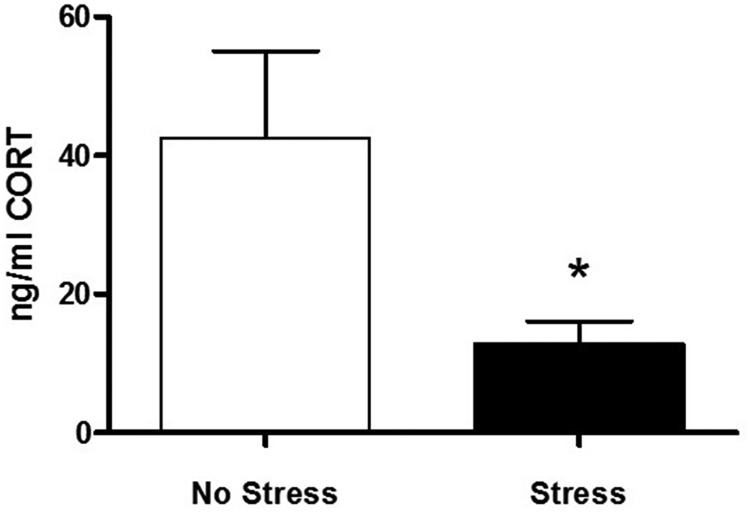
Figure 1.
Basal CORT day after CUS. Graph of basal CORT measured from plasma morning after the last stressor indicate a significant decrease in CORT in the Stress group (n = 6) compared with the NoStress group (n = 5; *p < 0.05).
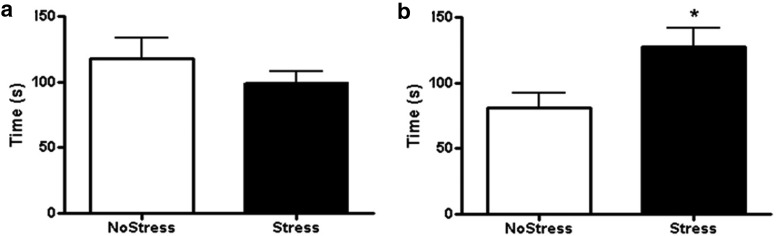
Figure 3.
Forced-swim test 1 month after last CUS. a, Latency to immobility during forced-swim test in the NoStress and Stress groups. (NoStress, n = 6; Stress, n = 8). b, Duration spent in immobile behavior during the 10 min forced-swim test. Stressed rats had significantly longer duration of immobility compared with the NoStress group (*p < 0.05; NoStress, n = 5; Stress, n = 8).
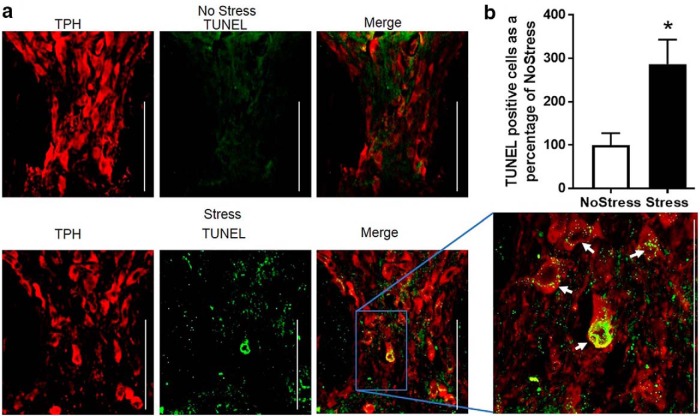
Figure 6.
TUNEL-positive cells in the DRN after 17 d of CUS. a, Top and bottom panels show representative immunofluorescence images of the DRif in the NoStress and Stress groups, respectively, of TPH immunoreactivity (red), TUNEL stain (green), and the merge (yellow). There was minimal to no distinct TUNEL staining in the NoStress group but punctate TUNEL staining surrounding the nuclei of TPH-positive cells in the Stress group. The white arrows point to TUNEL-positive cells in the DRif of the Stress group. The expanded image is 60× magnification. White line/bar, 100 μm. Variations in TUNEL intensity is attributed to differences in the plane and angle of section of TUNEL-labeled soma in the DRif. b, Graph of TUNEL/TPH-positive cells normalized to NoStress (*p < 0.05; NoStress, n = 8; Stress, n = 8).
Results
Basal CORT concentrations in plasma indicate that 21 d of CUS significantly decreased CORT (NoStress = 42.53 ± 12.56 ng/ml, Stress = 12.89 ± 3.26 ng/ml, t (9) = 2.49, p < 0.034; Fig. 1).
