Abstract
Aim:
The aim of the study was to evaluate the local anesthetic activity of root extracts of the Anacyclus pyrethrum, and to check its effect of interaction at the site of injection in guinea pigs.
Materials and Methods:
The study sample included thirty guinea pigs each weighing 450–500 g, maintained under standard conditions. The root extracts were prepared using three solvents, in 1% and 2% concentration and injected in guinea pigs. The animals were divided into five groups, six in each group based on the type of extract used along with a control and a standard drug. All the animals received 0.5 ml of intradermal injection of the prepared extract, with 1% concentration in the left and 2% in the right dorsal flank of the animal and were checked for local anesthetic activity by a pinprick test. After 72 h, biopsy was done from the injected site to check for drug interaction.
Results:
The number of negative response obtained from 2% ethanol extract is more effective when compared to other extracts. Histological samples showed inflammatory changes in 1% aqueous extract in a single animal.
Conclusion:
Among the test compounds, 2% ethanol showed more significant effect; hence, it is suggested to synthesize more compounds in this series and evaluate their pharmacological actions.
Keywords: Anacyclus pyrethrum, local anesthesia, root extract
INTRODUCTION
One of the most paramount and challenging aspects while performing dental procedure for the child is the pain control. To minimize the likelihood of the child experiencing discomfort during restorative and surgical procedures dentists turn to the utilization of local anesthetics to control pain. Lignocaine is the most popularly used local anesthetic agent with rare incidence of toxicity.[1] Even though local anesthetic toxicity is profoundly infrequent in infants and children, they may be at an increased risk when compared with adults, as the metabolism and elimination of local anesthetics can be delayed in young children.[2] Hence, understanding of the biology and pharmacology of local anesthetic agent is required for quality dental practice.[3]
Over the past three decades, the use of herbal medicinal products has tremendously increased.[4] Even though they have shown promising potential; its safety continues to be a major issue. Unlike their synthetic counterparts, herbal medicines remain untested and yet to be proved clinically. Hence, establishing a scientific proof for its therapeutic actions may help in the development of more effective drug.[5]
Anacyclus pyrethrum is a plant that belongs to Asteraceae family. It has been identified to have properties of a sialagogue, to treat tongue paralysis, throat muscles, epilepsy, and rheumatism, aphrodisiac, and toothache reliever. The cortical portion of the root contains about 5% of the acrid compound called pyrethrin (pellitorine), which is responsible for the various medicinal values of this plant, especially its local anesthetic activity.[6] It was Beckman and Allen 1966, isolated six different components called pyrethrin I, cinerin I, jasmolin I and pyrethrin II, cinerin II and jasmolin II, from this plant extract.[7] Gopalakrishna et al. (1987) in an animal study confirmed that the local anesthetic activity from the root extract of this plant is mainly due to the main ingredient pyrethrin in the plant root.[8] Hence, the present study was designed to evaluate the effectiveness of different root extracts of A. pyrethrum, in comparison with standard lignocaine solution, and to check its interaction at the site of injection in guinea pigs.
MATERIALS AND METHODS
The study design was approved by the Institutional Review Board and Institutional Animal Ethics Committee (Ref no: 1826/Po/EreBi/S/15/CPCSEA). The roots of A. pyrethrum were obtained from an authenticated ayurvedic supplier. They were then dried, powdered, and subjected to successive extraction by soxhlet extraction method. The preparation was done with different organic solvents of varying polarity such as petroleum ether, ethanol, and aqueous (distilled water).[9]
The extract obtained was evaporated using rotary evaporator, to get a fine powdered residue. This powder was stored in a refrigerator, and fresh sterile solutions were prepared as and when required, by dissolving the residue in physiological saline. Each extract from the three solvents was prepared in 1% and 2% concentration.
Thirty albino guinea pigs, weighing 450–500 g, were selected for the study. They were kept under standard conditions (day/night rhythm), 22°C + 2°C room temperature, fed standard pelleted diet, and housed for 1 week in polypropylene cages before experiment to acclimatize to the laboratory conditions. The guinea pigs were randomly divided into five groups of six in each, based on injection given.
Group I - Physiological saline
Group II - 1% and 2% petroleum ether extract in physiological saline
Group III - 1% and 2% ethanol extract in physiological saline
Group IV - 1% and 2% aqueous extract in physiological saline
Group V - 1% and 2% lignocaine hydrochloride.
