Abstract
During neuronal migration, cells that do not reach their normal destination or fail to establish proper connections are eliminated through an apoptotic process. Recent studies have shown that the proinflammatory cytokine tumor necrosis factor α (and its second messengers ceramides) and the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) play a pivotal role in the histogenesis of the cerebellar cortex. However, the effects of ceramides and PACAP on migration of cerebellar granule cells have never been investigated. Time-lapse videomicroscopy recording showed that C2-ceramide, a cell-permeable ceramide analog, and PACAP induced opposite effects on cell motility and neurite outgrowth. C2-ceramide markedly stimulated cell movements during the first hours of treatment and inhibited neuritogenesis, whereas PACAP reduced cell migration and promoted neurite outgrowth. These actions of C2-ceramide on cell motility and neurite outgrowth were accompanied by a disorganization of the actin filament network, depolarization of tubulin, and alteration of the microtubule-associated protein Tau. In contrast, PACAP strengthened the polarization of actin at the emergence cone, increased Tau phosphorylation, and abolished C2-ceramide-evoked alterations of the cytoskeletal architecture. The caspase-inhibitor Z-VAD-FMK, like PACAP, suppressed the “dance of the death” provoked by C2-ceramide. Finally, Z-VAD-FMK and the PP2A inhibitor okadaic acid both prevented the impairment of Tau phosphorylation induced by C2-ceramide. Taken together, these data indicate that the reverse actions of C2-ceramide and PACAP on cerebellar granule cell motility and neurite outgrowth are attributable to their opposite effects on actin distribution, tubulin polymerization, and Tau phosphorylation.
Keywords: neuronal migration, sphingolipid
During neurodevelopment, newly generated neurons migrate to reach their appropriate location in the brain and undergo neurite outgrowth to establish cell connections. Cell migration and synaptogenesis thus represent pivotal phases in maturation of the central nervous system. Neurons that have not established proper connection will be eliminated through apoptosis (1), and defects in the differentiation processes can lead to severe neuronal malformations. For instance, in the developing cerebellum, overexpression of the cytokine TNFα markedly alters granule cell migration and induces aberrant cerebellar cytoarchitecture (2). TNF stimulates the biosynthesis of ceramides, a class of second messengers that play a pivotal role during neurodevelopment (3, 4). In cerebellar granule neurons, ceramides trigger cell death through activation of the mitochondrial apoptotic pathway (5). Invalidation of acid sphingomyelinase, which catalyses the formation of ceramides, causes cerebellar axonal dystrophy and neurodegeneration (6), indicating that ceramides are involved in the control of neurogenesis and programmed cell death.
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a 38-aa neuropeptide that belongs to the vasoactive intestinal polypeptide family (7, 8). PACAP and its receptors are actively expressed in the central nervous system during development, notably in the germinative neuroepithelia and migratory zones (9, 10), suggesting that PACAP could be involved in the regulation of neurogenesis and/or cell differentiation (11). In agreement with this hypothesis, in vitro studies have shown that PACAP exerts growth cone attraction in cultured neural tube precursors from Xenopus laevis (12) and promotes survival and differentiation of rat cerebellar granule cells (13–15). In vivo, PACAP injection at the surface of the cerebellum of 8-day-old rats increases the number of granule neurons in the internal granule cell layer (16). Whereas these observations suggest a role for PACAP in brain plasticity, the possible effect of PACAP on neuronal migration has not yet been investigated.
Here, we demonstrate that PACAP prevents the deleterious effects of C2-ceramide on migration and neurite outgrowth in cerebellar granule cells. Our data also reveal that the effects of PACAP on neuronal motility and differentiation can be accounted for by phosphorylation of the microtubule-associated protein Tau.
Materials and Methods
Chemicals. The 38-aa form of PACAP was synthesized by solid phase methodology as previously described (17). C2-ceramide, BSA (fraction V), DTT, Ham's F12 medium, and the N-1 supplement were obtained from Sigma. The caspase inhibitor Z-VAD-FMK was purchased from Promega. DMEM, Ham's F12, and the antibiotic-antimycotic solution (10,000 units/ml penicillin, 10 mg/ml Eheim 2211 fungizone) were from Life Technologies (Cergy Pontoise, France).
Cell Culture. Granule cell suspensions were prepared from cerebellums of 8-day-old Wistar rats as previously described (18). Neurons were cultured at a density of 4 × 103 cells per mm2 in a chemically defined medium. Cells were grown at 37°C in a humidified incubator with an atmosphere of 5% CO2/95% air. For Western blot analysis and videomicroscopy, dispersed cells were seeded in 35-mm and 60-mm Falcon dishes (Becton Dickinson), respectively. For immunochemistry and measurements of neurite outgrowth, cells were seeded in 24-well plates containing a glass coverslip.
Real-Time Videomicroscopy. For imaging of living granule cells, neurons were cultured during 24 h at constant temperature (37°C) in a controlled atmosphere (5% CO2) under an inverted microscope (Leica, Rueil-Malmaison, France) equipped with an incubation chamber (Solent Scientific), standard phase-contrast optics, and computer-controlled illumination shutters and filter wheels. Images were acquired with a ×20 objective every 5 min by using a CoolSNAPfx camera (Princeton Instruments, Trenton, NJ). Migration parameters, including cell velocity and distance covered, were analyzed by using metamorph software (Universal Imaging, Media, PA).
