Abstract
Background and Aims:
Elective lower segment cesarean section under spinal anesthesia is frequently associated with shivering. Ondansetron has been shown to be effective for postspinal shivering. In the present study, we compare the efficacy of ondansetron to prevent postspinal shivering in parturients undergoing cesarean delivery under spinal anesthesia.
Materials and Methods:
A total of eighty full-term parturients scheduled for elective lower segment cesarean section under spinal anesthesia were randomly allocated into two groups. Group O received 8 mg/4 ml ondansetron, and Group S received 4 ml normal saline intravenously immediately before induction of spinal anesthesia. The level of sensory block, core body temperature, shivering score, and presence or absence of nausea and vomiting during the perioperative period, 1st and 5th min neonates Apgar scores were recorded. The data analysis was carried out with Z-test and Chi-square test.
Results:
Ten percent (4/40) of patients in Group O and 42.5% (17/40) of patients in Group S had Grade III shivering during the perioperative period and that was treated with intravenous injection tramadol (P = 0.001). Two patients (5%) in ondansetron and 19 patients (47.5%) in control group had nausea and vomiting (P < 0.001) and was treated with intravenous 10 mg metoclopramide. 1st and 5th min Apgar scores of neonates were not statistically different in the groups.
Conclusions:
Ondansetron is an effective way to prevent shivering, nausea and vomiting during lower segment cesarean section under spinal anesthesia with no effect on Apgar score.
Keywords: Anesthesia, cesarean section, female, ondansetron, pregnancy, shivering, spinal
INTRODUCTION
Shivering, the “big little problem” during anesthesia has an incidence of 30%–40% following regional anesthesia.[1] Apart from nausea and vomiting, postspinal shivering is one of the leading causes of discomfort for patients. Shivering not only causes psychological stress to the patient but also physiologically leads to an increase in oxygen consumption by 200%–600% and increased carbon dioxide production, increased chances of myocardial ischemia, infection, bleeding, and increase in minute ventilation. It also produces hypoxemia, lactic acidosis, increased intraocular pressure, intracranial pressure and interferes with patient monitoring such as electrocardiogram (ECG), noninvasive blood pressure (NIBP), and peripheral oxygen saturation (SpO2).
The etiology of shivering is not clearly understood. The mechanisms chiefly responsible for shivering in patients undergoing surgery are intraoperative temperature loss, increased sympathetic tone, pain, and systemic release of pyrogens.[2] The best way to avoid the intraoperative and postoperative shivering induced complications is to prevent shivering. Perioperative hypothermia and shivering are usually prevented by physical methods such as surface warming and pharmacologically by drugs such as pethidine, tramadol, clonidine, and ketamine.
The neurotransmitter pathways involved in the mechanism of shivering are complex and still poorly understood. Serotonin (5-hydroxytryptamine [5-HT3]), a biologic amine found in the brain and spinal cord, plays a part in neurotransmission of shivering. Many studies explained that the serotonergic system plays an important role in the pathogenesis of perioperative shivering.[3,4] Serotonin antagonism seems to lower the human thermal set range thereby reducing metabolic cold defenses and discomfort associated with postoperative hypothermia.
Ondansetron, 5-HT3 antagonist, is a widely used antiemetic drug. It can be used safely during pregnancy and surgery. Some studies showed its anti-shivering effect following both general and regional anesthesia.[5] It has a potential advantage in obstetric anesthesia because of its very low incidence of sedation, hypotension, bradycardia, or risk to the neonate. The mechanism of action of ondansetron as anti-shivering is not clear, and it is proposed to act centrally at the level of the preoptic anterior hypothalamic region by inhibition of serotonin reuptake.[6]
On the other hand, nausea and vomiting during spinal anesthesia for cesarean section are very common and unpleasant complications. During spinal anesthesia, ondansetron has been shown to be effective in the prevention of nausea and vomiting.[7]
Based on a previous study, we hypothesized that ondansetron can reduce postspinal shivering in pregnant women undergoing lower segment cesarean section under spinal anesthesia.[8] The primary aim of our study is to evaluate the efficacy of ondansetron in reducing the postspinal shivering in pregnant patients undergoing elective lower segment cesarean section under spinal anesthesia. The secondary aim is to evaluate the effectiveness of ondansetron in reducing intraoperative nausea and vomiting.
