Abstract
Background
Better surgical techniques, chemotherapy and biological therapy have improved survival in patients with colorectal cancer (CRC), most markedly in younger patients. About half of patients over 70 years receive dose reductions or early treatment discontinuation of the planned adjuvant or first-line treatment due to side effects. The Comprehensive Geriatric Assessment (CGA) is a multidisciplinary evaluation of an elderly individual’s health status. This assessment in older patients with cancer can predict survival, chemotherapy toxicity and morbidity.
Methods
This randomized phase II trial (GERICO) is designed to investigate whether comprehensive geriatric assessment and intervention before and during treatment with chemotherapy in frail elderly patients with stages II–IV CRC will increase the number of patients completing chemotherapy. All patients ≥70 years in whom chemotherapy for CRC is planned to start at Herlev and Gentofte Hospital are screened for frailty using the G8 questionnaire at the first visit to the outpatient clinic. The G8 questionnaire is a multi-domain screening tool to identify frail or vulnerable patients at risk of increased toxicity and morbidity. Frail patients are offered inclusion and are then randomized to two groups (the intervention group and the control group). Patients in the intervention group receive a full geriatric assessment of comorbidity, medication, psycho-cognitive function, physical, functional and nutrition status, and interventions are undertaken on identified health issues. Simultaneously, they are treated for their cancer according to international guidelines. Patients in the control group receive the same chemotherapy regimens and standard of care. Primary outcome is number of patients completing scheduled chemotherapy at starting dose. Secondary outcomes are dose reductions, treatment delays, toxicity, time to recurrence, survival, cancer-related mortality and quality of life.
Discussion
This ongoing trial is one of the first to evaluate the effect of geriatric intervention in frail elderly patients with CRC. The trial will provide new and valuable knowledge about whether it is beneficial for the elderly patient undergoing chemotherapy to be treated simultaneously by a geriatrician.
Trial registration
ClinicalTrials.gov ID: NCT02748811. The trial was registered retrospectively; registration date 04/28/2016.
Keywords: Chemotherapy, Colorectal cancer, Comprehensive geriatric assessment, Elderly, Frail, Intervention
Background
The incidence of colorectal cancer (CRC) increases with age [1], and as populations are getting older [2], an increasing number of elderly patients will be diagnosed with CRC. In recent years, mortality has decreased in patients with CRC due to better surgical techniques, chemotherapy and new biological therapy, but in elderly patients, the mortality remains higher than in younger patients [3, 4].
Adjuvant chemotherapy after surgery for stages II and III colon cancer (CC) with 5-fluorouracil (5-FU) [5, 6] or capecitabine [7, 8] reduces the recurrence rate and improves overall survival (OS). The evidence for the recommendation of adjuvant chemotherapy in rectal cancer is sparse, but one meta-analysis supports the use of 5-FU-based postoperative treatment [9]. For patients older than 70 years, adjuvant treatment also prolongs time to recurrence [10], and OS is higher in CRC patients >75 years receiving adjuvant chemotherapy compared to patients not treated with chemotherapy [11–13]. There are divergent results in elderly (defined as age > 70 years) regarding the addition of oxaliplatin to 5-FU (FOLFOX) or capecitabine treatment (CAPEOX) on disease-free survival (DFS) and OS [14–16]. However, combination chemotherapy is considered standard therapy for patients with stage III CRC, but the beneficial effect for elderly patients and patients with stage II CC remains controversial [5, 17].
Although the benefit of a least single-agent treatment seems to be the same for elderly and younger patients in clinical trials, elderly patients are less frequently treated [18]. In a study reported by Sanoff HK, 63% of patients aged 75–79 years with CC stage III received adjuvant chemotherapy, whereas only 14% of patients ≥85 years received chemotherapy [11]. Elderly patients do not receive adjuvant treatment because of high age, co-morbidity and poor performance status (PS) [12], but this could also be due to lack of social support and concerns regarding toxicity and efficiency. The underuse of this potentially lifesaving treatment may explain a higher prevalence of recurrence and the higher mortality seen in the elderly with CRC [4]. About half of patients over 70 years receive dose reductions or early treatment discontinuation of the planned treatment due to severe side effects [12].
For patients with metastatic CRC (mCRC), treated with combinations of chemotherapy drugs (5-FU, capecitabine, oxaliplatin or irinotecan) and new biological drugs in clinical trials, the median OS has increased from 6 to 8 months to about 24 months over the last decades [4, 19]. But, in clinical practice, the median survival is only 10–11 months, mainly due to poor OS in elderly patients and patients with low PS who do not receive any treatment [20].
In elderly patients, irinotecan as second-line therapy has the same efficacy as that seen in younger patients, but gives an increased risk of grades 3–4 neutropenia and diarrhea. [21]. Irinotecan/5-FU combinations are well tolerated and their efficacy is similar to that seen in younger patients [22].
Retrospective studies suggest that combination chemotherapy for elderly patients with mCRC is associated with longer progression-free survival (PFS) [23], even if 71% of all patients with CAPEOX received dose reductions. In another study of elderly and frail patients, no significant improvement was seen when reduced doses of oxaliplatin were added to capecitabine or 5-FU [24].