Neurite Outgrowth Measurement. Immature granule cells were treated during 24 h in the absence or presence of C2-ceramide, PACAP, or caspase-3 inhibitor. Neurites were visualized by immunohistochemistry by using a neurofilament 200 antiserum (Sigma) as previously described (19). The length of the neurites borne by granule cells was measured by means of an image analysis system (Nikon) by using mercator software (Explora Nova, La Rochelle, France).
Immunohistochemistry. Immature granule cells were treated during 24 h in the absence or presence of PACAP, C2-ceramide, or both. Granule cells were fixed with 4% paraformaldehyde in PBS and incubated at 4°C overnight with antisera against actin (Santa Cruz Biotechnology), tubulin (Sigma), phospho-Tau (Santa Cruz Biotechnology), or Tau (Santa Cruz Biotechnology). Immunolabeling was revealed by using the appropriate FITC-conjugated secondary antibody (Sigma) incubated 5 min with 4′,6-diamidino-2-phenyl indole (1 μg/ml) in the same buffer and observed with a fluorescence-equipped microscope (Nikon).
Western Blot Analysis. Cerebellar granule cells were incubated for various durations ranging from 2 to 24 h in the absence or presence of C2-ceramide (20 μM), PACAP (10-7 M), or the caspase-3 inhibitor Z-VAD-FMK (20 μM). Total cellular proteins were extracted as previously described and electrophoresed on a 10% SDS/PAGE (5). After separation, proteins were electrically transferred onto a nitrocellulose membrane (Amersham Pharmacia) and revealed with antibodies against Tau or phospho-Tau by using a chemiluminescence detection kit (ECL System, Amersham Pharmacia). Autoradiographic films were quantified by using an image analysis system (Samba, Grenoble, France).
Statistical Analysis. Data are presented as the mean ± SEM from at least three independent experiments performed in triplicate. Statistical analysis of the data was performed by using an ANOVA followed by Tukey's post hoc test.
Results
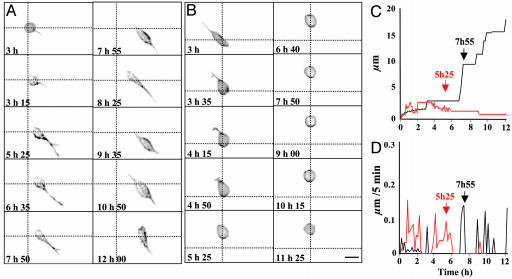
Effects of PACAP and C2-Ceramide on the Morphology, the Distance of Migration, and the Velocity of Granule Cells. Individual granule cell recordings were performed during 12 h by time-lapse microscopy (Figs. 1, 2, 3). In control conditions, granule cells typically emitted a single neurite within the first 5 h; thereafter, a second process appeared (Fig. 1A). Granule cell bodies moved by sliding along the neurites (Fig. 1A). The distance covered from origin was characterized by a scale pattern (Fig. 1C, black curve), whereas the velocity presented a jerky profile with maximum pikes of 2.5 μm/h (Fig. 1D, black curve). When treated with C2-ceramide (20 μM), granule cells emitted one neurite within the first 5 h, but rapidly, this neurite regressed and, in most cases, the cell conserved a round shape (Fig. 1B). The distance covered was short, and cell velocity decreased with time (Fig. 1 C and D, red curves). Morphological changes of granule cells treated with PACAP (10-7 M) were similar to control cells (data not shown).
Fig. 1.
Time-lapse videomicroscopy recording of cerebellar granule cells cultured during 12 h in control conditions (A, C, and D)orinthe presence of C2-ceramide (B–D). (A and B) Microphotographs illustrating time-dependent changes of the cell morphology and motility in control conditions (A) or in the presence of 20 μM C2-ceramide (B). (C and D) Diagrams showing the distance covered by the recorded cells (C) and their velocity (D) in control conditions (black curves) or in the presence of 20 μM C2-ceramide (red curves). Scale bar, 8 μm.
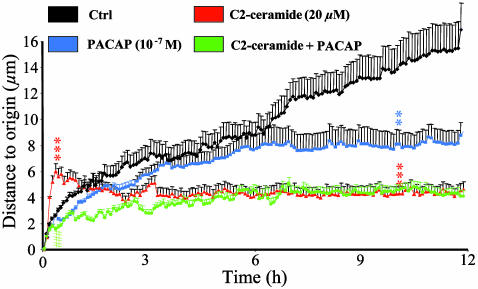
Fig. 2.
Effect of C2-ceramide, PACAP, or both on the distance to origin covered by cultured cerebellar granule cells. The curves represent the mean distance to origin covered by granule cells cultured in control conditions (black curve, n = 100) or in the presence of 20 μM C2-ceramide (red curve, n = 90), 10-7 M PACAP (blue curve, n = 80), or C2-ceramide plus PACAP (green curve, n = 85). **, P < 0.01; ***, P < 0.001 vs. control; ###, P < 0.001 vs. C2-ceramide.