The animals belonging to different groups were kept in separate cages.
The standard operating procedure for testing local anesthesia as recommended by Bulbring and Wajda was followed in this present study. The method involved the use of intradermal wheal method in guinea pigs and eliciting pain response by gentle pinprick. A day before the injection, the hair on the back of guinea pigs was clipped and removed, using depleting agent, to observe the wheal formation induced by intradermal injection.[10,11]
All the animals received 0.5 ml of intradermal injection irrespective of the group they belonged to. One percent drug concentration was injected in the left dorsal flank and 2% concentration in the right dorsal flank of the animal. The time of injection was noted, and the wheal formation was marked with permanent marker [Figure 1].[10,11]
Figure 1.
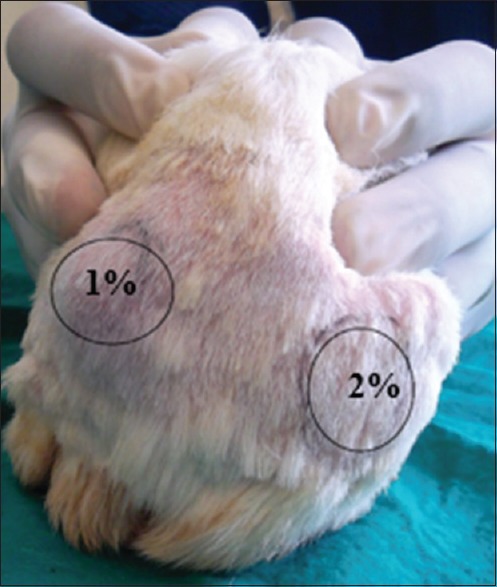
The shaved area in the back of the animal, where 1% drug concentration is injected in the left dorsal flank and 2% drug concentration injected in right dorsal flank, of the animal
A tuberculin syringe (1 ml) with a 3/8 inch, 27-gauge needle was used for the intradermal injection. The skin was pulled taut, and needle was inserted at 5°-15° angle (parallel to skin). Injection was given slowly, and the site was observed for blanching or appearance of wheal. Bleeding from the site or no wheal formation indicated that injection was given incorrectly.[12]
Before the injection was given, the skin was tested to verify the twitching on pricking with a needle. The middle of the back generally is more sensitive than the posterior and anterior areas, so the normal pain response was observed first by pricking in the midline of animal.
The reaction to pinprick was checked every 5 min for 30 min after injection. The pain was evoked by pricking the skin at the center of the wheal. The number of pricks when the guinea pig failed to react was recorded. Each prick was applied at the intervals of about 3–5 s. Six pricks were applied every 5 min in the 30 min duration. A localized skin twitch, usually accompanied by squeak, was considered as the normal response to pinprick. When the animal failed to respond either by twitching or squeaking, a negative response (absence of pain) was recorded. The number of times the prick failed to elicit a response during the 30 min period was added up. The sum out of the possible 42 gave an indication of the degree of anesthesia.[11] After a period of seventy 2 h, tissue sample from the injected site was taken, to check the histopathological changes due to drug interaction [Figure 2]. The biopsy site was then sutured using nonabsorbable monofilament suture material (reverse cutting needle) for skin closure that gets degraded by hydrolysis. The animals were monitored periodically, kept under medication and rehabilitated after the experiment.[13]
Figure 2.
Tissue sample taken from the injected site
RESULTS
The local anesthetic activity of different extracts has been shown in Tables 1 and 2. Table 1 shows 1% lignocaine was effective followed by 1% ethanol and 1% petroleum ether extract. 1% aqueous and control saline solutions showed pain response throughout the period.
Table 1.
Local anesthetic activity with 1% drug concentration for 30 min
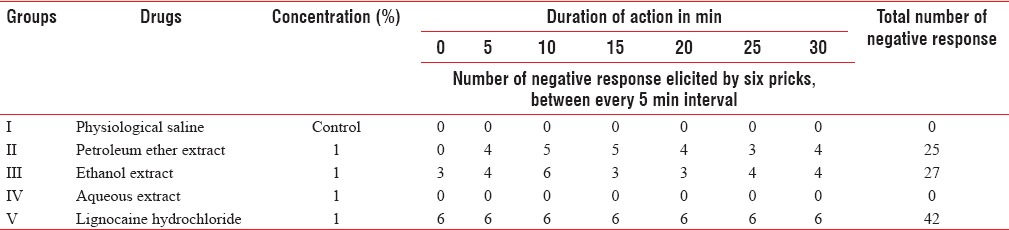
Table 2.