In elderly patients with mCRC, adding bevacizumab to standard chemotherapy improves PFS and OS [25, 26]. However, an increase in thromboembolic events is observed in patients >65 years [25, 27, 28]. For cetuximab and panitumumab, efficacy and safety are similar for patients ≤65 and >65 years [29]. Single-agent (off-label) panitumumab has been shown to be an effective and well-tolerated first-line treatment for frail elderly patients deemed unfit for chemotherapy [30].
Geriatric oncology
Oncologists and geriatricians have begun to cooperate to individualize and improve treatment for elderly patients with cancer [31]. Elderly vulnerable patients are at high risk of severe toxicity [32]. During the last decade, there has therefore been an increased focus on the importance of individual patient assessment before treatment decisions are made. Chronological age per se should not be an exclusion criterion for adjuvant or palliative chemotherapy [33–35]. Comorbidity and PS more than age alone influence treatment outcomes for both patients in adjuvant [36] and palliative therapy [23, 37]. The Comprehensive Geriatric Assessment (CGA) is a multidisciplinary comprehensive evaluation of an elderly individual’s functional status, physical performance, comorbidity, polypharmacy, cognitive and emotional function, nutritional status and need of social support [38, 39]. CGA in older patients with cancer can predict survival, chemotherapy toxicity and morbidity [40–42]. The assessment can detect unknown geriatric problems and lead to change in treatment strategy for 20% to 49% of patients [43, 44]. To assess all those important factors, there is a need for a multidisciplinary approach in decision making, before and during treatment with chemotherapy, in elderly patients with CRC [31, 35].
Geriatric frailty assessment can predict 1- and 5-year survival in patients after surgery for CRC, and the impact of frailty on 5-year survival is comparable with the impact of TNM stage [45]. Interventions on identified health issues found by the CGA could reduce the side effects and thus increase the number of patients who complete chemotherapy. The supportive care could also improve coping strategies in dealing with adverse events and thereby improve treatment compliance. This could increase survival of the patients. In a recent randomized study, the impact of geriatric intervention on tolerance to chemotherapy in elderly patients (age > 70 years) was investigated. The patients who received CGA in the intervention group (N = 46) were more likely to complete planned chemotherapy without dose modifications, but there was no difference in grade 3+ toxicity between the two groups [46].
Screening for frailty
CGA is considered as the most appropriate way to examine the overall health situation of the elderly patient, but it is time consuming and not required for all patients, and other frailty screenings tools have been developed and validated. In the recommendations from the International Society for Geriatric Oncology (SIOG) from June 2014 [47], the G8 questionnaire is found to be the more or equally sensitive than other screening tools compared to CGA. For patients with an abnormal G8 score (≤ 14/17), full CGA is recommended [48].
Geriatric intervention in non-oncological settings
The effect of geriatric intervention in patients undergoing chemotherapy for CRC has not been fully investigated. However, there is evidence for a beneficial effect of geriatric assessment for elderly admitted to hospitals for medical conditions. In a meta-analysis of randomized trials, CGA after appropriate interventions during hospitalization increased the likelihood of being alive and living at home 6 months after hospital discharge [49]. The beneficial effect was greatest for geriatric wards, but geriatric teams had also a positive effect.
Several studies have evaluated the cooperation between geriatricians and surgeons from different specialties, and a beneficial effect in terms of cost reduction and treatment outcomes was found, e.g. in elderly patients with hip fractures randomized to comprehensive geriatric care compared to usual orthopedic care, 4-month mobility improves [50]. Multi-disciplinary preoperative CGA with post-operative in-ward follow-up by geriatricians in elderly patients at risk of complications due to elective orthopedic surgery decreased the risk of complications and reduced length of hospital stay [51]. For elderly patients undergoing elective orthopedic, urological or gastrointestinal surgery, a preoperative CGA led to fewer cancelled procedures, a decrease in postoperative complications and a reduced length of stay [52].
To our knowledge, there are no intervention studies evaluating the beneficial effect of geriatric intervention on chemotherapy completion in patients undergoing chemotherapy for CRC. No such studies are described on clinical trials.gov (search July 15, 2016). The present trial will therefore provide new and valuable knowledge about whether it is beneficial for the elderly patient with CRC to be simultaneously treated by a geriatrician.
There is no evidence for the effect of the full geriatric intervention in patients with CRC undergoing chemotherapy; however, different parts of the geriatric assessment have been shown to be predictive, and intervention has been shown to be beneficial.