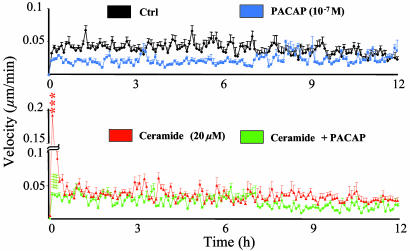
Fig. 3.
Effect of C2-ceramide, PACAP, or both on cerebellar granule cell velocity. The curves represent the mean velocity measured in control conditions (black curve, n = 90) or in the presence of 20 μM C2-ceramide (red curve, n = 90), 10-7 M PACAP (blue curve, n = 80), or PACAP plus C2-ceramide (green curve, n = 85). ***, P < 0.001 vs. control; ###, P < 0.001 vs. C2-ceramide.
Series of similar recordings were performed on granule cells in the absence or presence of C2-ceramide, PACAP, or C2-ceramide plus PACAP (at least 40 cells in each group). In control conditions, granule neurons moved regularly from their origin during the whole recording period (Fig. 2, black curve). Exposure of granule cells to C2-ceramide (20 μM) induced an immediate and transient increase of cell motility (Fig. 2, red curve). After 10–30 min of C2-ceramide treatment, the distance to origin was significantly higher (P < 0.001) than in control conditions. In contrast, longer treatment with C2-ceramide inhibited cell motility (P < 0.001 after 10 h). Treatment with PACAP (10-7 M) did not affect cell motility during the first 6 h but abolished displacement during the next 6 h (Fig. 2, blue curve). From 9 to 12 h, PACAP significantly reduced (P < 0.01) the distance covered by granule cells. Coincubation of granule cells with C2-ceramide and PACAP abolished the early stimulatory effect of C2-ceramide on cell motility (Fig. 2, green curve; P < 0.001). In contrast, PACAP did not affect C2-ceramide-induced inhibition of cell movements during a prolonged incubation. Analysis of the mean velocity revealed that, in control conditions, cultured granule cells moved at a mean speed of 0.04 μm/min over a 12-h period (Fig. 3, black curve). Incubation of cells with C2-ceramide (20 μM) provoked an immediate and transient increase of the velocity that peaked after 30 min of treatment (0.17 μm/min; P < 0.001). Then, the mean velocity returned to control level within 30 min (Fig. 3, red curve). Exposure of granule cells to PACAP (10-7 M) induced a sustained and significant reduction of the mean velocity (0.02 μm/min; P < 0.001 at 30 min; Fig. 3, blue curve). Coincubation of granule cells with C2-ceramide and PACAP totally suppressed the early stimulation of cell velocity induced by C2-ceramide alone (Fig. 3, green curve).
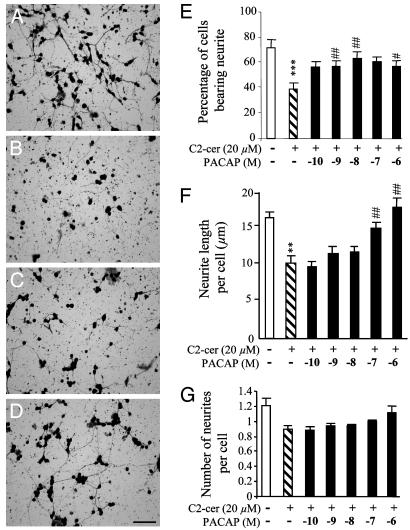
Effects of C2-Ceramide and PACAP on Neurite Outgrowth. After a 12-h culture in control conditions, 72% of cerebellar granule cells possessed neurites (Fig. 4 A and E) and the mean number of neurites per cell was 1.2 ± 0.1 with a mean length of 17 μm (Fig. 4 F and G). Treatment of cells with C2-ceramide (20 μM) for 12 h reduced the proportion of cells bearing neurites to 47% (Fig. 4 B and E). These cells possessed a single neurite (Fig. 4G) with a mean length of 10 μm (Fig. 4F). Addition of graded concentrations of PACAP significantly augmented the number of cells bearing neurites (Fig. 4 C–E) and induced a dose-dependent increase in the mean neurite length (Fig. 4 C, D, and F). However, PACAP did not prevent the reduction of the number of neurites evoked by C2-ceramide (Fig. 4 C, D, and G).
Fig. 4.
Effects of C2-ceramide, PACAP, or both on neurite outgrowth in cultured cerebellar granule cells. (A–D) Microphotographs illustrating the growth of neuritic processes after 12 h of culture in control conditions (A) or in the presence of 20 μM C2-ceramide (B), C2-ceramide plus 10-8 M PACAP (C), or C2-ceramide plus 10-7 M PACAP (D). Scale bar, 50 μm. (E–G) Effect of C2-ceramide (20 μM) in the absence or presence of graded concentrations of PACAP (10-10 to 10-6 M) on the mean number of granule cells bearing neurites (E), the mean length of neurites (F), and the mean number of neurites per cell (G). **, P < 0.01; ***, P < 0.001 vs. control; #, P < 0.05; ##, P < 0.01 vs. C2-ceramide.