Local anesthetic activity with 2% drug concentration for 30 min
The ethanol extract at 1% concentration showed an immediate onset of action and 27 negative responses (no pain) out of 42 pricks. Petroleum ether 1% had onset of action at 5 min and 25 negative responses.
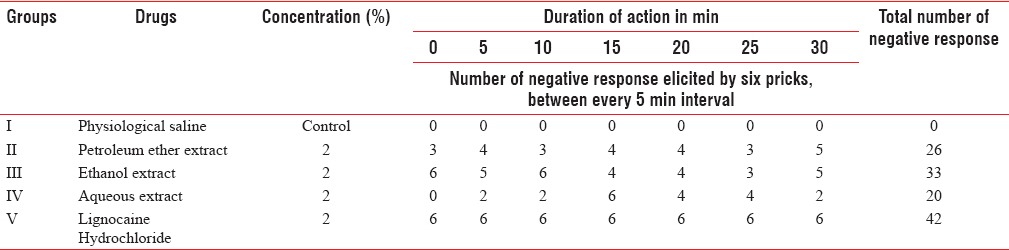
Table 2 shows that 2% lignocaine was more effective followed by 2% ethanol and 2% petroleum ether extract. Two percentage aqueous extract showed negative response at different time intervals but was more effective than 1% aqueous extract.
The ethanol extract at 2% concentration showed an immediate onset of action and 33 negative responses. Petroleum ether 2% had immediate onset of action and 26 negative responses. Two percent aqueous extract showed an onset of action at 5 min and twenty negative responses.
When histopathological changes of injected site were compared with normal tissue sample [Figure 3a], they showed the presence of inflammatory cells, with multinucleated giant cells and granulomatous cells in deep dermal layer of 1% aqueous extract, in a single animal in group IV [Figure 4a–c]. Whereas all other animals treated with 1% and 2% concentration of ethanol, petroleum ether, and aqueous extracts showed histological characteristics similar to the normal tissue sample [Figure 3b–d].
Figure 3.
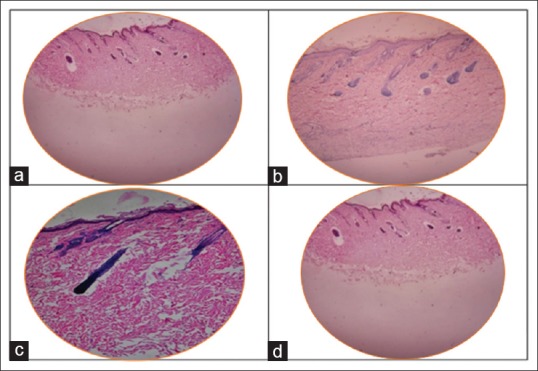
(a) Tissue sample taken from normal skin of the animal. (b) Tissue sample of animal injected with ethanol extract shows no pathological findings. (c) Tissue sample of animal injected with petroleum ether extract shows no pathological findings. (d) Tissue sample of animal injected with aqueous extract shows no pathological findings
Figure 4.
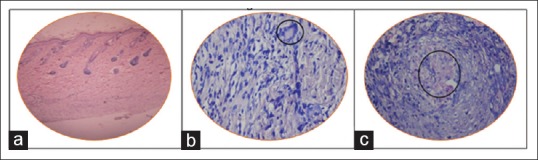
Tissue sample of animal injected with aqueous 1% extract in a single animal in Group IV, shows inflammatory changes (a) with multinucleated giant cells (b), and granulation tissues (c)
DISCUSSION
This study was planned as a preclinical trial, to demonstrate the local anesthetic activity of extracts of A. pyrethrum. Guinea pigs maintained under standard conditions were given intradermal injection using different concentration of extracts of A. pyrethrum and lignocaine, for 30 min.
A. pyrethrum, commonly referred to as Akarkara, is widely recognized in ayurvedic system of Indian medicine for its rejunvenative properties. Different parts of the plant A. pyrethrum, such as flower, root, stem, and leaves have been used traditionally, for various health problems. It has been used as a sialagogue for simulating salivary glands, curing chronic catarrah of the head or nostrils. It has also been used as a tonic for digestion, epilepsy, lethargy, constipation, malaria, and chronic rheumatism.[6]
The plant root is a hard, compact, fusiform root, about the size of the little finger. Research has to be carried out to identify the biological activity of its phytoconstituents so that development of an effective, safe, and cost-effective herbal drug is possible. Alkaloids and other components of A. pyrethrum root might be the cause of local anesthetic action, and this needs further investigation.