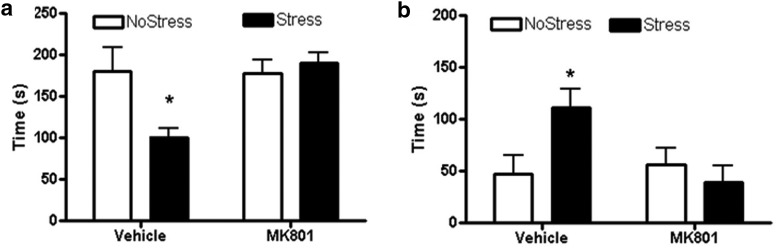
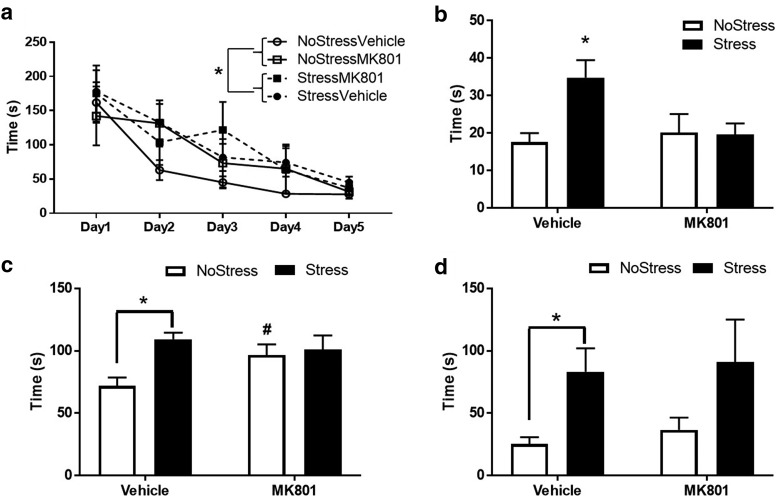
To determine whether NMDA receptor activity during CUS played a role in stress-induced depressive-like symptoms, rats underwent the forced-swim test. NMDA receptor inhibition with MK801 treatments during CUS blocked the decrease in latency to immobility and increase in duration of immobility examined 4 d after CUS. There was a main effect of MK801 treatment (F(1,20) = 5.76, p = 0.026) and an interaction between MK801 and stress (F(1,20) = 6.39, p = 0.020) in the latency to immobility. Vehicle treatment during stress significantly decreased latency to immobility (q = 4.17, p = 0.008) compared with NoStressVehicle and MK801 during stress significantly increased the latency to immobility compared with StressVehicle (q = 5.15, p = 0.002; Fig. 2a). In addition, there was an interaction between MK801 and stress (F(1,21) = 5.53, p = 0.029) in the duration of immobility. Vehicle treatment during stress significantly increased duration of immobility (q = 3.59, p = 0.019) compared with NoStressVehicle and MK801 during stress significantly decreased the duration of immobility compared with StressVehicle (q = 4.47, p = 0.005; Fig. 2b).
Figure 2.
Forced-swim test 4 d after CUS. MK801 or vehicle saline was injected 15 min before each stressor. (NoStressVehicle, n = 5; StressVehicle, n = 8; NoStressMK801, n = 6; and NoStressMK801, n = 7). a, The StressVehicle group showed significantly lower latency to immobility during forced-swim test compared with all other groups (*p < 0.01, StressVehicle vs all other groups). b, The StressVehicle group showed significantly longer duration spent in immobile behavior during forced-swim test compared with all other groups (*p < 0.05, StressVehicle vs all other groups).
The forced-swim test was conducted 1 month after CUS exposure to determine whether stress exposure caused persistent changes in mood and depression-like behavior. Although no difference in the latency to immobility was observed between the Stress and NoStress groups (Fig. 3a), there was a significant increase in the duration of immobility in the Stress group (t (11) = 2.25, p < 0.05; Fig. 3b).
Rats were tested in the Barnes maze to determine whether CUS exposure caused long-lasting cognitive deficits. A three-way ANOVA showed a significant main effect of stress (F(1,223) = 4.40, p = 0.037) and day of acquisition training (F(4,223) = 14.65, p < 0.001; Fig. 4a). The Stress group had longer latencies to find the goal box compared with NoStress rats (q = 2.97, p = 0.036). The rats exhibited longer latencies to find the goal box on day 1 compared with all other days (day 1 vs day 2: q = 4.35, p = 0.018; day 1 vs day 3: q = 6.44, p < 0.001; day 1 vs day 4: q = 8.19, p < 0.001; day 1 vs day 5: q = 9.88, p < 0.001).
Figure 4.
Barnes maze testing 1 month after CUS. (NoStressVehicle, n = 16; StressVehicle, n = 17; NoStressMK801, n = 8; StressMK801, n = 8). a, Acquisition over 5 d of training in the maze show an effect of stress. *p < 0.05. b, After a 2 d break from acquisition training, rats were tested for recall function in the maze. The StressVehicle group showed significantly longer latency to find the goal box (*p < 0.05, StressVehicle vs all other groups). c, Rats were tested for perseverative behavior by recording duration spent in the quadrant that usually contained the goal box in the absence of a goal box in the maze. StressVehicle rats showed significantly longer duration spent in the quadrant compared with NoStressVehicle (*p < 0.001, StressVehicle vs NoStressVehicle) and the NoStressMK801 group showed significantly longer duration spent in the quadrant compared with the NoStressVehicle group (#p < 0.05, NoStressMK801 vs NoStressVehicle). d, Rats were tested for reversal learning by moving the goal box from its standard position to a new location on the maze and the latency to find the goal box in the new location was recorded. StressVehicle rats took significantly longer time to find the goal box compared with the NoStressVehicle group (*p < 0.01, StressVehicle vs NoStressVehicle).
To ascertain whether CUS caused deficits in memory recall, the latency to find the goal box after a 2 d break from acquisition training was recorded. Results indicate an interaction between stress and treatment (F(1,45) = 4.08, p < 0.049). Vehicle treatment during CUS significantly increased the latency to find the goal box compared with the NoStressVehicle group (q = 4.86, p = 0.001) and MK801 treatment during stress decreased the latency compared with StressVehicle (q = 3.47, p = 0.018; Fig. 4b).