Physical exercise
In the last decade, there has been an increased focus on physical performance, exercise and their relation with a diagnosis of CC, treatment outcomes and quality of life during and after chemotherapy. Physical activity prevents risk of CC [53], and several studies suggest that physical activity after cancer diagnosis increases DFS and OS [54], and it is found to be associated with CC-and CRC-specific mortality [55, 56]. In mouse models, physical exercise reduces tumor growth, probably by increasing immune cell infiltration [57]. Jung et al. found that decreased muscle mass at the start of treatment was significantly associated with toxicity grades 3 to 4 in patients undergoing adjuvant chemotherapy [58]. Others have reported the beneficial effect of physical training on quality of life and fatigue during chemotherapy [59, 60]. In a recent study of 33 patients with CC, an 18-week exercise program significantly reduced physical and general fatigue [61]. In patients undergoing adjuvant chemotherapy for CRC, supervised exercise compared to usual care was superior in improvements of physical activity level, functional status and QOL, but the patients were younger than in GERICO (mean 56.5 years) [62]. Among breast cancer patients undergoing adjuvant treatment, both moderate to high-intensity exercise and low intensity home training programs resulted in better physical performance but also less pain and toxicity (e.g. nausea and vomiting) due to the treatment [63]. In patients med lung cancer, home-based walking has been shown to be feasible and have a beneficial effect on anxiety and depression [64]. In a meta-analysis, Meneses-Echáves et al. conclude that supervised physical activity interventions reduce cancer-related fatigue and suggest that combined aerobic and resistance exercise regimens should be included as a part of rehabilitation in people diagnosed with cancer [65].
Many elderly patients suffer from sarcopenia, an age-related decrease in muscle mass and strength leading to decline in physical performance. Sarcopenia is associated with falls, disabilities and increased risk of death [66]. Muscle strength is measured with handgrip strength, with a cut off of 20 kg for women and 30 kg for men [67]. Muscle mass can be measured with DXA scanning, and physical performance is assessed by different tests. Usual gait speed, which is commonly used, is associated with OS [68], and a gait speed over 4 m (at a threshold of 1 m/s) can predict adverse outcomes in community-dwelling older people [69]. Gait speed is also recommended as a frailty screening test in cancer patients 65 years of age and older [70]. Low handgrip strength and slow usual gait speed are independently associated with higher mortality [71].
Nutritional intervention
Loss of weight in patients with cancer is associated with a poor prognosis [72], and nutritional support to malnourished patients with gastrointestinal cancer is recommended [73]. There are few studies evaluating the effect of nutrition intervention in cancer patients. In 358 patients with metastatic or locally advanced gastrointestinal, non-small cell lung cancer or mesothelioma undergoing chemotherapy, nutritional advice and supplements had no impact on 1-year mortality or quality of life [74]. In a meta-analysis of 1414 malnourished patients with different cancers included in 13 randomized controlled trials, nutritional intervention was found to be effective in increasing nutritional intake and improving some aspects of QOL, but the interventions had no effect on mortality [75]. In a randomized trial of 336 patients with cancer at risk of malnutrition, dietary counseling increased nutritional intake but had no impact on mortality, toxicity or other treatment outcomes [76]. The authors suggest that cancer cachexia anti-anabolism is responsible for the lack of effect.
Methods /Design
Aim
The aim of the study is to investigate whether frail elderly patients (defined as age ≥ 70 years) with stages II–IV CRC will benefit from full comprehensive geriatric assessment and intervention before and during treatment with adjuvant or palliative first-line chemotherapy. We will evaluate whether optimizing all health conditions can increase the number of patients completing 6 months of adjuvant chemotherapy or first-line chemotherapy until disease progression.
Hypothesis
Our hypothesis is that geriatric intervention can optimize the health and functional status of frail elderly patients with stages II–IV CRC. Due to this geriatric intervention, more patients will receive the scheduled doses and series of chemotherapy. As a consequence, the patients will have a longer OS and an improved quality of life.
Design
The study is an open, randomized, prospective trial performed at the departments of oncology and medicine at Copenhagen University Hospital, Herlev and Gentofte, Denmark.
Participants
A total of 140 participants will be included: 70 participants in the intervention group and 70 participants in the control group.
All patients ≥70 years [77] who meet the criteria to either receive adjuvant chemotherapy after surgery for stage III and high-risk stage II CRC or palliative first-line chemotherapy for inoperable or metastatic CRC will be screened for frailty with the G8 questionnaire [78] at their first visit to the oncology outpatient clinic. Frail patients, defined as patients with ≤14/17 points in the G8 questionnaire, will be offered inclusion in the study. Other inclusion criteria are PS 0–2 and a life expectancy ≥3 months. All patients must provide informed signed consent to be eligible for inclusion. Exclusion criteria are other malignancies during the last 5 years (except basal cell carcinoma, squamous cell carcinoma in situ and cervical cancer), and simultaneous participation in a trial of a medical product. The patients with recurrence or metastatic disease must not have received prior adjuvant treatment in the GERICO protocol.
To achieve adequate participant enrolment, the single center research team notifies the oncologists about all patients eligible for screening. The research team obtains informed consent and collects all data.
Randomization
After inclusion, the participants are randomized to either the intervention group or the control group. The randomization is made using ARRACT (A Real-time Randomization Application for Clinical Trials), a computer program developed and operated by the Department of Oncology Clinical Research at Herlev and Gentofte Hospital. The method used is "Stratified Balanced Allocation Method (n- Treatments)" [79, 80]. In the randomization, the PS of the patients (0 or 1+), and chemotherapy treatment (adjuvant or metastatic setting) are used as stratification variables.