MATERIALS AND METHODS
After approval of our Institutional Ethical Committee and obtaining written informed consents, eighty American Society of Anesthesiologists (ASA) physical Status I and II full-term parturients, 22–32 years old, scheduled for elective lower segment cesarean section surgery under spinal anesthesia were enrolled in this prospective, randomized study. The study was carried out in RIMS, Kadapa general hospital from March to November 2016. Patients with contraindications to spinal anesthesia, cardiopulmonary disease, psychological disorder, and thyroid disorders, patients who are likely to receive blood transfusion intraoperatively and with body temperature more than 38°C or <36.5°C were excluded from the study.
Eighty full-term parturients were randomized using a computer-generated randomization list [Figure 1]. The randomization scheme was generated using the website randomization.com (http://www.randomization.com). Random group assigned was enclosed in a sealed envelope to ensure concealment of allocation sequence. Sealed envelope was opened by an anesthesiologist not involved in the study to prepare the drug solution in a masked 5 ml syringe according to randomization. The anesthesiologist performing the block and observing the patient were blinded to the treatment group. Data collection was done by the same anesthesiologist who was unaware of the group allocation. Patients were randomly assigned to one of the two equal groups. All patients received an intravenous bolus of the tested drug in 4 ml volume, immediately before induction of spinal anesthesia. Group O (forty patients) received 4 ml (8 mg) ondansetron while Group S (forty patients) received 4 ml of normal saline 0.9%.
Figure 1.
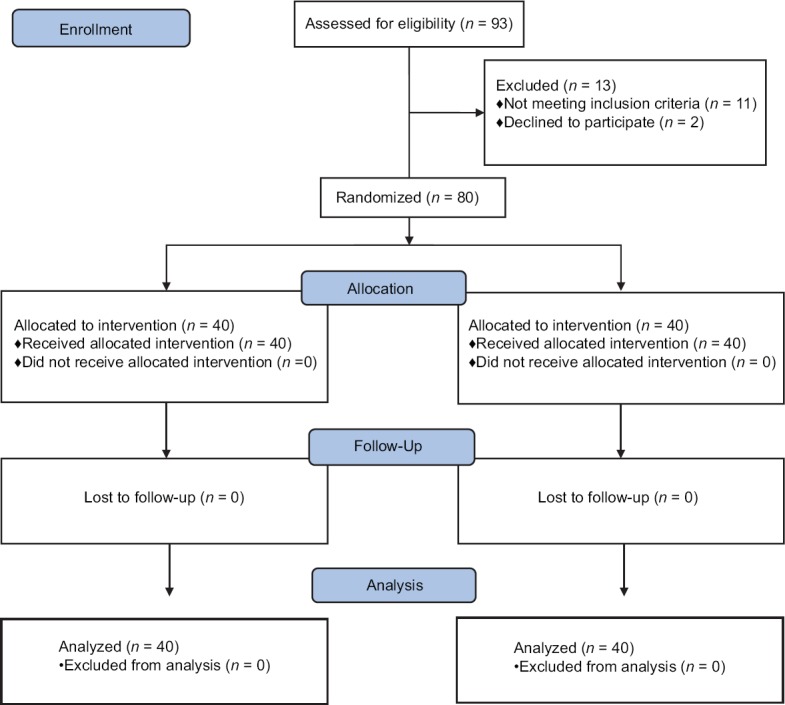
CONSORT diagram showing the number of patients included and analyzed
Patients were routinely fasted and premedicated with ranitidine 150 mg orally night before surgery. On arrival to the operating room, standardized monitoring was done throughout the perioperative period. Heart rate (HR), ECG, NIBP, respiratory rate, and SpO2 were recorded. Core body temperature was measured by tympanic thermometer (Thermoscan IRT 3020; Braun, Kronberg, Germany), and skin temperature was measured using skin probe. Operation theatre temperature was maintained at 24°C by air-conditioning.