Perseverative behavior measured as duration spent in the quadrant that originally contained the goal box but then was removed before the test showed a main effect of stress (F(1,45) = 7.11, p = 0.011) and an interaction between stress and treatment (F(1,45) = 4.45, p = 0.040). Vehicle treatment during CUS significantly increased the duration spent in the quadrant that originally contained the goal box compared with NoStressVehicle group (q = 5.91, p < 0.001) and MK801 treatment by itself increased perseverative behavior in the NoStress group (q = 3.14, p = 0.031; Fig. 4c).
Cognitive flexibility was assessed in the rats by moving the goal box from the 2:00 to the 5:00 position in the maze and latency to find the goal box in the new position was timed. A significant main effect of stress was observed (F(1,45) = 8.72, p = 0.005) with the StressVehicle rats exhibiting a longer latency to find the goal box in the new position compared with the NoStressVehicle rats (q = 3.77, p = 0.011; Fig. 4d).
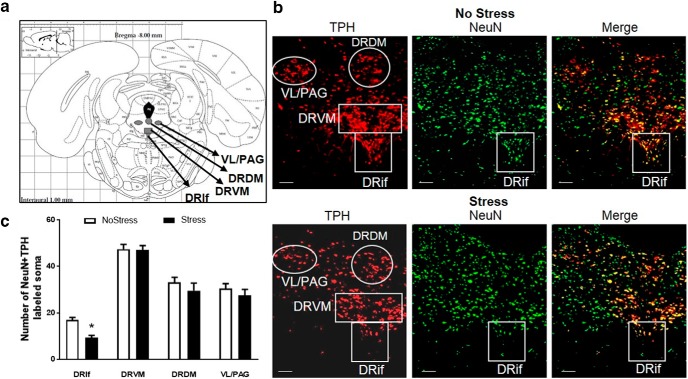
To analyze the effects of CUS on 5HT soma in the DRN (Fig. 5a), cells positive for NeuN and TPH were counted in the DRif and the ventromedial (DRVM), dorsomedial (DRDM), and ventrolateral/periaqueductal gray (VL/PAG) subregions of the DRN (Fig. 5b). There was a significant main effect of stress (F(1,50) = 6.72, p = 0.012) and subregion (F(3,50) = 114.87, p < 0.001). Post hoc test indicated a significant decrease in NeuN + TPH-positive cells to 54.8 ± 5.6% in the DRif of the Stress group (cell counts = 9.4 ± 0.9; q = 4.19, p = 0.005) compared with the NoStress group (cell counts = 17.1 ± 0.1). No significant differences were observed in the DRVM, DRDM, or VL/PAG between the Stress and NoStress groups (Fig. 5c).
Figure 5.
NeuN + TPH-positive cells in the DRN. 5HT cells in the DRN were counted in Stress and NoStress rats 1 week after CUS. (NoStressDRif, n = 8; NoStressDRVM, n = 8; NoStressDRDM, n = 8; NoStressVL/PAG, n = 8; StressDRif, n = 8; StressDRVM, n = 8; Stress DRDM, n = 5; and StressVL/PAG, n = 5). a, Identification of subregions analyzed within the DRN based on the rat brain atlas (Paxinos and Watson, 2004). b, Representative images (10× magnification) of NeuN + TPH-positive cells in the DRN. Red indicates TPH; green, NeuN; and yellow, NeuN + TPH colocalization in No Stress and Stress rats. c, Graph of NeuN + TPH-positive cells in the DRN subregions. DRif subregion shows a significant decrease in the number of NeuN + TPH cells after Stress (*p < 0.001). White scale line indicates 100 μm.
TUNEL-positive staining was assessed in the DRN to determine whether the decrease in NeuN + TPH-positive cells is due to cell death through apoptosis (Fig. 6a). A significant increase of 286.4 ± 56.8% in TUNEL + TPH compared with NoStress (t (14) = 2.95, p = 0.010) was observed in the DRif 1 d after exposure to 17 d of CUS (Fig. 6b).
The role of the NMDA receptor in mediating the loss of NeuN + TPH immunoreactivity in the DRif was examined by administering the NMDA receptor antagonist MK801 during CUS. DRif cells that colabeled NeuN and TPH were counted 1 week after the end of CUS (Fig. 7a). A two-way ANOVA showed a significant main effect of stress (F(1,18) = 16.74, p < 0.001; Fig. 7b). Vehicle treatment during CUS significantly decreased NeuN + TPH-immunoreactive cells in the DRif (q = 5.83, p < 0.001) and MK801 treatment during CUS significantly increased NeuN + TPH cell counts compared with the StressVehicle group (q = 3.56, p = 0.022).
Figure 7.