Description of the two groups
The patients in the control group receive standard treatment with 6 months of adjuvant chemotherapy after surgery for CRC or palliative first-line chemotherapy until disease progression, operation or other scheduled change in treatment. If the patient has other health problems, those issues will be assessed either by the oncologist or by the general practitioner according to standard of care.
The patients in the intervention group also receive standard treatment with 6 months of adjuvant chemotherapy after surgery for CRC or first-line chemotherapy until disease progression, operation or other scheduled change of treatment. These patients will simultaneously be examined and treated by a geriatrician (CL), who will assess all other health issues.
Patients in both groups will fill out quality of life questionnaires (QLQ) prior to the start of chemotherapy, after 2 months, and at the end of the treatment using the validated QLQ C30 and QLQ ELD14 questionnaires from the European Organization for Research and Treatment of Cancer (EORTC). The adverse events are routinely recorded by the oncologist using the standardized CTC criteria version 4.0.
Geriatric assessment and intervention
All patients in the intervention group will receive full geriatric assessment and a clinical examination at start of chemotherapy, after 2 months, and more frequently if needed. Invention will be performed on identified issues (Table 1).
Table 1.
The geriatric assessment performed at the start of treatment
| Domain | Assessment | Possible intervention |
|---|---|---|
| Multimorbidity | Medical record review Clinical examination |
Treatment or referrals |
| Medication | Assessment of medication list based on START/STOPP criteria | Discontinuation Change in dosage Change in prescription of medication |
| Psychological function | Geriatric depression scale (GDS) | Referral to therapy or medication |
| Cognitive function | Minimal Mental State Examination (MMSE) | Cognitive evaluation Medication Social support |
| Nutritional status | Local nutritional screening based on minimal nutrition assessment (MNA) | Referral to dietitian |
| Physical function | Gait speed 10 m: (cut off 1 m/s) Handgrip assessed with the Jamar Dynamometer: (cut off 20 kg for women and 30 kg for men) |
Referral to physiotherapist and scheduled program |
| Functional status | Activities of daily living (ADL) Instrumental activities of daily living (IADL) | Social support Occupational therapy assessment for equipment needs Transport support |
| Laboratory parameters | TSH, cbalamin, folat, albumin, vitamin D | Treat deficiencies |
In the present study, all patients in the intervention group are screened, as part of the geriatric assessment, with a Jamar Dynamometer for handgrip strength (cut off 20 kg for women and 30 kg for men) and with a 10-m gait speed (cut-off 1 m/s). With a test result under cut off, patients are referred to a standardized physical exercise program twice weekly at the hospital and home exercise once weekly for 12 weeks.
Furthermore, the patients in the intervention group are screened for risk of malnutrition, and the patients are referred to a dietitian if needed.
Review of patients’ medication lists will be performed with focus on polypharmacy and interactions [81] and inappropriate medication based on START STOPP criteria [82].
The oncological treatment
The standard adjuvant chemotherapy is outlined in Table 2. The standard dose is based on body surface area (BSA), with a possible 25% primary dose reduction in frail patients.
Table 2.
Regimens and doses of chemotherapy
| Adjuvant setting | |||
| Regimen | Drug | Dose | Frequency |
| Capecitabine | Capecitabine | 2000 mg/m2 p.o. daily for 14 days | Every 3 weeks* |
| 5-FU | 5-FU Calcium folinate |
400 mg/m2 i.v. Infusion 46 h: 2400 mg/ m2 i.v. 400 mg/m2 i.v. |
Every 2 weeks** |
| Capeox | Capecitabine Oxaliplatin |
2000 mg/m2 p.o. daily for 14 days 130 mg/m2 i.v. |
Every 3 weeks* |
| Folfox | 5-FU Calcium folinate Oxaliplatin |
400 mg/m2 i.v. Infusion 46 h: 2400 mg/ m2 i.v. 400 mg/m2 i.v. 85 mg/m2 i.v. |
Every 2 weeks** |
| Metastatic setting | |||
| Regimens | Drug | Dose | Frequency |
| Capecitabine | Capecitabine | 2000 mg/m2 p.o. daily for 14 days | Every 3 weeks* |
| 5-FU | 5-FU Calcium folinate |
400 mg/m2 i.v. Infusion 46 h: 2400 mg/ m2 i.v. 400 mg/m2 i.v. |
Every 2 weeks** |
| Capeox | Capecitabine Oxaliplatin |
2000 mg/m2 p.o. daily for 14 days 130 mg/m2 i.v. |
Every 3 weeks* |
| Folfox | 5-FU Calcium folinate Oxaliplatin |
400 mg/m2 i.v. Infusion 46 h: 2400 mg/ m2 i.v. 400 mg/m2 i.v. 85 mg/m2 i.v. |
Every 2 weeks*** |
| Irinotecan | Irinotecan | 200 mg/m2 i.v. | Every 2 weeks**** |
| Capiri | Capecitabine Irinotecan |
1600 mg/m2 p.o. daily for 14 days 200 mg/m2 i.v. |
Every 3 weeks * |
| Folfiri | 5-FU Calcium folinate Irinotecan |
400 mg/m2 i.v. Infusion 46 h: 2400 mg/ m2 i.v. 400 mg/m2 i.v. 180 mg/m2 i.v. |
Every 2 weeks**** |
| Irox | Irinotecan Oxaliplatin |
165 mg/m2 i.v. 85 mg/m2 i.v. |
Every 2 weeks**** |
*maximum 8 series
**maximum 12 series
i.v. intravenous, p.o. per os
*optional addition of bevacizumab 7.5 mg/m2 i.v
**optional addition of bevacizumab 5 mg/m2 i.v
***optional addition of bevacizumab 5 mg/m2 i.v., irinotecan 165 mg/m2 i.v., cetuximab 500 mg/m2 i.v., or panitumumab 6 mg/kg i.v
****optional addition of bevacizumab 5 mg/m2 i.v., cetuximab 500 mg/m2 i.v., or panitumumab 6 mg/kg i.v
Study objectives
Primary outcome
The primary endpoint of the study is the proportion of patients completing scheduled chemotherapy with the same dose as at the start of treatment.