Peripheral intravenous access was secured using an 18-gauge cannula on the dorsum of the nondominant arm. All patients were preloaded with warm Ringer's lactate solution of 10 ml/kg before spinal anesthesia. Patients received respective drugs intravenously just before initiation of spinal anesthesia. All patients were blocked in the sitting position, in which a 25-gauge Quincke needle was inserted by midline approach into the L3–L4 or L4–L5 interspaces and after ensuring the correct position of the needle, 12.5 mg of hyperbaric 0.5% bupivacaine was injected. Patients were immediately placed in the supine position after the block. The administration of pre- or intra-operative opioids was not permitted except injection tramadol as rescue anti-shivering drug. Supplemental oxygen (3 L/min) was applied through a nasal cannula till the end of the surgery. All patients were covered with one layer of paper surgical drapes and one layer of a cotton blanket positioned over the thighs and calves. In addition, one layer of a cotton blanket was placed over the chest and arms. No other warming devices were used.
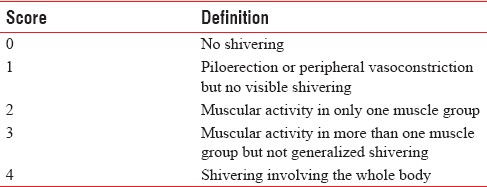
The level of sensory block, core body temperature, shivering score, and presence or absence of nausea and vomiting during perioperative period, 1st and 5th min Apgar scores were recorded. Body temperatures were monitored just before intrathecal injection and then with every 15 min intervals up to 6 h. Shivering was graded using a 5-item scale [Table 1]. Grades III and IV shivering for at least 3 min were considered positive, and prophylaxis was regarded as ineffective. An intravenous bolus of tramadol 1 mg/kg was used as a rescue drug.
Table 1.
Five-item scale of assessing shivering
The incidence of hypotension, bradycardia, nausea and vomiting were recorded. Hypotension was defined as fall in blood pressure by 20% from the baseline or an absolute mean arterial pressure (MAP) <60 mmHg; which was managed by increments of intravenous ephedrine 6 mg. Bradycardia was defined as a decrease in HR by 20% from the baseline value or an absolute HR <50 beats/min; which was managed by 0.5 mg intravenous bolus of atropine. Patients with refractory nausea or vomiting were treated with 10 mg metoclopramide intravenous as a rescue medication.
Power analysis was based on the results of a previous study conducted by Kelsaka et al., they have shown that ondansetron can reduce the incidence of postspinal shivering to 8% compared with 36% in the control group.[9] A sample size was calculated based on these findings, with a value of 0.05 and power (1− β) of 0.80. It was calculated that 36 subjects were required per group. We included forty patients in each group for better validation of results. Data were checked, entered, and analyzed using SPSS version 19 for Windows (IBM Corp., Armonk, NY, USA). Data were expressed as mean ± standard deviation for quantitative variables, and for categorical variables as number and percentages. Z-test and Chi-square tests were used for comparison in between groups. P < 0.05 was considered statistically significant.
RESULTS
A total of 93 full-term pregnant women who were posted for elective lower segment cesarean section under spinal anesthesia were included in the study. Finally, eighty patients were analyzed [Figure 1].
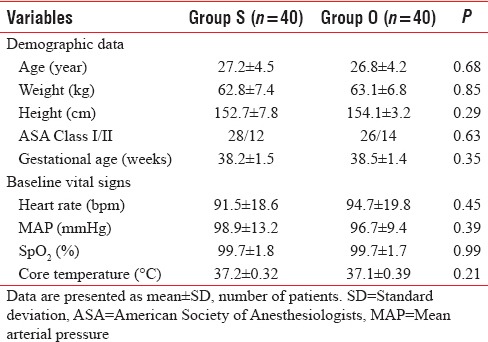
The demographic characteristics such as age, weight, height, ASA grade, and gestational age were comparable in both groups (P > 0.05) [Table 2]. There was no difference in baseline vital signs (HR, MAP, and SpO2). In addition, the preoperative core body temperature was not statistically different between the study groups [Table 2].
Table 2.
Preoperative variables
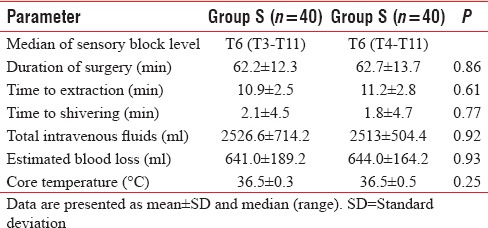
Duration of surgery was 62.7 ± 13.7 min in Group O and 62.2 ± 12.3 min in Group S and was comparable in both groups.(P = 0.86). The level of sensory block, time to extraction, time to shivering, total intravenous fluids, and estimated blood loss were also not statistically significant [Table 3]. There was no difference in intraoperative mean core body temperature in both groups (P = 0.25).