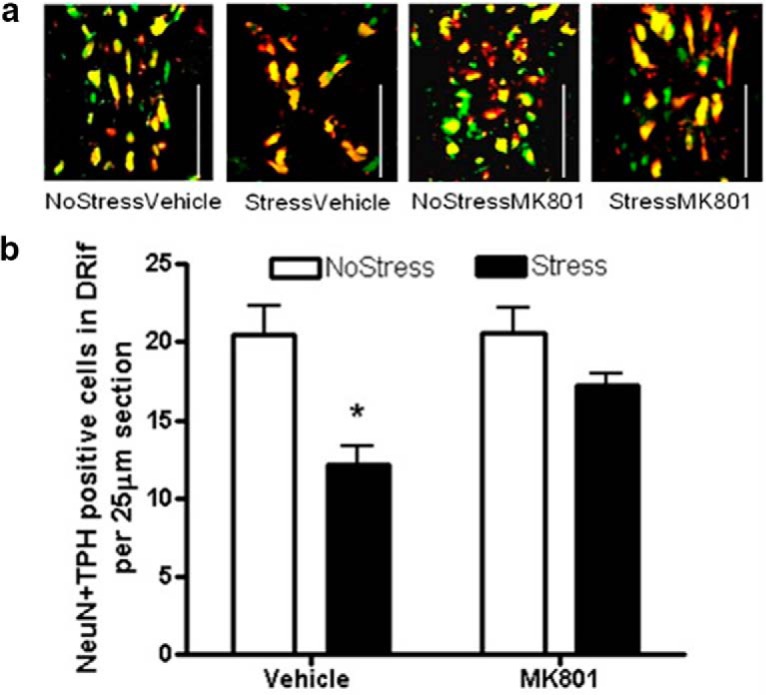
NeuN + TPH-positive cells in the DRif 1 week after MK801 treatment during stress. MK801 (0.3 mg/kg) or vehicle saline was injected 15 min before each stressor. 5HT cells in the DRif were counted in Stress and NoStress rats 1 week after the last stressor (NoStressVehicle, n = 6; NoStressMK801, n = 5; StressVehicle, n = 5; StressMK801, n = 6). a, Representative images (10× magnification) of NeuN + TPH-positive cells in the DRif. Red indicates TPH; green, NeuN; yellow, NeuN + TPH colocalization. White line, 100 μm. b, Graph of NeuN + TPH-positive cells counted in the DRif in MK801 or vehicle (saline) pretreated rats illustrates a significant decrease in StressVehicle group that is blocked by MK801 (*p < 0.001 vs NoStress).
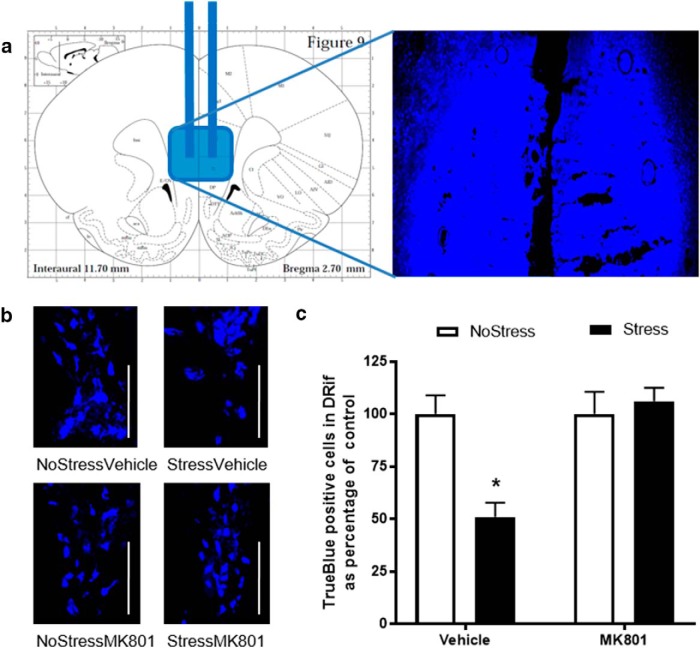
To elucidate whether CUS affected 5HT neurons that projected from the DRif to the mPFC, the retrograde tracer TrueBlue was injected into the mPFC (Fig. 8a) and tracer-positive cells in the DRif were counted in rats that were pretreated with either vehicle or MK801 (Fig. 8b). Results indicated a main effect of stress (F(1,22) = 5.58, p = 0.027) and MK801 treatment (F(1,22) = 9.21, p = 0.06) and an interaction between stress and MK801 treatment (F(1,22) = 9.21, p = 0.006; Fig. 8c). The StressVehicle group had significantly fewer TrueBlue-positive cells in the DRif (q = 4.83, p < 0.003) compared with NoStressVehicle and MK801 treatment during CUS significantly increased the number of TrueBlue-positive cells counted in the DRif compared with the StressVehicle group (q = 6.49, p < 0.001).
Figure 8.
Retrograde tracer TrueBlue injection into the mPFC in MK801-pretreated rats. a, Schematic representation of tracer injection into the mPFC according to the rat brain atlas (Paxinos and Watson, 2004). b, Representative images of TrueBlue-positive cells in the DRif in the NoStressVehicle, StressVehicle, NoStressMK801, and StressMK801 groups. White line, 100 μm (NoStressVehicle, n = 4; StressVehicle, n = 6; NoStressMK801, n = 8; and StressMK801, n = 8). c, Percentage of TrueBlue positive cells in the DRif in Stress and NoStress groups after intraperitoneal injection with either 1 ml/kg vehicle (saline) or 0.3 mg/kg MK801. The StressVehicle had a significantly decreased number of TrueBlue-positive cells in the DRif (*p < 0.01, StressVehicle vs all other groups).