Secondary outcomes
Secondary endpoints include adverse events (registered for every chemotherapy cycle after CTC criteria version 4.0), dose reductions, treatment delays (which follow the standardized guidelines of the department), DFS, PFS, OS, CRC mortality and quality of life.
Statistical power and analyzes
The power calculation is made based on the expected impact of the geriatric intervention. The proportion of patients who complete the planned treatment is assumed to increase from 50%, the percentage given in the literature [12], to 75% after geriatric intervention. With 70 patients included in each group (total 140 evaluable patients) such an increase can be detected at a 5% significance level with a probability (power) of 87%. The proportion in the two groups will be compared using a chi-square test.
Dose intensity (DI), the cumulative given dose compared to planned total dose per week, and relative dose intensity (RDI), defined as 100* DI/ planned dose intensity (PDI) (mg/ week), will be analyzed.
DFS is defined as time from surgery to time of recurrence or death. PFS is defined as time from start of first-line chemotherapy to date of progression or death according to RECIST criteria 1.1. OS is defined as time from surgery or start of first-line treatment to time of death. CRC mortality will be analyzed in all patients who are followed until outcome of interest or end of follow-up using statistical methods for censored time-to-event data.
All statistical analyzes will be performed by a statistician (CD). The statistical software package R (http://www.rproject.org) is used for all analyses. Disease-free survival, PFS and OS will be estimated with the Kaplan–Meier method and compared with a log-rank test. Additional subgroup analyses based on chemotherapy regimen will be performed if feasible. Missing data will be handled with multiple imputations.
Discussion
In spite of the high prevalence of CRC and the high incidence of CRC-related mortality, elderly patients are under-presented in clinical trials [38]. Nevertheless, elderly patients are the largest group of cancer patients, and their number is rapidly growing.
When treating elderly patients with comorbidity and poor PS, oncologists today possess a limited number of treatment options. Due to concerns regarding toxicity and the lack of guidelines for treating elderly patients, there is a trend toward less aggressive treatment and exclusion of elderly patients especially in the adjuvant setting, where chemotherapy is potentially lifesaving [83]. There is, however, evidence that chemotherapy is also effective in elderly patients, and in order to improve the poorer cancer prognosis, it is of great importance to find the elderly patients who will profit from chemotherapy and to perform dose adjustments in case of side effects [84, 85].
The cooperation between geriatricians and oncologists in order to individualize and improve treatment for elderly patients with cancer could be the solution. However, there is a lack of randomized intervention studies that evaluate the effect of geriatric intervention treatment outcomes in elderly patients with CRC.
In the present GERICO study, only elderly frail patients with CRC receiving chemotherapy are included. There may be a group of patients who are deemed too frail, too old or to have too much comorbidity to receive chemotherapy, which could profit from the geriatric assessment. An optimization of health conditions in those patients could bring them into consideration for treatment.
Our long-term aim is to integrate the geriatric function into the oncology setting. Optimizing the overall health of elderly patients will potentially allow more intensive treatment, which would lead to increased survival and a higher quality of life. The project will provide new and valuable information for better treatment of frail, elderly patients with CRC.
Trial status
The first participant was included in April 2015. The recruitment period is 2–3 years. The last participant included is expected in June 2018. A total of 190 patients have been screened for frailty with the G8 questionnaire, and (131) 69% of the patients were frail, a little lower percentage than seen in other studies [86]. To date, 81 patients have been included. The number of included patients is lower than expected. One of the reasons is that 32 patients were not screened at all, since they were found to be too frail to receive treatment. Of the 131 patients screened frail with the G8 questionnaire, 19 patients did not start any treatment due to performance status, high age, comorbidity or patients’ refusal (Fig. 1). An additional five patients were excluded because of other malignancies. Since the start of the trial, eight patients that could have been included were missed.
Fig. 1.