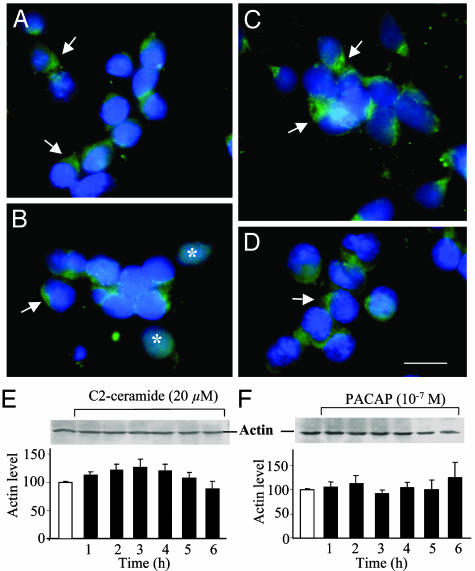
Effects of C2-Ceramide and PACAP on Distribution and Expression of Actin and Tubulin. After 12 h of culture in control conditions, actin immunoreactivity was mainly localized in the emergence cone of the process (Fig. 5A). Exposure of granule cells to C2-ceramide (20 μM) provoked the disorganization of the actin network in most cells, although polarized labeling could still be observed in a few neurons (Fig. 5B). Treatment with PACAP (10-7 M) alone reinforced the polarization of actin at the emergence cone (Fig. 5C). Cotreatment of granule cells with C2-ceramide and PACAP partially restored the distribution pattern of actin (Fig. 5D). Western blot analysis showed that C2-ceramide and PACAP had no effect on the expression of actin during the first 6 h of treatment (Fig. 5 E and F), a period during which cell survival is not modified by either compound (20).
Fig. 5.
Effect of C2-ceramide, PACAP, or on both actin distribution and expression in cerebellar granule cells. (A–D) Immunohistochemical micrographs illustrating the distribution of actin in control conditions (A)orinthe presence of 20 μM C2-ceramide (B), 10-7 M PACAP (C), or C2-ceramide plus PACAP (D). The arrows show accumulation of actin at the emergence cones, and the asterisks point out diffuse immunoreactivity in ceramide-treated cells. Scale bar, 10 μm. (E and F) Western blot analysis showing the absence of effect of 20 μM C2-ceramide (E) or 10-7 M PACAP (F) on actin expression.
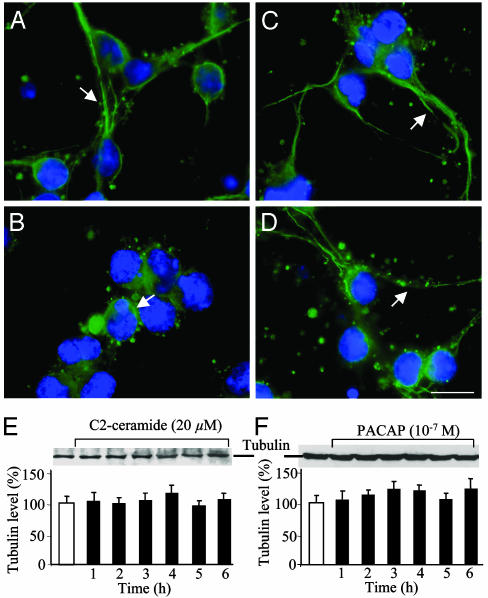
In control conditions, tubulin immunoreactivity was localized at the periphery of the soma and in neuronal processes (Fig. 6A). Exposure of granule cells to C2-ceramide provoked accumulation of the immunolabeling in the soma (Fig. 5B). In contrast, treatment with PACAP did not affect the cellular distribution of tubulin (Fig. 6C). Coincubation of C2-ceramide and PACAP restored, in most cells, the distribution pattern of tubulin observed in control conditions (Fig. 6D). Western blot analysis revealed that the expression level of tubulin was not modified by treatment with C2-ceramide or PACAP (Fig. 6 E and F).
Fig. 6.
Effect of C2-ceramide, PACAP, or both on tubulin distribution and expression in cerebellar granule cells. (A–D) Immunohistochemical micrographs illustrating the distribution of tubulin in control conditions (A)orinthe presence of 20 μM C2-ceramide (B), 10-7 M PACAP (C), or C2-ceramide plus PACAP (D). Arrows show association of tubulin with neuritic processes. Scale bar, 10 μm. (E and F) Western blot analysis showing the absence of effect of 20 μM C2-ceramide (E) or 10-7 M PACAP (F) on tubulin expression.