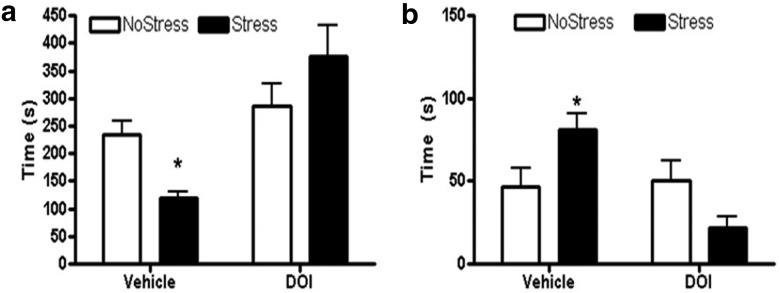
The 5HT2A/C agonist DOI was injected into the mPFC of rats 4 d after CUS and tested in the forced swim task to examine the possibility of blocking depressive behavior that was associated with decreased in 5HT innervation to the mPFC. A significant main effect of DOI treatment was observed (F(1,24) = 16.33, p < 0.001) and there was a interaction between DOI treatment and stress (F(1,24) = 7.4, p = 0.012) in the latency to immobility. aCSF treatment during stress decreased latency to immobility (q = 3.1, p = 0.041) compared with NoStress-aCSF and DOI during stress significantly increased the latency to immobility compared with Stress-aCSF (q = 6.8, p < 0.001; Fig. 9a). In addition, a significant main effect of DOI was observed (F(1,25) = 8.1, p = 0.009) and there was a interaction between DOI and stress (F(1,25) = 10.1, p = 0.004) in the duration of immobility. Stress increased the duration of immobility (q = 3.45, p = 0.022) compared with NoStress-aCSF and DOI treatment during stress decreased the duration of immobility compared with stress-aCSF (q = 6.1, p < 0.001; Fig. 9b).
Figure 9.
Forced-swim test 3 d after CUS. The 5HT2A/C agonist DOI or vehicle aCSF was injected bilaterally into the mPFC 15 min before forced-swim testing (NoStressVehicle, n = 7; StressVehicle, n = 7; NoStressDOI, n = 7; and StressDOI, n = 7). a, The StressVehicle group showed significantly lower latency to immobility during the forced-swim test compared with all other groups (*p < 0.05, StressVehicle vs all other groups). b, The StressVehicle group showed significantly longer duration spent in immobile behavior during the forced-swim test compared with all other groups (*p < 0.05, StressVehicle vs all other groups).
Discussion
The results show that CUS produced multiple and persistent neuroanatomical, behavioral, and neurochemical changes. Basal CORT was significantly decreased by CUS. Rats exposed to CUS exhibited long-lasting learned helplessness as indicated by shorter latency to immobility and longer durations spent immobile in the forced-swim test, all of which were blocked by MK801 treatment during CUS. Furthermore, deficits in acquisition, recall, perseveration, and reversal learning were observed. Cell death in the DRif specifically and its projections to the mPFC were associated with these behavioral changes in a manner blocked by MK801 pretreatment during CUS. In addition, the depression-like behaviors were blocked by injection of the acute 5HT2A/C agonist into the mPFC before forced-swim testing.
CUS and depression-like behavior
Basal CORT was lower in rats exposed to CUS (Fig. 1). These results are consistent with low basal plasma CORT levels observed in mood disorders such as posttraumatic stress disorder and chronic fatigue syndrome that is comorbid with depression and cognitive dysfunction (Oquendo et al., 2003; Torres-Harding et al., 2008; Papadopoulos and Cleare, 2011). Moreover, forced-swim testing conducted 4 d after CUS indicated that CUS rats exhibited depression-like behavior reflected by decreased latency to immobility and increased duration of immobility (Fig. 2a,b). The longer durations of immobility persisted when measured 1 month after the termination of CUS and paralleled the findings that chronic stress increases the likelihood for development of depression long after exposure to stressful events has ceased (Hammen et al., 1992; Kessler, 1997). In contrast, there was no significant change in latency to immobility (Fig. 3a). This lack of change in latency to immobility appeared to be due to a decrease in latency to immobility in the NoStress control rats tested in forced swim at 1 month versus 3 d after CUS. It is possible that prior exposure to the forced-swim testing at 3 d after CUS could have affected performance 1 month later. In fact, a similar effect over this time span has been observed in young-adult versus middle-aged female rats (Récamier-Carballo et al., 2012). Therefore, age could interact with CUS to cause a persistent increase in duration of immobility and is consistent with long-lasting deficits in mood exhibited by patients diagnosed with depressive disorder (Hammen et al., 1992).
CUS and cognition
Persistent-stress-induced depression increases the likelihood for the development of cognitive impairments (Jorm, 2000) and can persist even after depressive behavior is no longer apparent (Reppermund et al., 2009), indicating that stress can cause long-lasting cognitive impairments. Our results show that CUS-induced impairment in acquisition was not blocked by MK801 treatment and suggest an NMDA-independent mechanism (Fig. 4a); however, MK801 blocked the stress induced deficit in recall function (Fig. 4b). This finding is consistent with the role of the PFC in long-term memory storage and retrieval (Simons and Spiers, 2003; Jo et al., 2007). In addition, exposure to stress causes dendritic atrophy in the PFC through an NMDA-receptor-dependent manner (Martin and Wellman, 2011) and may be the cause of the behavioral changes produced by CUS in the current study.
CUS rats also spent a longer time in perseverative behavior (Fig. 4c,d), as did the NoStress/MK801 group, compared with the NoStressVehicle group. This finding suggests that long-term NMDA receptor antagonism alone can cause lasting dysfunction in perseverative behavior. However, MK801 treatment during stress increased perseverative behavior similar to the NoStressMK801 group, indicating that MK801 may have blocked the stress-induced enhancement in perseverative behavior. In contrast, MK801 treatment during CUS did not block the CUS-induced impairments in reversal learning and is consistent with the findings that NMDA receptor antagonists by themselves cause long-term deficits in reversal learning without affecting acquisition (Zajaczkowski et al., 2000).