Badhe et al. 2010 determined the antidepressant activity of A. pyrethrum root extract. They performed tail suspension test and forced swim test, which is a mouse behavioral test used in the screening of potential antidepressant drugs. A. pyrethrum produced significant antidepressant effect, which was attributed to its interaction with adrenergic and dopamine receptor thereby increasing the level of noradrenaline and dopamine in brains of mice.[14]
Müller-Jakic et al., 1994, reported the antibacterial and anti-inflammatory activities of this plant root.[15] Kumar et al. 2012 compared the alcoholic extract of A. pyrethrum with antibiotic ciprofloxacin and concluded that the plant extract had more effective antimicrobial potential against Bacillus subtilis, Escherichia coli, Salmonella typhi, Klebsiella pneumoniae, and Staphylococcus aureus.[16]
Venkatakrishna-Bhatt et al., 1988 conducted a toxicity analysis with aqueous extract of Pyrethrum in mice. The extract was injected in four doses 400, 550, 750, and 1000 mg/kg intraperitoneal in mice and concluded that a maximum dose of 750 mg/kg is safe and nonlethal to use as a local anesthesia in mice.[17]
Barbero and Frasch, in an in vitro study, compared the permeability of pig and guinea pig skin as surrogate for human skin. They suggested that both pig and guinea pig were good models for human skin permeability and had less variability compared to human skin model.[18]
The local anesthetic test by intradermal injection, inducing wheal formation and pinprick was the standard procedure to check in guinea pigs and was followed in several literatures.[11] Gopalakrishna et al. 1987 stated that 2% aqueous and alcoholic extract of A. pyrethrum showed greater anesthetic activity than lignocaine (0.2%) while tested in guinea pig dermis and rabbit cornea. The local anesthetic activity lasted 12 h longer than lignocaine.[8] In this present study other than aqueous extract, two other solvents were used. In general, the solvent system used plays an important role in the solubility of the active components of plant materials present and this in turn influences the medicinal properties of the extracts. Pathmanathan et al. 2010 have shown that sequential extraction of plant material with solvents of various polarities is useful to extract the biologically active elements from plants.[9] In the present study, sequential extraction was carried out with different solvents such as petroleum ether, ethanol, and distilled water (aqueous). The extracts were prepared in 1% and 2% concentrations, and compared with gold standard 1% and 2% lignocaine (which are used for dental treatments) as control rather than 0.2% lignocaine used by Gopalakrishna et al. Histopathological analysis was also done in this study and this was not done in the previous studies.
The results in this study suggest that ethanol derived extract was more effective in 2% concentration followed by 1%. Similarly, 2% petroleum ether extract was more effective followed by 1% petroleum ether and 2% aqueous extract while 1% aqueous showed no reaction. Turner et al.[19] 2011 suggested a dosage of 0.05–0.1 ml for intradermal injection. In the present study, we used a maximum dose of 0.5 ml to check any adverse toxic effect.[19]
No adverse effects such as sunken eyes, changes in respiration, timidity, and arched back were seen in this study.
Tissue samples were collected from the injected site, 72 h after injection. Histopathological findings showed no inflammatory changes in all experimental groups, except one sample where 1% aqueous extract was injected, inflammatory changes in deep dermal layers were seen. This was assumed to be due to needle stick injury while injecting the animal. The guinea pigs were then observed for 10 days and showed no adverse effects. As there have been no developments in the use of new local anesthetic agents in dentistry in the recent years, the root of A. pyrethrum can be taken for extensive research for the development of the same.
Limitations of the study: Since the study was in preclinical phase, nonhuman subjects (guinea pigs) were used in the present study. To better understand the plant extract constituents, isolation of the active compound (pyrethrin), followed by cell line (nerve block models) evaluation should be done.
CONCLUSION
The present study discloses that the ethanol extract in both 1% and 2% concentration shows significant local anesthetic activity, with rapid onset of action and 33 negative responses in 2% and 27 negative response in 1% concentration. Petroleum ether 2% showed rapid onset of action and equal number of negative responses in both 1% and 2% concentrations. In aqueous extract, 2% exhibited comparatively least number of negative response 20, while 1% aqueous showed a positive response, i.e. there is no loss of sensation.