Table 3.
Intraoperative clinical characteristics
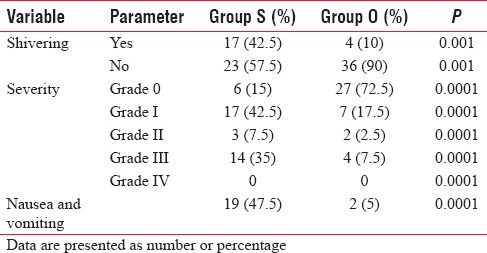
Grade IV shivering was not observed in any patient in both groups. Ten percent (4/40) of patients in Group O and 42.5% (17/40) of patients in Group S had Grade III shivering during the perioperative period and that was treated with intravenous injection tramadol (P < 0.001) [Table 4]. Two patients (5%) in ondansetron and 19 patients (47.5%) in control group had nausea and vomiting (P < 0.001) and was treated with intravenous 10 mg metoclopramide.
Table 4.
Incidence and severity of shivering and incidence of nausea and vomiting
None of the patients in both groups received atropine. Eighteen patients in the Group S and six patients in the Group O received the intravenous injection ephedrine 6 μg for hypotension (P = 0.016). The hemodynamic profiles between the two groups were statistically significant (P = 0.016).
First minute Apgar scores were 10 ± 3 and 10 ± 2 and 5th min Apgar scores were10 ± 1 and 10 ± 1 in Group O and Group S, respectively (P = 0.17, P = 0.16). There were no adverse effects of ondansetron on fetuses after delivery compared to the saline group regarding the Apgar scores.
DISCUSSION
The results of this prospective, randomized, double-blinded study demonstrate that statistically significant higher incidence of shivering was seen in Group S compared to Group O (P = 0.001). The incidence of maximum shivering score was also high in Group S compared to Group O. In addition, the incidence of nausea and vomiting was significantly high in Group S compared to Group O. 1st and 5th min Apgar scores of neonates in both groups were also not statistically significant.
It has been reported that shivering occurs in up to 40% of patients undergoing surgery under regional anesthesia.[10] In our study, the incidence of shivering was similar (42.5% [17/40]) in the control group. During the perioperative period, core body temperature should be maintained within the limit of 36.5°C–37.5°C because shivering is a response of hypothermia. Under regional anesthesia, shivering may also seen in normothermic patients.[11] A number of factors including age, level of sensory block, type and volume of infusion solution, and temperature of the operating room are risk factors for developing hypothermia in regional anesthesia.[12] In our study, patients between 22 and 32 years age (pregnant patients) were included, the temperature of the operating room was maintained about 24°C during the intraoperative period, and infusions of cold crystalloid solutions were avoided.
Ondansetron, granisetron, and dolasetron, all are 5-HT3-receptor antagonists. Recently, they have been used very effectively to decrease postspinal shivering. The mechanism of 5-HT3-receptor antagonists in preventing postspinal shivering is still not clearly understood but is thought to be related to inhibition of serotonin reuptake on the preoptic anterior hypothalamic region.[13]
In a double-blinded, placebo-controlled study by Powell and Buggy, two doses of ondansetron (4 mg vs. 8 mg) were compared with placebo for prevention of shivering after general anesthesia, in which 82 patients (age, 18–60 years) were randomized into three groups.[14] Postanesthetic shivering was observed in 16/28 (57%) patients in Group C compared with 9/27 (33%) in Group O 4 (P = 0.13) and 4/27 (15%) patients in Group O 8 (P = 0.003).
In regional anesthesia such as spinal anesthesia, Kelsaka et al. reported the incidence of postspinal shivering in ondansetron 8 mg group was 8% compared to 36% in the control group, which was very similar to our results of 10% in the ondansetron group versus 42.5% in the control group.[9] In another study by Kim et al. on 52 patients who had undergone knee arthroscopy under spinal anesthesia, ramosetron, a selective serotonin 5-HT3 receptor antagonist effectively prevented shivering during spinal anesthesia.[15] These results are corroborating with the results of our study.