CUS and 5HT soma in the DRN
Several studies have shown that dysregulation of 5HT in the mPFC causes deficits in memory formation, memory retrieval, and behavioral flexibility (Incisa della Rocchetta and Milner, 1993; Tulving et al., 1994; Runyan et al., 2004; Clarke et al., 2007). Because DRN 5HT neurons innervate the mPFC, it can be reasoned that the loss of DRN cells can affect cognitive behavior. Cell counts of the DRN 1 week after 21 d of stress indicate that CUS decreased the number of NeuN + TPH-immunoreactive cells in the DRif by ∼40% without having any effect on cells in other subregions of the DRN (Fig. 5c). Furthermore, TUNEL was detected in the DRif cells during CUS (day 17 of CUS) indicative of existing cell soma undergoing apoptosis, unlike 1 week after CUS at a time when cell soma have already been lost (Fig. 6). The reason for the specificity of neuronal cell death in the DRif is unclear, but there are several factors that are unique to the DRif that could underlie its vulnerability. The DRif contains morphologically and electrophysiologically distinct, stress-sensitive 5HT neurons that project to the mPFC (Hale and Lowry, 2011; Kelly et al., 2011). Furthermore, the DRif neurons contain glucocorticoid (Ahima and Harlan, 1990), corticotropin releasing factor (CRF) (Lukkes et al., 2011), and AMPA/NMDA receptors (Laurie et al., 1995) that are activated by stress-induced increases in CORT, CRF innervation from the amygdala (Gray, 1993), and glutamatergic input originating from the mPFC (Commons et al., 2005), respectively. Therefore, the combination of differential sensitivity to stress (Kawahara et al., 1993; Adell et al., 1997; Lowry, 2002; Maier and Watkins, 2005; Crawford et al., 2010) due to distinct electrophysiological properties, afferents, and receptor distribution of the DRif neurons may underlie their selective vulnerability to CUS.
The presence of AMPA/NMDA receptors (Laurie et al., 1995) on DRif neurons suggests a glutamatergic component to the stress-induced damage of DRif cells (McEwen et al., 1995). Stress and the activation of glucocorticoid receptors increases calcium channel and NMDA receptor activity, potentiates glutamate release in the hippocampus, and increases cytosolic calcium concentrations to cause cell damage (Sapolsky, 1986; Landfield and Eldridge, 1994; Moghaddam et al., 1994; Bartanusz et al., 1995; Weiland et al., 1997). In fact, neuronal atrophy and cell death in the hippocampus have been attributed to stress hormones and NMDA receptor activity (Goodman et al., 1996; McEwen, 1999). Our results indicate that NMDA receptor antagonism by MK801 effectively blocked cell death in the DRif (Fig. 7) and suggest that a mechanism similar to that which occurs in the hippocampus may be responsible for the decrease in NeuN + TPH immunoreactivity in the DRif after CUS.
CUS and 5HT innervation of the mPFC
To determine whether the projections from the DRif to the mPFC were disrupted by CUS, we used TrueBlue retrograde tracer injections into the mPFC and identified tracer-positive cell soma within the DRif. Figure 8a illustrates that, when TrueBlue was injected into the mPFC of CUS rats, the number of tracer-positive cells in the DRif was lower compared with NoStress rats (Fig. 8c). Because TrueBlue does not require active transport, confounds relating to poor tracer uptake and transport due to damaged terminals or transporter proteins at the injection site are minimized. Therefore, a decrease in tracer-filled soma would indicate loss of DRif cells that project to the mPFC. Treatment with MK801 during CUS effectively blocked the loss of tracer-positive cells in the DRif (Fig. 8c), similar to the blockade of decreases in NeuN + TPH cells (Fig. 7), suggesting that NMDA receptor activation causes the decrease in 5HTergic cells of DRif and their innervations to the mPFC.
The decrease in 5HT within the mPFC due to the loss of innervations from the DRif can underlie the behavioral deficits after CUS. In fact, Fontenot et al. (1995) have shown that chronic social stress in macaques caused persistent decreases mPFC 5HT and its metabolite 5HIAA. In addition, a decrease in 5HT transporter has been observed in the PFC of depressed suicide victims (Linnet et al., 1995; Austin et al., 2002) and further highlights the role of 5HT in modulating mood-related behavior. Interestingly, our results show that CUS-induced depression-like behavior was reversed after the development of depressive symptoms by acute bilateral injection of 5HT2A/C agonist DOI into the mPFC (Fig. 9), indicating that 5HT receptor stimulation in the mPFC by DRif-mPFC 5HT projections is critical for the expression of normal mood-related behavior.
In conclusion, the results presented in this study are the first to show that CUS is toxic to DRif cells and its innervations to the mPFC in a manner that is dependent upon the NMDA receptor. Furthermore, the DRif–mPFC innervations are involved differentially in depression and memory recall function, but not in acquisition of spatial learning or in behavioral flexibility. These results have important implications for understanding the effects of chronic stress and the differential functions of subregions within the dorsal raphe and help to identify the distinct neuroanatomical pathways and mechanisms that contribute to cognitive and mood dysfunctions.
Footnotes
This work was supported by the National Institutes of Health (Grant DA007606).
The authors declare no competing financial interests.