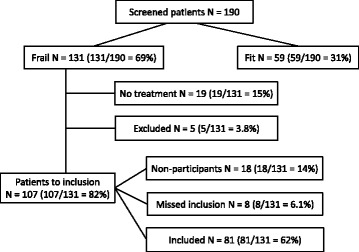
Trial status
Acknowledgements
The authors want to thank Hanne Elkjær Andersen for collaborating and contributing with knowledge about Comprehensive Geriatric Assessment, Anders Winther for input to the screening to physiotherapy, Lars Matzen for comments and ideas along the way. We also want to thank our Clinical Research Department for help with the development of the clinical database and randomization program.
Funding
We thank the Danish Cancer Society, Aase and Ejnar Danielsens Foundation, Sofus Carl Emil Friis og hustru Olga Doris Friis’ Grant, and Aage and Johanne Louis-Hansens Foundation for externally funding. None of the funders have any role in designing the study, neither in data collection, management, analysis or interpretation, nor in writing or submitting the manuscript.
Availability of data and materials
Plans for data entry, coding, security and storage are approved by the Danish Data Protection Agency (j.nr. HEH-2014-112). Clinical data and study material (e.g. patients’ questionnaires) will be available from Dr. Cecilia Lund on request.
Abbreviations
- BSA
Body surface area
- CC
Colon cancer
- CGA
Comprehensive geriatric assessment
- CRC
Colorectal cancer
- DFS
Disease-free survival
- i.v.
Intraveneus
- mCRC
metastatic CRC
- OS
Overall survival
- p.o.
Per os
- PFS
Progression-free survival
- PS
Performance status
- QLQ
Quality of life questionnaire
Authors’ contributions
Authorship follows the Vancouver guidelines. CL, DN, JJ, KV and FN designed the study; CD did the power calculation and the data analysis; CL and DN wrote the protocol; CL, DN and JJ handled ethics approval; CL and KV are trial coordinators, responsible for the daily running of the trial; CL wrote the first paper draft and JJ, DN, KV, CD and FN reviewed it critically. All authors contributed to and approved the final version of the manuscript.
Authors’ information
CL and FR are geriatricians. KK, DN and JSJ are oncologists. CD is a statistician.
Ethics approval and consent to participate
The name of the study is GERICO, and the study protocol “The effect of geriatric intervention in frail elderly patients treated with chemotherapy for colorectal cancer” version 1.3 has been approved by the Regional Ethics Committee (VEK ref. H-7-2014-015). Any protocol modifications will be communicated to the Regional Ethics Committee and to all financially supportive organizations. The research team will obtain oral and written consent from all participants before inclusion. Trial results will be communicated to all participants and in publications.
Consent for publication
Not applicable.
Competing interests
None of the authors have any conflicts of interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
C. M. Lund, Email: cecilia.margareta.lund.01@regionh.dk
K. K. Vistisen, Email: Kirsten.Vistisen@regionh.dk
C. Dehlendorff, Email: cdchr395@hotmail.com
F. Rønholt, Email: Finn.Roenholt@regionh.dk
J. S. Johansen, Email: Julia.Sidenius.Johansen@regionh.dk
D. L. Nielsen, Email: Dorte.Nielsen.01@regionh.dk
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quaglia A, Tavilla A, Shack L, et al. The cancer survival gap between elderly and middle-aged patients in Europe is widening. Eur J Cancer. 2009;45:1006–1016. doi: 10.1016/j.ejca.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Braendegaard Winther S, Baatrup G, Pfeiffer P, Qvortrup C. Trends in colorectal cancer in the elderly in Denmark, 1980-2012. Acta Oncol. 2016;55(Suppl 1):29–39. doi: 10.3109/0284186X.2015.1114674. [DOI] [PubMed] [Google Scholar]
- 5.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 6.Gray R, Barnwell J, McConkey C, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61058-7. [DOI] [PubMed] [Google Scholar]
- 7.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 8.Schmoll HJ, Twelves C, Sun W, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 2014;15:1481–1492. doi: 10.1016/S1470-2045(14)70486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen SH, Harling H, Kirkeby LT et al. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 2012;(3):CD004078. [DOI] [PMC free article] [PubMed]
- 10.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 11.Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol. 2012;30:2624–2634. doi: 10.1200/JCO.2011.41.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoeben KW, van Steenbergen LN, van de Wouw AJ, et al. Treatment and complications in elderly stage III colon cancer patients in the Netherlands. Ann Oncol. 2013;24:974–979. doi: 10.1093/annonc/mds576. [DOI] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol. 2002;20:3992–3998. doi: 10.1200/JCO.2002.03.083. [DOI] [PubMed] [Google Scholar]
- 14.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 15.Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 17.McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol. 2013;31:2600–2606. doi: 10.1200/JCO.2013.49.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 19.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorbye H, Cvancarova M, Qvortrup C, et al. Age-dependent improvement in median and long-term survival in unselected population-based Nordic registries of patients with synchronous metastatic colorectal cancer. Ann Oncol. 2013;24:2354–2360. doi: 10.1093/annonc/mdt197. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs CS, Moore MR, Harker G, et al. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol. 2003;21:807–814. doi: 10.1200/JCO.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Jackson NA, Barrueco J, Soufi-Mahjoubi R, et al. Comparing safety and efficacy of first-line irinotecan/fluoropyrimidine combinations in elderly versus nonelderly patients with metastatic colorectal cancer: findings from the bolus, infusional, or capecitabine with camptostar-celecoxib study. Cancer. 2009;115:2617–2629. doi: 10.1002/cncr.24305. [DOI] [PubMed] [Google Scholar]