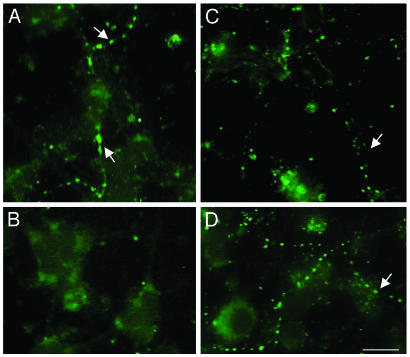
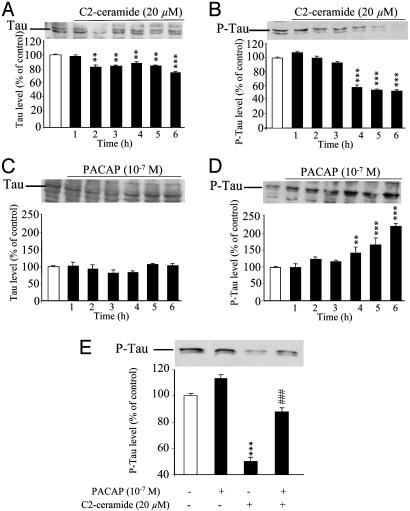
Effects of C2-Ceramide and PACAP on Cellular Distribution, Expression, and Phosphorylation of Tau. Immunohistochemical labeling with a panTau antibody revealed that the Tau protein was concentrated in varicosities along neuritic processes (Fig. 7A). Exposure of cells to C2-ceramide markedly reduced the intensity of labeling, and the residual Tau immunoreactivity was diffusely distributed in the soma (Fig. 7B). Treatment of granule cells with PACAP did not modify the distribution of Tau (Fig. 7C). Coincubation of granule cells with C2-ceramide plus PACAP yielded a dotted distribution similar to that observed in control conditions (Fig. 7D). Western blot analysis showed that C2-ceramide induced a slight but significant decrease of the total amount of Tau after 2 h (Fig. 8A) and caused a marked decrease of phospho-Tau after 4 h (Fig. 8B). Conversely, exposure of cells to PACAP (10-7 M) did not affect the total amount of Tau (Fig. 8C) but provoked a significant increase of the amount of the phosphorylated form of Tau (Fig. 8D). The inhibitory effect of C2-ceramide on Tau phosphorylation was prevented by coadministration of PACAP (Fig. 8E).
Fig. 7.
Effects of C2-ceramide, PACAP, or both on Tau distribution in cerebellar granule cells. Shown is visualization by immunohistochemistry of Tau in control conditions (A) and in granule cells treated with 20 μM C2-ceramide (B), 10-7 M PACAP (C), or C2-ceramide plus PACAP (D). Arrows show the dotted distribution of Tau along neuritic processes. Scale bar, 10 μm.
Fig. 8.
Western blot analysis showing the effect of C2-ceramide, PACAP, or both on the concentration of Tau and phospho-Tau in cerebellar granule cells. (A and B) Time course of the effect of 20 μM C2-ceramide on the levels of Tau (A) and phospho-Tau (B). (C and D) Time course of the effect of 10-7 M PACAP on the levels of Tau (C) and phospho-Tau (D). (E) Effect of a 6-h incubation of cells in the absence or presence of C2-ceramide plus PACAP on the level of phospho-Tau. **, P < 0.01; ***, P < 0.001 vs. control; ###, P < 0.001 vs. C2-ceramide.
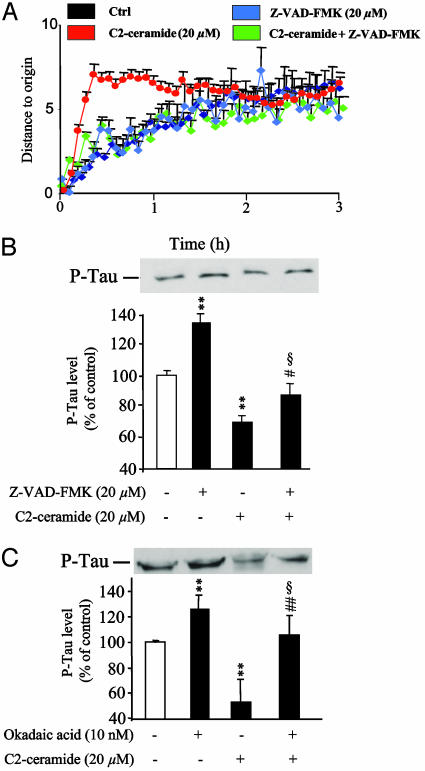
Effects of a Caspase Inhibitor on Cell Migration, Neurite Outgrowth, and Tau Expression. The caspase inhibitor Z-VAD-FMK did not affect migration of granule cells by its own but totally suppressed the transient stimulatory effect induced by C2-ceramide on cell motility (Fig. 9A). Z-VAD-FMK increased the level of phospho-Tau and significantly reduced the inhibitory effect of C2-ceramide on Tau phosphorylation (Fig. 9B). These effects of Z-VAD-FMK were mimicked by okadaic acid, a PP2A phosphatase inhibitor (Fig. 9C).
Fig. 9.
Effect of the caspase inhibitor Z-VAD-FMK on motility and Tau phosphorylation level in cerebellar granule cell. (A) The curves represent the mean distance to origin covered by granule cells cultured in control conditions (black curve, n = 80) or in the presence of 20 μM C2-ceramide (red curve, n = 80), 20 μM Z-VAD-FMK (blue curve, n = 80), or C2-ceramide plus Z-VAD-FMK (green curve, n = 80). (B) Western blot analysis showing the effect of 20 μM C2-ceramide, 20 μM Z-VAD-FMK, or C2-ceramide plus Z-VAD-FMK on the level of phospho-Tau. (C) Western blot analysis showing the effect of 20 μM C2-ceramide, 10 nM okadaic acid on the level of phospho-Tau. **, P < 0.01 vs. control; ##, P < 0.01 vs. C2-ceramide; §, not statistically different from control.