Histopathological analysis showed no adverse inflammatory changes in all the extracts when compared with the normal tissue sample.
Therefore, among the test compounds, 2% ethanol showed more significant action; hence, it is suggested to synthesize more compounds in this series and evaluate their pharmacological actions, bio-availability, and dosage. Hence, a new molecule with local anesthetic activity similar to lignocaine can be developed as plant extracts may have higher safety margins with minimum or no side effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Setnikar I. Ionization of bases with limited solubility. Investigation of substances with local anesthetic activity. J Pharm Sci. 1966;55:1190–5. doi: 10.1002/jps.2600551104. [DOI] [PubMed] [Google Scholar]
- 2.Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649–72. doi: 10.2165/00128072-200204100-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kalow W. Hydrolysis of local anesthetics by human serum cholinesterase. J Pharmacol Exp Ther. 1952;104:122–34. [PubMed] [Google Scholar]
- 4.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saboo S, Tapadiya R, Khadabadi S, Deokate U. In vitro antioxidant activity and total phenolic, flavonoid contents of the crude extracts of Pterospermum acerifolium wild leaves. J Chem Pharm Res. 2010;2:417–23. [Google Scholar]
- 6.Annalakshmi R, Uma R, Subash G, Muneeswaran A. A treasure of medicinal herb-Anacyclus pyrethrum a review. Indian J Drugs Dis. 2012;1:59–67. [Google Scholar]
- 7.Beckman HF, Allen PT. Analysis of pyrethrins. In: Crosby DG, editor. Natural Pest Control Agents. 1st ed. Washington, DC: American Chemical Society; 1966. pp. 51–64. [Google Scholar]
- 8.Venkatakrishna-Bhatt H, Panchal GM, Devasankariah G, Gopalakrishna G, Patel VK. Local anaesthetic activity of Anacyclus pyrethrum in animals. Asian J microbiol Biotechnol Environ Sci. 2001;3:83–5. [Google Scholar]
- 9.Pathmanathan M, Uthayarasa K, Jeyadevan J, Jeyaseelan E. In vitro antibacterial activity and phytochemical analysis of some selected medicinal plants. Int J Pharm Biol Arch. 2010;1:291–9. [Google Scholar]
- 10.Bulbring E, Wajda I. Biological comparison of local anaesthetics. J Pharmacol Exp Ther. 1945;85:78–84. [PubMed] [Google Scholar]
- 11.Gerald Vogel H. Effect of peripheral nerve function. In: Gerald Vogel H, Bernward A, editors. Drug Discovery and Evaluation-Pharmacological Assay. 2nd ed. Heidelberg: Springer; 2008. p. 957. [Google Scholar]
- 12.Perry AG. Injection techniques. In: Perry AG, Potter PA, editors. Clinical Nursing Skills and Techniques. 6th ed. St. Louis: Elsvier Mosby; 2006. pp. 712–5. [Google Scholar]
- 13.Jindra NM, Imholte ML. The potential application of hairless guinea pigs as a replacement for the Yucatan mini-pig in animal studies. Proc SPIE. 2008;6854:1117. [Google Scholar]
- 14.Badhe SR, Badhe RV, Ghaisas MM, Chopade VV, Deshpande AD. Evaluations of antidepressant activity of Anacyclus pyrethrum root extract. Int J Green Pharm. 2010;4:79–82. [Google Scholar]
- 15.Müller-Jakic B, Breu W, Pröbstle A, Redl K, Greger H, Bauer R. In vitro inhibition of cyclooxygenase and 5-lipoxygenase by alkamides from Echinacea and Achillea species. Planta Med. 1994;60:37–40. doi: 10.1055/s-2006-959404. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Verma U, Ritesh Antimicrobial activity of alcoholic extract of Anacyclus pyrethrum. Indian J Plant Sci. 2012;1:120–3. [Google Scholar]
- 17.Venkatakrishna-Bhatt H, Panchal GM, Patel VK. Toxicity of A. pyrethrum in mice. Curr Sci. 1988;57:912–3. [Google Scholar]
- 18.Barbero AM, Frasch HF. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol In Vitro. 2009;23:1–13. doi: 10.1016/j.tiv.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: Routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 2011;50:600–13. [PMC free article] [PubMed] [Google Scholar]