References
- Adell A, Casanovas JM, Artigas F (1997) Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology 36:735–741. 10.1016/S0028-3908(97)00048-8 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE (1990) Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience 39:579–604. 10.1016/0306-4522(90)90244-X [DOI] [PubMed] [Google Scholar]
- Austin MC, Whitehead RE, Edgar CL, Janosky JE, Lewis DA (2002) Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience 114:807–815. 10.1016/S0306-4522(02)00289-0 [DOI] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Gobbi G (2009) Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. European Neuropsychopharmacology 19:215–228. 10.1016/j.euroneuro.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Barnes CA. (1979) Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93:74–104. 10.1037/h0077579 [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Aubry JM, Pagliusi S, Jezova D, Baffi J, Kiss JZ (1995) Stress-induced changes in messenger RNA levels of N-methyl-D-aspartate and AMPA receptor subunits in selected regions of the rat hippocampus and hypothalamus. Neuroscience 66:247–252. 10.1016/0306-4522(95)00084-V [DOI] [PubMed] [Google Scholar]
- Bell AA, Butz BL, Alper RH (1999) Cardiovascular responses produced by microinjection of serotonin-receptor agonists into the paraventricular nucleus in conscious rats. J Cardiovasc Pharmacol 33:175–180. 10.1097/00005344-199902000-00001 [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA (2008) Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33:320–331. 10.1038/sj.npp.1301410 [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC (2007) Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex 17:18–27. [DOI] [PubMed] [Google Scholar]
- Commons KG, Beck SG, Bey VW (2005) Two populations of glutamatergic axons in the rat dorsal raphe nucleus defined by the vesicular glutamate transporters 1 and 2. Eur J Neurosci 21:1577–1586. 10.1111/j.1460-9568.2005.03991.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LK, Craige CP, Beck SG (2010) Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: a mechanism for selective activation in stress circuits. J Neurophysiol 103:2652–2663. 10.1152/jn.01132.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJ, Chrousos GP, Gold PW (1991) Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab 73:1224–1234. 10.1210/jcem-73-6-1224 [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118. 10.1007/s00429-008-0189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot MB, Kaplan JR, Manuck SB, Arango V, Mann JJ (1995) Long-term effects of chronic social stress on serotonergic indices in the prefrontal cortex of adult male cynomolgus macaques. Brain Res 705:105–108. 10.1016/0006-8993(95)01146-3 [DOI] [PubMed] [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, Mattson MP (1996) Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid β-peptide toxicity in hippocampal neurons. J Neurochem 66:1836–1844. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF (1999) Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res 826:35–43. 10.1016/S0006-8993(99)01208-1 [DOI] [PubMed] [Google Scholar]
- Gray TS. (1993) Amygdaloid CRF pathways: role in autonomic, neuroendocrine, and behavioral responses to stress. Ann N Y Acad Sci 697:53–60. 10.1111/j.1749-6632.1993.tb49922.x [DOI] [PubMed] [Google Scholar]
- Hale MW, Lowry CA (2011) Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 213:243–264. 10.1007/s00213-010-2089-z [DOI] [PubMed] [Google Scholar]
- Hammen C, Davila J, Brown G, Ellicott A, Gitlin M (1992) Psychiatric history and stress: predictors of severity of unipolar depression. Journal of Abnormal Psychology 101:45–52. 10.1037/0021-843X.101.1.45 [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Stefovska V, Turski L (2000) Neuronal death enhanced by N-methyl-D-aspartate antagonists. Proc Natl Acad Sci U S A 97:12885–12890. 10.1073/pnas.220412197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incisa della Rocchetta A, Milner B (1993) Strategic search and retrieval inhibition: the role of the frontal lobes. Neuropsychologia 31:503–524. 10.1016/0028-3932(93)90049-6 [DOI] [PubMed] [Google Scholar]
- Jo YS, Park EH, Kim IH, Park SK, Kim H, Kim HT, Choi JS (2007) The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J Neurosci 27:13567–13578. 10.1523/JNEUROSCI.3589-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. (2000) Is depression a risk factor for dementia or cognitive decline? A review. Gerontology 46:219–227. 10.1159/000022163 [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ (1981) Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev 5:247–251. 10.1016/0149-7634(81)90005-1 [DOI] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M (1993) Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett 162:81–84. 10.1016/0304-3940(93)90565-3 [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Donner NC, Hale MW, Lowry CA (2011) Swim stress activates serotonergic and nonserotonergic neurons in specific subdivisions of the rat dorsal raphe nucleus in a temperature-dependent manner. Neuroscience 197:251–268. 10.1016/j.neuroscience.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. (1997) The effects of stressful life events on depression. Annu Rev Psychol 48:191–214. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Eldridge JC (1994) The glucocorticoid hypothesis of age-related hippocampal neurodegeneration: role of dysregulated intraneuronal calcium. Ann N Y Acad Sci 746:308–321; discussion 321–326. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Putzke J, Zieglgänsberger W, Seeburg PH, Tölle TR (1995) The distribution of splice variants of the NMDARI subunit rnRNA in adult rat brain. Mol Brain Res 32:94–108. 10.1016/0169-328X(95)00067-3 [DOI] [PubMed] [Google Scholar]
- Lin MT, Tsay HJ, Su WH, Chueh FY (1998) Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am J Physiol 274:R1260–R1267. [DOI] [PubMed] [Google Scholar]
- Linnet K, Koed K, Wiborg O, Gregersen N (1995) Serotonin depletion decreases serotonin transporter mRNA levels in rat brain. Brain Res 697:251–253. 10.1016/0006-8993(95)00906-7 [DOI] [PubMed] [Google Scholar]