Discussion
Recent studies have demonstrated that ceramides induce apoptosis of cerebellar granule cells (4, 20), whereas PACAP promotes survival and neurite outgrowth in granule neurons (13, 21). However, the effects of ceramides and PACAP on neuronal migration have never been investigated. During histogenesis of the cerebellum, migration of granule cells to reach their final destination occurs through both glia-independent and glia-dependent mechanisms (22). Here, we show that C2-ceramide markedly affects glia-independent granule cell motility and that PACAP prevents the deleterious effects of C2-ceramide on granule neuron migration.
Effects of C2-Ceramide and PACAP on Granule Cell Motility and Neurite Outgrowth. During neurodevelopment, migration of neurons can be schematically divided into three steps (23, 24). First, the cell produces filipodia and lamellipodia that explore the microenvironment and then extend leading edges in response to attractive and repulsive signals that are detected. Second, nucleokinesis occurs; i.e., the nucleus moves into the leading process. Third, the cell retracts the trailing process. Thereafter, neurons that have completed migration acquire their mature morphological characteristics.
Ceramides play a pivotal role during histogenesis of the central nervous system (3, 25). In particular, ceramides act as second messengers mediating the neurotoxic effects of the proinflammatory cytokine TNFα that has been shown to control programmed cell death (26). Ceramides have been recently reported to inhibit growth cone formation (27), but the effects of ceramides on neuronal migration have not been explored. Using time-lapse microscopy, the present study shows that the cell-permeable analog C2-ceramide produces a robust transient stimulation of cell motility that mimicked the “dance of the death” observed during the early stage of apoptosis of retinal ganglion cells (28). During prolonged treatment, C2-ceramide suppressed cell movements and totally inhibited neurite outgrowth. Altogether, these observations strongly suggest that cytokines, acting through ceramide production, contribute to histogenesis of the cerebellar cortex. In support of this notion, it has been shown that overexpression of TNFα alters the cytoarchitecture of the mouse cerebellum (2).
In contrast to C2-ceramide, PACAP markedly inhibited nucleokinesis, reduced cell velocity, and stimulated neurite outgrowth, indicating that the differentiating activity of PACAP is associated with an inhibitory effect on cell migration. Previous studies have shown that PACAP-induced differentiation is mediated through cAMP production in all cell types investigated, including cerebellar granule cells (19), pheochromocytoma cells (29), neuroblastoma cells (30), and embryonic stem cells (31). Increased formation of cAMP induced by PACAP can likely account for its inhibitory effect on cell migration because somatostatin, a peptide that inhibits adenylyl cyclase activity, has been shown to stimulate the early phase of granule cell migration (22). Recently, PACAP has been reported to activate the expression of genes associated with neuronal differentiation, such as synaptophysin (31) and β-tubulin III (30), suggesting that PACAP favors a shift of granule cells from a migrating to a differentiated state.
Cotreatment of cerebellar granule cells with C2-ceramide and PACAP suppressed the transient stimulation of cell motility induced by C2-ceramide and restored neurite outgrowth. In contrast, PACAP could not prevent the long-term action of C2-ceramide on cell migration, suggesting that determination of the cell fate by ceramide and PACAP occurs during the first hours of treatment. Consistent with this hypothesis, it has been previously shown that a 3-h treatment of granule cells with PACAP is sufficient to induce neurotrophic effects (32).
Effects of C2-Ceramide and PACAP on Cytoskeleton Organization. Cytoskeleton components play a pivotal role in cell migration and neurite outgrowth. For instance, leading edge extension largely depends on actin-rich protrusions, whereas nucleokinesis requires microtubule elongation (1). Here, we show that neither C2-ceramide nor PACAP has an effect on de novo synthesis of cytoskeletal proteins but markedly alters their distribution in cerebellar granule cell; C2-ceramide treatment caused a disorganization of the microfilament network, whereas PACAP treatment provoked accumulation of actin in the protrusion region of the neurites and attenuated the dispersion of actin induced by C2-ceramide. These observations suggest that the effects of C2-ceramide and PACAP on actin assembly may eventually affect growth cone dynamics and neuritogenesis. In support with this notion, it has been recently reported that PACAP induces growth cone attraction through a cAMP-dependent mechanism (12), whereas C2-ceramide inhibits growth cone formation through activation of a protein phosphatase (27).
Concurrently, exposure of granule cells to C2-ceramide provoked depolimerization of microtubules, and the organization of the microtubular system was largely restored by coadministration of PACAP. As nucleokinesis is critically dependent on tubulin polymerization (23), our results strongly suggest that inhibition of granule cell nucleokinesis induced by long-term (6–12 h) C2-ceramide treatment results from massive destructuration of tubulin.