In a study by Sagir et al. on 160 patients undergoing urological surgery under spinal anesthesia, the patients were randomly allocated to receive saline (Group P, n = 40), ketamine 0.5 mg/kg (Group K, n = 40), granisetron 3 mg (Group G, n = 40), or ketamine 0.25 mg/kg + granisetron 1.5 mg (Group KG, n = 40). The number of patients with observed shivering was 22 in Group P, 6 in Group G, 7 in Group GK, and 0 in Group K. The number of patients with a shivering score of 3 was statistically significantly higher in Group P compared with the other groups. They concluded that prophylactic use of ketamine and granisetron separately and in combination was effective in preventing shivering developed during regional anesthesia that emphasizes the effect of a serotonin 5-HT3 receptor antagonist on the prevention of shivering.[16]
In a prospective double-blinded study by Sajedi et al. on 132 ASA I–II, patients undergoing elective orthopedic surgery under standardized general anesthesia patients were randomly assigned to one of the four equal groups. Group T received 1 mg/kg tramadol; Group G received 40 μg/kg granisetron, Group M received 0.4 mg/kg meperidine, and Group P received saline 0.9% as placebo. They showed that prophylactic use of granisetron 40 μg/kg is as effective as meperidine (0.4 mg/kg) and tramadol (1 mg/kg) in preventing postanesthetic shivering without prolonging the emergence time from anesthesia. They concluded that granisetron was as effective drug as pethidine in preventing postanesthetic shivering which was comparable to our study.[17]
Recent studies showed that serotonin receptor antagonists (ondansetron, granisetron) are highly effective for nausea, retching, and vomiting during regional anesthesia for cesarean delivery in parturients and correlated with our results.[18] Nausea and vomiting was observed in 19/40 patients (47.5%) in the normal saline group while 2/40 patients (5%) in the ondansetron group. Owczuk et al. found that intravenous ondansetron attenuates spinal-induced hypotension.[19] They advised the use of ondansetron in high-risk population, including pregnant women, in whom administration of vasoconstrictors can produce adverse effects on uterine blood flow, as well as elderly persons, in whom excess fluid administration is contraindicated due to the risk of cardiovascular decompensation. Sajedi et al. reported a higher incidence of nausea (27.3%), vomiting (3%), and respiratory depression (12.1%) in meperidine group. The study also showed nausea in 3% patients in granisetron group. Mohammadi et al. study concluded granisetron prevents shivering, and it also reduced nausea and vomiting. The distinctive feature of this study is that we assessed the effectiveness of one drug for prevention of both shivering, nausea and vomiting at the same time during neuraxial block.
CONCLUSIONS
On the basis of the findings of the present study, we concluded that ondansetron 8 mg intravenously is an effective prophylactic means of prevention of postspinal anesthesia induced shivering during lower segment cesarean section delivery, with no effect on Apgar score. It is also very effective against nausea and vomiting. Intravenous ondansetron also reduces hemodynamic changes following spinal anesthesia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Iqbal A, Ahmed A, Rudra A, Wankhede RG, Sengupta S, Das T, et al. Prophylactic granisetron vs. pethidine for the prevention of postoperative shivering: A randomized control trial. Indian J Anaesth. 2009;53:330–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Bozgeyik S, Mizrak A, Kiliç E, Yendi F, Ugur BK. The effects of preemptive tramadol and dexmedetomidine on shivering during arthroscopy. Saudi J Anaesth. 2014;8:238–43. doi: 10.4103/1658-354X.130729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tie HT, Su GZ, He K, Liang SR, Yuan HW, Mou JH. Efficacy and safety of ondansetron in preventing postanesthesia shivering: A meta-analysis of randomized controlled trials. BMC Anesthesiol. 2014;14:12. doi: 10.1186/1471-2253-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammadi SS, Jabbarzadeh S, Movafegh A. Efficacy of granisetron on prevention of shivering, nausea and vomiting during cesarean delivery under spinal anesthesia. J Obstet Anaesth Crit Care. 2015;5:22–6. [Google Scholar]