- Littrell JL. (2012) Taking the perspective that a depressive state reflects inflammation: implications for the use of antidepressants. Front Psychol 3:297. 10.3389/fpsyg.2012.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA. (2002) Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol 14:911–923. 10.1046/j.1365-2826.2002.00861.x [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Staub DR, Dietrich A, Truitt W, Neufeld-Cohen A, Chen A, Johnson PL, Shekhar A, Lowry CA (2011) Topographical distribution of corticotropin-releasing factor type 2 receptor-like immunoreactivity in the rat dorsal raphe nucleus: co-localization with tryptophan hydroxylase. Neuroscience 183:47–63. 10.1016/j.neuroscience.2011.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR (2005) Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 29:829–841. 10.1016/j.neubiorev.2005.03.021 [DOI] [PubMed] [Google Scholar]
- Mann JJ. (1999) Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology 21. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V (2000) A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Archives of General Psychiatry 57:729–738. 10.1001/archpsyc.57.8.729 [DOI] [PubMed] [Google Scholar]
- Marshall PS, Watson D, Steinberg P, Cornblatt B, Peterson PK, Callies A, Schenck CH (1996) An assessment of cognitive function and mood in chronic fatigue syndrome. Biol Psychiatry 39:199–206. 10.1016/0006-3223(95)00131-X [DOI] [PubMed] [Google Scholar]
- Martin KP, Wellman CL (2011) NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex 21:2366–2373. 10.1093/cercor/bhr021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. (1999) Stress and hippocampal plasticity. Annu Rev Neurosci 22:105–122. 10.1146/annurev.neuro.22.1.105 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Albeck D, Cameron H, Chao HM, Gould E, Hastings N, Kuroda Y, Luine V, Magariños AM, McKittrick CR (1995) Stress and the brain: a paradoxical role for adrenal steroids. Vitam Horm 51:371–402. 10.1016/S0083-6729(08)61045-6 [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R (1994) Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res 655:251–254. 10.1016/0006-8993(94)91622-5 [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Echavarria G, Galfalvy HC, Grunebaum MF, Burke A, Barrera A, Cooper TB, Malone KM, John Mann J (2003) Lower cortisol levels in depressed patients with comorbid post-traumatic stress disorder. Neuropsychopharmacology 28:591–598. 10.1038/sj.npp.1300050 [DOI] [PubMed] [Google Scholar]
- Papadopoulos AS, Cleare AJ (2011) Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol 8:22–32. 10.1038/nrendo.2011.153 [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E (1994) Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol 263:1–7. 10.1016/0014-2999(94)90516-9 [DOI] [PubMed] [Google Scholar]
- Paul ED, Lowry CA (2013) Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol (Oxford) 27:1090–1106. 10.1177/0269881113490328 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2004) The rat brain in stereotaxic coordinates, Ed 5 Burlington, MA: Elsevier Academic. [Google Scholar]
- Popke EJ, Patton R, Newport GD, Rushing LG, Fogle CM, Allen RR, Pearson EC, Hammond TG, Paule MG (2002) Assessing the potential toxicity of MK-801 and remacemide: chronic exposure in juvenile rhesus monkeys. Neurotoxicol Teratol 24:193–207. 10.1016/S0892-0362(02)00206-4 [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732. 10.1038/266730a0 [DOI] [PubMed] [Google Scholar]
- Récamier-Carballo S, Estrada-Camarena E, Reyes R, Fernández-Guasti A (2012) Synergistic effect of estradiol and fluoxetine in young adult and middle-aged female rats in two models of experimental depression. Behav Brain Res 233:351–358. 10.1016/j.bbr.2012.05.034 [DOI] [PubMed] [Google Scholar]
- Reppermund S, Ising M, Lucae S, Zihl J (2009) Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med 39:603–614. 10.1017/S003329170800411X [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK (2004) A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci 24:1288–1295. 10.1523/JNEUROSCI.4880-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. (1986) Glucocorticoid toxicity in the hippocampus: reversal by supplementation with brain fuels. J Neurosci 6:2240–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ (2003) Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci 4:637–648. 10.1038/nrn1178 [DOI] [PubMed] [Google Scholar]
- Soiza-Reilly M, Commons KG (2011) Glutamatergic drive of the dorsal raphe nucleus. J Chem Neuroanat 41:247–255. 10.1016/j.jchemneu.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Harding S, Sorenson M, Jason L, Maher K, Fletcher MA, Reynolds N, Brown M (2008) The associations between basal salivary cortisol and illness symptomatology in chronic fatigue syndrome. J Appl Biobehav Res 13:157–180. 10.1111/j.1751-9861.2008.00033.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FI, Habib R, Houle S (1994) Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc Natl Acad Sci U S A 91:2012–2015. 10.1073/pnas.91.6.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E (2010) Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res 1314:29–37. 10.1016/j.brainres.2009.09.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel VE, Meijer OC, Steenbergen PJ, Joëls M (2003) Chronic unpredictable stress causes attenuation of serotonin responses in cornu ammonis 1 pyramidal neurons. Neuroscience 120:649–658. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Orchinik M, Tanapat P (1997) Chronic corticosterone treatment induces parallel changes in N-methyl-D-aspartate receptor subunit messenger RNA levels and antagonist binding sites in the hippocampus. Neuroscience 78:653–662. 10.1016/S0306-4522(96)00619-7 [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M (1992) Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16:525–534. 10.1016/S0149-7634(05)80194-0 [DOI] [PubMed] [Google Scholar]
- Zajaczkowski W, Hetman M, Nikolaev E, Quack G, Danysz W, Kaczmarek L (2000) Behavioural evaluation of long-term neurotoxic effects of NMDA receptor antagonists. Neurotox Res 1:299–310. [DOI] [PubMed] [Google Scholar]