Effects of C2-Ceramide and PACAP on Tau Expression and Tau Phosphorylation. Tau, a major microtubule-associated protein found in neurons, stimulates tubulin polymerization and thus maintains the microtubule structure. Tau possesses many phosphorylation sites, and the biological activity of Tau is regulated by its degree of phosphorylation. The present data show that C2-ceramide produces a marked inhibition of phospho-Tau after 4 h of treatment and a moderate reduction of the total level of Tau after 6 h. In contrast, PACAP increased Tau phosphorylation and had no effect on the total amount of Tau. In addition, PACAP prevented the effect of C2-ceramide on both phosphorylated and total Tau. Tau can be phosphorylated by various kinases including PKA and mitogen-activated protein kinases (33) and dephosphorylated by several protein phosphatases such as PP2A, PP2B, and PPC (34). A recent study has also shown that Tau can be cleaved in its C-terminal domain by caspase-3 (35). The fact that ceramides stimulate PP-2A (36) and activate caspase-3 (5), whereas PACAP stimulates PKA and mitogen-activated protein kinase and inhibits caspase-3 activity (16), strongly suggested that C2-ceramide and PACAP could regulate phosphorylation and proteolysis of Tau. To test this hypothesis, we have studied the effects of caspase and protein phosphatase inhibitors on Tau expression and Tau phosphorylation. The cell-permeable caspase inhibitor Z-VAD-FMK suppressed the effect of C2-ceramide on the level of phospho-Tau, indicating that the early action of C2-ceramide on granule cell dance can be accounted for by a caspase-dependent decrease in phospho-Tau level. Consistent with this observation, it has been recently shown that Tau can be hydrolyzed by caspase-3 at the Asp-421 residue (35). On the other hand, there is now evidence that stimulation of the mitochondrial apoptotic pathway by ceramides necessitates activation of the phosphatase PP2A (36). Here, we show that the PP2A inhibitor okadaic acid abolished the inhibitory effect of C2-ceramide on phospho-Tau, indicating that C2-ceramide-induced apoptosis is attributable to activation of a PP2A-like kinase that causes dephosphorylation of Tau.
In conclusion, the present study has demonstrated that C2-ceramide and PACAP exert opposite effects on granule cell motility and neurite outgrowth; C2-ceramide provokes an immediate and transient activation of cell movements, inhibits neuritogenesis, and induces apoptosis, whereas PACAP inhibits cell migration and promotes neurite outgrowth. These opposite effects of C2-ceramide and PACAP on cell motility and neurite extension can be ascribed to their reverse actions on the cytoskeletal architecture and Tau integrity through caspase- and PP2A-dependent mechanisms. Taken together, these data strongly suggest that TNFα and PACAP are involved in the control of granule cell migration and synaptogenesis during the histogenesis of the cerebellar cortex.
Acknowledgments
This study was supported by the Institut National de la Santé et de la Recherche Médicale (U-413), an exchange program from the Institut National de la Santé et de la Recherche Médicale and the Fonds de la Recherche en Santé du Québec (to A.F. and H.V.), and the Conseil Régional de Haute-Normandie. A.F.M. and M. Benard are recipients of a fellowship from the Lille-Amiens-Rouen-Caen-Neuroscience network. N.A. is the recipient of a fellowship from the Centre International de Toxicologie and the Conseil Régional de Haute-Normandie. H.V. is an affiliated Professor at the INRS-Institut Armand Frappier (Montreal).
Author contributions: A.F.-M., D.V., N.A., and B.J.G. designed research; A.F.-M., N.A., L.G., M. Benard, M. Basille, A.F., and B.J.G. performed research; A.F.-M., D.V., M. Benard, M.F., H.V., and B.J.G. analyzed data; and A.F.-M., D.V., H.V., and B.J.G. wrote the paper.
Abbreviation: PACAP, pituitary adenylate cyclase-activating polypeptide.
References
- 1.Tojima, T. & Ito, E. (2004) Prog. Neurobiol. 72, 183-193. [DOI] [PubMed] [Google Scholar]
- 2.Ye, P., Price, W., Kassiotis, G., Kollias, G. & D'Ercole, A. J. (2003) J. Neurosci. Res. 71, 721-731. [DOI] [PubMed] [Google Scholar]
- 3.Bieberich, E., MacKinnon, S., Silva, J. & Yu, R. K. (2001) J. Biol. Chem. 276, 44396-44404. [DOI] [PubMed] [Google Scholar]
- 4.Toman, R. E., Movsesyan, V., Murthy, S. K., Milstien, S., Spiegel, S. & Faden, A. I. (2002) J. Neurosci. Res. 68, 323-330. [DOI] [PubMed] [Google Scholar]
- 5.Falluel-Morel, A., Aubert, N., Vaudry, D., Basille, M., Fontaine, M., Fournier, A., Vaudry, H. & Gonzalez, B. J. (2004) J. Neurochem. 91, 1231-1243. [DOI] [PubMed] [Google Scholar]