- 23.Bosse D, Vickers M, Lemay F, Beaudoin A. Palliative chemotherapy for patients 70 years of age and older with metastatic colorectal cancer: a single-centre experience. Curr Oncol. 2015;22:e349–e356. doi: 10.3747/co.22.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737–743. doi: 10.1007/s00432-009-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol. 2009;27:199–205. doi: 10.1200/JCO.2008.17.7931. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani F, Cunningham D. Bevacizumab in elderly patients with metastatic colorectal cancer. J Geriatr Oncol. 2014;5:78–88. doi: 10.1016/j.jgo.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Rouyer M, Fourrier-Reglat A, Smith D, et al. Effectiveness and safety of first-line bevacizumab plus FOLFIRI in elderly patients with metastatic colorectal cancer: Results of the ETNA observational cohort. J Geriatr Oncol. 2016;7:187–194. doi: 10.1016/j.jgo.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Jehn CF, Boning L, Kroning H, et al. Cetuximab-based therapy in elderly comorbid patients with metastatic colorectal cancer. Br J Cancer. 2012;106:274–278. doi: 10.1038/bjc.2011.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietrantonio F, Cremolini C, Aprile G, et al. Single-Agent Panitumumab in Frail Elderly Patients With Advanced RAS and BRAF Wild-Type Colorectal Cancer: Challenging Drug Label to Light Up New Hope. Oncologist. 2015;20:1261–1265. doi: 10.1634/theoncologist.2015-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papamichael D, Audisio RA, Glimelius B, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26:463–476. doi: 10.1093/annonc/mdu253. [DOI] [PubMed] [Google Scholar]
- 32.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kordatou Z, Kountourakis P, Papamichael D. Treatment of older patients with colorectal cancer: a perspective review. Ther Adv Med Oncol. 2014;6:128–140. doi: 10.1177/1758834014523328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. 2015;21:5158–5166. doi: 10.3748/wjg.v21.i17.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Power DG, Lichtman SM. Chemotherapy for the elderly patient with colorectal cancer. Cancer J. 2010;16:241–252. doi: 10.1097/PPO.0b013e3181e07690. [DOI] [PubMed] [Google Scholar]
- 36.Hermosillo-Rodriguez J, Anaya DA, Sada Y, et al. The effect of age and comorbidity on patient-centered health outcomes in patients receiving adjuvant chemotherapy for colon cancer. J Geriatr Oncol. 2013;4:99–106. doi: 10.1016/j.jgo.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Lieu CH, Renfro LA, de Gramont A, et al. Association of Age With Survival in Patients With Metastatic Colorectal Cancer: Analysis From the ARCAD Clinical Trials Program. J Clin Oncol. 2014;32:2975–2984. doi: 10.1200/JCO.2013.54.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients With Cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohile SG, Velarde C, Hurria A, et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Cancer Netw. 2015;13:1120–1130. doi: 10.6004/jnccn.2015.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 41.Wildes TM, Ruwe AP, Fournier C, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol. 2013;4:227–234. doi: 10.1016/j.jgo.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aparicio T, Jouve JL, Teillet L, et al. Geriatric factors predict chemotherapy feasibility: ancillary results of FFCD 2001-02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. J Clin Oncol. 2013;31:1464–1470. doi: 10.1200/JCO.2012.42.9894. [DOI] [PubMed] [Google Scholar]
- 43.Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29:3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 44.Hamaker ME, Schiphorst AH, ten Bokkel HD, et al. The effect of a geriatric evaluation on treatment decisions for older cancer patients-a systematic review. Acta Oncol. 2014;53:289–296. doi: 10.3109/0284186X.2013.840741. [DOI] [PubMed] [Google Scholar]
- 45.Ommundsen N, Wyller TB, Nesbakken A, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014;19:1268–1275. doi: 10.1634/theoncologist.2014-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer. 2015;112:1435–1444. doi: 10.1038/bjc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26:288–300. doi: 10.1093/annonc/mdu210. [DOI] [PubMed] [Google Scholar]
- 48.Leo S, Accettura C, Gnoni A, et al. Systemic treatment of gastrointestinal cancer in elderly patients. J Gastrointest Cancer. 2013;44:22–32. doi: 10.1007/s12029-012-9447-5. [DOI] [PubMed] [Google Scholar]
- 49.Ellis G, Whitehead MA, Robinson D, et al. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343:d6553. doi: 10.1136/bmj.d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prestmo A, Hagen G, Sletvold O, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet. 2015;385:1623–1633. doi: 10.1016/S0140-6736(14)62409-0. [DOI] [PubMed] [Google Scholar]