- 5.Shakya S, Chaturvedi A, Sah BP. Prophylactic low dose ketamine and ondansetron for prevention of shivering during spinal anaesthesia. J Anaesthesiol Clin Pharmacol. 2010;26:465–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Asl ME, Isazadefar K, Mohammadian A, Khoshbaten M. Ondansetron and meperidine prevent postoperative shivering after general anesthesia. Middle East J Anaesthesiol. 2011;21:67–70. [PubMed] [Google Scholar]
- 7.Choi DK, Chin JH, Lee EH, Lim OB, Chung CH, Ro YJ, et al. Prophylactic control of post-operative nausea and vomiting using ondansetron and ramosetron after cardiac surgery. Acta Anaesthesiol Scand. 2010;54:962–9. doi: 10.1111/j.1399-6576.2010.02275.x. [DOI] [PubMed] [Google Scholar]
- 8.Badawy AA, Mokhtar AM. The role of ondansetron in prevention of post-spinal shivering in obstetric patients. Egypt J Anaesth. 2017;33:29–3. [Google Scholar]
- 9.Kelsaka E, Baris S, Karakaya D, Sarihasan B. Comparison of ondansetron and meperidine for prevention of shivering in patients undergoing spinal anesthesia. Reg Anesth Pain Med. 2006;31:40–5. doi: 10.1016/j.rapm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Joshi SS, Arora A, George A, Shidhaye RV. Comparison of intravenous butorphanol, ondansetron and tramadol for shivering during regional anesthesia. Anaesth Pain Intensive Care. 2013;17:33–9. [Google Scholar]
- 11.Sayed AM, Ezzat SM. Preoperative granisetron for shivering prophylaxis in cesarean section under spinal anesthesia. Ain Shams J Anaesthesiol. 2014;7:151–5. [Google Scholar]
- 12.Wang M, Zhuo L, Wang Q, Shen MK, Yu YY, Yu JJ, et al. Efficacy of prophylactic intravenous ondansetron on the prevention of hypotension during cesarean delivery: A dose-dependent study. Int J Clin Exp Med. 2014;7:5210–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Marashi SM, Soltani-Omid S, Soltani Mohammadi S, Aghajani Y, Movafegh A. Comparing two different doses of intravenous ondansetron with placebo on attenuation of spinal-induced hypotension and shivering. Anesth Pain Med. 2014;4:e12055. doi: 10.5812/aapm.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell RM, Buggy DJ. Ondansetron given before induction of anesthesia reduces shivering after general anesthesia. Anesth Analg. 2000;90:1423–7. doi: 10.1097/00000539-200006000-00032. [DOI] [PubMed] [Google Scholar]
- 15.Kim MS, Kim DW, Woo SH, Yon JH, Lee S. Effect of ramosetron on shivering during spinal anesthesia. Korean J Anesthesiol. 2010;58:256–9. doi: 10.4097/kjae.2010.58.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagir O, Gulhas N, Toprak H, Yucel A, Begec Z, Ersoy O. Control of shivering during regional anaesthesia: Prophylactic ketamine and granisetron. Acta Anaesthesiol Scand. 2007;51:44–9. doi: 10.1111/j.1399-6576.2006.01196.x. [DOI] [PubMed] [Google Scholar]
- 17.Sajedi P, Yaraghi A, Moseli HA. Efficacy of granisetron in preventing postanesthetic shivering. Acta Anaesthesiol Taiwan. 2008;46:166–70. doi: 10.1016/S1875-4597(09)60004-7. [DOI] [PubMed] [Google Scholar]
- 18.Rai S, Verma S, Pandey HP, Yadav P, Patel A. Role of butorphanol and ondansetron premedication in reducing postoperative shivering after general and spinal anesthesia: A randomized comparative study from North India. Anesth Essays Res. 2016;10:319–23. doi: 10.4103/0259-1162.172724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owczuk R, Wenski W, Polak-Krzeminska A, Twardowski P, Arszulowicz R, Dylczyk-Sommer A, et al. Ondansetron given intravenously attenuates arterial blood pressure drop due to spinal anesthesia: A double-blind, placebo-controlled study. Reg Anesth Pain Med. 2008;33:332–9. doi: 10.1016/j.rapm.2008.01.010. [DOI] [PubMed] [Google Scholar]