- 6.Lozano, J., Morales, A., Cremesti, A., Fuks, Z., Tilly, J. L., Schuchman, E., Gulbins, E. & Kolesnick, R. (2001) Cell Death Differ. 8, 100-103. [DOI] [PubMed] [Google Scholar]
- 7.Sherwood, N. M., Krueckl, S. L. & McRory, J. E. (2000) Endocr. Rev. 21, 619-670. [DOI] [PubMed] [Google Scholar]
- 8.Vaudry, D., Gonzalez, B. J., Basille, M., Yon, L., Fournier, A. & Vaudry, H. (2000) Pharmacol. Rev. 52, 269-324. [PubMed] [Google Scholar]
- 9.Sheward, W. J., Lutz, E. M., Copp, A. J. & Harmar, A. J. (1998) Dev. Brain Res. 109, 245-253. [DOI] [PubMed] [Google Scholar]
- 10.Basille, M., Vaudry, D., Coulouarn, Y., Jégou, S., Lihrmann, I., Fournier, A., Vaudry, H. & Gonzalez, B. J. (2000) J. Comp. Neurol. 425, 495-509. [PubMed] [Google Scholar]
- 11.Hu, Z., Lelievre, V., Rodriguez, W. I., Tam, J., Cheng, J. W., Cohen-Cory, S. & Waschek, J. A. (2001) J. Comp. Neurol. 441, 266-275. [DOI] [PubMed] [Google Scholar]
- 12.Guirland, C., Buck, K. B., Gibney, J. A., DiCicco-Bloom, E. & Zheng, J. Q. (2003) J. Neurosci. 23, 2274-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaudry, D., Gonzalez, B. J., Basille, M., Pamantung, T. F., Fontaine, M., Fournier, A. & Vaudry, H. (2000) Proc. Natl. Acad. Sci. USA 97, 13390-13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicot, A. & DiCicco-Bloom, E. (2001) Proc. Natl. Acad. Sci. USA 98, 4758-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicot, A., Lelievre, V., Tam, J., Waschek, J. A. & DiCicco-Bloom, E. (2002) J. Neurosci. 22, 9244-9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaudry, D., Gonzalez, B. J., Basille, M., Fournier, A. & Vaudry, H. (1999) Proc. Natl. Acad. Sci. USA 96, 9415-9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chartrel, N., Tonon, M. C., Vaudry, H. & Conlon, J. M. (1991) Endocrinology 129, 3367-3371. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez, B. J., Leroux, P., Lamacz, M., Bodenant, C., Balazs, R. & Vaudry, H. (1992) Proc. Natl. Acad. Sci. USA 89, 9627-9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez, B. J., Basille, M., Vaudry, D., Fournier, A. & Vaudry, H. (1997) Neuroscience 78, 419-430. [DOI] [PubMed] [Google Scholar]
- 20.Vaudry, D., Falluel-Morel, A., Basille, M., Pamantung, T. F., Fontaine, M., Fournier, A., Vaudry, H. & Gonzalez, B. J. (2003) J. Neurosci. Res. 72, 303-316. [DOI] [PubMed] [Google Scholar]
- 21.Tabuchi, A., Koizumi, M., Nakatsubo, J., Yaguchi, T. & Tsuda, M. (2001) J. Neurosci Res. 39, 85-93. [DOI] [PubMed] [Google Scholar]
- 22.Yacubova, E. & Komuro, H. (2002) Nature 415, 77-81. [DOI] [PubMed] [Google Scholar]
- 23.Lambert de Rouvroit, C. & Goffinet, A. M. (2001) Mech. Dev. 105, 47-56. [DOI] [PubMed] [Google Scholar]
- 24.Komuro, H. & Rakic, P. (1998) J. Neurosci. 18, 1478-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herget, T., Esdar, C., Oehrlein, S. A., Heinrich, M., Schutze, S., Maelicke, A. & van Echten-Deckert, G. (2000) J. Biol. Chem. 275, 30344-30354. [DOI] [PubMed] [Google Scholar]
- 26.Futerman, A. H. & Hannun, Y. A. (2004) EMBO Rep. 5, 777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geddis, M. S. & Rehder, V. (2003) J. Neurosci. Res. 74, 210-220. [DOI] [PubMed] [Google Scholar]
- 28.Cellerino, A., Galli-Resta, L. & Colombaioni, L. (2000) J. Neurosci. 20, 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaudry, D., Chen, Y., Ravni, A., Hamelink, C., Elkahloun, A. G. & Eiden, L. E. (2002) J. Neurochem. 83, 1272-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heraud, C., Hilairet, S., Muller, J. M., Leterrier, J. F. & Chadeneau, C. (2004) J. Neurosci. Res. 75, 320-329. [DOI] [PubMed] [Google Scholar]
- 31.Cazillis, M., Gonzalez, B. J., Billardon, C., Lombet, A., Fraichard, A., Samarut, J., Gressens, P., Vaudry, H. & Rostene, W. (2004) Eur. J. Neurosci. 19, 798-808. [DOI] [PubMed] [Google Scholar]
- 32.Vaudry, D., Gonzalez, B. J., Basille, M., Anouar, Y., Fournier, A. & Vaudry, H. (1998) Neuroscience 84, 801-812. [DOI] [PubMed] [Google Scholar]
- 33.Raghunandan, R. & Ingram, V. M. (1995) Biochem. Biophys. Res. Commun. 215, 1056-1066. [DOI] [PubMed] [Google Scholar]
- 34.Sun, L., Liu, S. Y., Zhou, X. W., Wang, X. C., Liu, R., Wang, Q. & Wang, J. Z. (2003) Neuroscience 118, 1175-1182. [DOI] [PubMed] [Google Scholar]
- 35.Gamblin, T. C., Chen, F., Zambrano, A., Abraha, A., Lagalwar, S., Guillozet, A. L., Lu, M., Fu, Y., Garcia-Sierra, F., LaPointe, N., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 10032-10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruvolo, P. P., Clark, W., Mumby, M., Gao, F. & May, W. S. (2002) J. Biol. Chem. 277, 22847-22852. [DOI] [PubMed] [Google Scholar]