- 51.Harari D, Hopper A, Dhesi J, et al. Proactive care of older people undergoing surgery ('POPS'): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing. 2007;36:190–196. doi: 10.1093/ageing/afl163. [DOI] [PubMed] [Google Scholar]
- 52.Ellis G, Spiers M, Coutts S, et al. Preoperative assessment in the elderly: evaluation of a new clinical service. Scott Med J. 2012;57:212–216. doi: 10.1258/smj.2012.012120. [DOI] [PubMed] [Google Scholar]
- 53.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 55.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Des Guetz G, Uzzan B, Bouillet T, et al. Impact of physical activity on cancer-specific and overall survival of patients with colorectal cancer. Gastroenterol Res Pract. 2013; doi:10.1155/2013/340851. [DOI] [PMC free article] [PubMed]
- 57.Pedersen L, Idorn M, Olofsson GH, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Jung HW, Kim JW, Kim JY, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer. 2015;23:687–694. doi: 10.1007/s00520-014-2418-6. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt ME, Wiskemann J, Armbrust P, et al. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int J Cancer. 2015;137:471–480. doi: 10.1002/ijc.29383. [DOI] [PubMed] [Google Scholar]
- 60.Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.VANV JK, Velthuis MJ, Steins Bisschop CN, et al. Effects of an Exercise Program in Colon Cancer Patients undergoing Chemotherapy. Med Sci Sports Exerc. 2016;48:767–775. doi: 10.1249/MSS.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 62.Lin KY, Shun SC, Lai YH, et al. Comparison of the effects of a supervised exercise program and usual care in patients with colorectal cancer undergoing chemotherapy. Cancer Nurs. 2014;37:E21–E29. doi: 10.1097/NCC.0b013e3182791097. [DOI] [PubMed] [Google Scholar]
- 63.van Waart H, Stuiver MM, van Harten WH, et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 64.Chen HM, Tsai CM, Wu YC, et al. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer. 2015;112:438–445. doi: 10.1038/bjc.2014.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Supervised exercise reduces cancer-related fatigue: a systematic review. J Physiother. 2015;61:3–9. doi: 10.1016/j.jphys.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 66.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 68.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 70.Pamoukdjian F, Paillaud E, Zelek L, et al. Measurement of gait speed in older adults to identify complications associated with frailty: A systematic review. J Geriatr Oncol. 2015;6:484–496. doi: 10.1016/j.jgo.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. doi: 10.1016/S0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 73.Senesse P, Assenat E, Schneider S, et al. Nutritional support during oncologic treatment of patients with gastrointestinal cancer: who could benefit? Cancer Treat Rev. 2008;34:568–575. doi: 10.1016/j.ctrv.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Baldwin C, Spiro A, McGough C, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet. 2011;24:431–440. doi: 10.1111/j.1365-277X.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- 75.Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:371–385. doi: 10.1093/jnci/djr556. [DOI] [PubMed] [Google Scholar]
- 76.Bourdel-Marchasson I, Blanc-Bisson C, Doussau A, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS One. 2014;9:e108687. doi: 10.1371/journal.pone.0108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hotta K, Ueoka H, Kiura K, et al. An overview of 48 elderly-specific clinical trials of systemic chemotherapy for advanced non-small cell lung cancer. Lung Cancer. 2004;46:61–76. doi: 10.1016/j.lungcan.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 78.Bellera CA, Rainfray M, Mathoulin-Pelissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 79.Zielhuis GA, Straatman H, van 't Hof-Grootenboer AE et al. The choice of a balanced allocation method for a clinical trial in otitis media with effusion. Stat Med 1990;9:237-246. [DOI] [PubMed]
- 80.Han B, Enas NH, McEntegart D. Randomization by minimization for unbalanced treatment allocation. Stat Med. 2009;28:3329–3346. doi: 10.1002/sim.3710. [DOI] [PubMed] [Google Scholar]
- 81.Walko CM, McLeod HL. Personalizing Medicine in Geriatric Oncology. J Clin Oncol. 2014;32:2581–2586. doi: 10.1200/JCO.2014.55.9047. [DOI] [PubMed] [Google Scholar]
- 82.Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46:72–83. doi: 10.5414/CPP46072. [DOI] [PubMed] [Google Scholar]
- 83.Muss HB, Bynum DL. Adjuvant chemotherapy in older patients with stage III colon cancer: an underused lifesaving treatment. J Clin Oncol. 2012;30:2576–2578. doi: 10.1200/JCO.2012.42.3780. [DOI] [PubMed] [Google Scholar]
- 84.Ramsdale E, Sanoff H, Muss H. Approach to the older patient with stage II/III colorectal cancer: who should get curative-intent therapy? Am Soc Clin Oncol Educ Book 2013.p.163–8. [DOI] [PubMed]
- 85.Wu C, Goldberg RM. Managing choices for older patients with colon cancer: adjuvant therapy. Am Soc Clin Oncol Educ Book 2013.p.190–3. [DOI] [PubMed]
- 86.Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24:1306–1312. doi: 10.1093/annonc/mds619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Plans for data entry, coding, security and storage are approved by the Danish Data Protection Agency (j.nr. HEH-2014-112). Clinical data and study material (e.g. patients’ questionnaires) will be available from Dr. Cecilia Lund on request.


