Abstract
We develop a discrete variant of a general model for adult mortality influenced by the delayed impact of early conditions on adult health and mortality. The discrete variant of the model builds on an intuitively appealing interpretation of conditions that induce delayed effects and is an extension of the discrete form of the standard frailty model with distinct implications. We show that introducing delayed effects is equivalent to perturbing adult mortality patterns with a particular class of time-/age-varying frailty. We emphasize two main results. First, populations with delayed effects could experience unchanging or increasing adult mortality even when background mortality has been declining for long periods of time. Although this phenomenon also occurs in a regime with standard frailty, the distortions can be more severe under a regime with Barker frailty. As a consequence, conventional interpretations of the observed rates of adult mortality decline in societies that experience Barker frailty may be inappropriate. Second, the observed rate of senescence (slope of adult mortality rates) in populations with delayed effects could increase, decrease, or remain steady over time and across adult ages even though the rate of senescence of the background age pattern of mortality is time- and age-invariant. This second result implies that standard interpretations of empirical estimates of the slope of adult mortality rates in populations with delayed effects may be misleading because they can reflect mechanisms other than those inducing senescence as conventionally understood in the literature.
Keywords: Barker hypothesis, Early origins of health and disease, Old-age mortality, Demographic frailty
Introduction
We derive a discrete model to represent delayed adult mortality effects of unfavorable early-life conditions. The model is built on an intuitively appealing interpretation of conditions that induce delayed effects, remove assumptions that limit the applicability of a continuous version (Palloni and Beltrán-Sánchez 2016), and formalize the implications for observed mortality patterns of several mechanisms producing delayed effects on adult health and mortality. Our study rests on two simplifications. First, although there are multiple pathways through which early-life conditions can manifest themselves as delayed effects, the current model treats them all as if they share the same dominant features and ignores dissimilarities. The simplification is needed to make the model tractable as a simple generalization of the standard frailty model (Vaupel and Missov 2014; Vaupel and Yashin 1987; Vaupel et al. 1979). Second, unlike the continuous version of the model, it assumes that early conditions can be treated as a 1/0 binary variable and that the population at birth can be classified into those who did and those who did not had adverse experiences.
Barker Frailty
Mechanisms
Solid empirical evidence and persuasive theoretical argumentation support the idea that early-life conditions—in utero, near birth, and during early childhood—exert an important influence on adult health and mortality (Barker 1998; Barouki et al. 2012; Bateson and Gluckman 2011; Beltrán-Sánchez et al. 2012; Gluckman and Hanson 2006; Langley-Evans 2004; McDade and Kuzawa 2004 ). The mechanisms that trigger these delayed effects involve perturbation of cell attrition, growth and functional differentiation, various classes of epigenetic changes (histone covalent modifications, noncoding RNA expression, methylation) allowing the expression of developmental plasticity, and (more generally) conditions including exposure to acute poverty, deprivation, and stress (Forsdahl 1977, 1978). The best known, albeit not the most general, version of the hypothesis of delayed health effects, was articulated by Barker (1998). The cornerstone idea of this version of the theory (“fetal programming”) is that nutritional deprivation in utero and soon after birth disrupts processes of organ formation (cell division, growth, and functional specialization) and exposes survivors to excess risk of a number of chronic conditions during late adulthood, including Type 2 diabetes mellitus (T2D), hypertension and other circulatory disorders, kidney and heart disease, and some diseases of the respiratory system.
A more recent formulation is associated with processes of predictive adaptive response (PAR), and more generally with theories of developmental origins of adult health and disease (DOHaD), operating either as homeostatic adaptation or epigenetic adjustment to experienced or predicted environmental challenges (Bateson and Gluckman 2011; Gluckman and Hanson 2006). Recent empirical research has identified the possibility that exposure in utero as well as perinatal experiences may lead to epigenetic changes that could result in permanent immune or metabolic dysfunctions later in life. The most intriguing of these are alterations of the microbiome during delivery and right after birth (Bateson and Gluckman 2011). Indeed, growing empirical evidence has shown a link between type of delivery, composition of the newborn’s gut bacterial population, and risks of adult T2D (Devaraj et al. 2013; Giongo et al. 2011).
An older and related yet better-established strand of the literature has identified links between exposure and contraction of early-life infections and the development of adult chronic conditions. Examples of this mechanism include the relation between Helicobacter pylori and colon cancer, human papilloma virus (HPV) and uterine cancer, hepatitis B and liver cirrhosis and cancer, and rheumatic heart fever and mitral valve stenosis. In all these cases, contraction of well-defined infections induces organ damage that manifests itself as adult chronic illnesses among survivors (Elo and Preston 1992; Fong 2000). A third mechanism linking early conditions and adult health via delayed response involves sustained inflammation that results from recurrent episodes of infectious diseases during early childhood; persistent low-level infections; and, more generally, continuous exposure to multiple infectious and parasitic diseases (Crimmins and Finch 2006; Danesh et al. 2000; Finch 2007; Finch and Crimmins 2004). The theory suggests that when the inflammatory processes promoted by these exposures are not episodic but are long-lasting, they are likely to increase susceptibility to a number of adult chronic illnesses (Finch 2007).
These mechanisms share one important feature and differ in one important respect. The commonality is the presence of deviant organ differentiation and development or damage and/or abnormal immune/metabolic responses with long latency periods and delayed manifestations in late adulthood. The difference between mechanisms is the role played by environments and experiences throughout life. Thus, whereas fetal programming and developmental plasticity working through epigenetic modifications require mismatches between prenatal and perinatal environment and postnatal experiences, the mechanisms working through infections or sustained inflammation require vulnerability to infectious diseases, perhaps established at birth, and exposure to and/or contraction of those diseases later in life. The discrete model that we introduce in this article enables us to capture both the presence of adverse prenatal and perinatal environments and susceptibility to disease using a single trait (defined at birth) with age-varying effects on mortality.
Conditions of Observability
Delayed expression of damage inflicted by early experiences will be observable in adult mortality patterns only if the following three conditions are met: (1) members of a birth cohort who experience the offending early conditions survive beyond some critical age (Y1), after which manifestation of the original damage begins to unfold; (2) the delayed effect must be significant in the sense that it should implicate a broad range of illnesses and conditions with relatively high fatality rates; and (3) the beneficial mortality-related effects of medical technological advances adopted and diffused between the time of onset of early adverse experiences and the time at which the birth cohort attains the critical age (Y1) are less than the excess mortality risks implied by the offending chronic conditions. In this article, we show formally that in populations where all three conditions are satisfied, adult mortality will exhibit two singularities: one affecting time trends, and the other distorting age patterns.1
In what follows we will use the term “Barker frailty” to refer to a trait that reflects adverse early-life experiences and the term “Barker effects” to refer to the impact of Barker frailty on adult mortality rates. The labels are shorthand to refer to a broad range of mechanisms, including but not reduced to those suggested by Barker, as if all of them shared the same properties. Although this simplification increases tractability, it should be improved upon.
Models for Discrete Barker Frailty2
The model for Barker frailty includes three properties. First, individuals are characterized by a propensity, 0 ≤ δ < ∞ (Barker frailty), acquired before or at birth and carried through life that makes them more or less likely to produce delayed health and mortality effects (Barker effect). In the discrete version of the model, a delayed manifestation occurs only if the trait’s value exceeds a threshold (Barker threshold).3 The actual expression of effects in the form of excess mortality and ill health throughout the life course could depend on individual experiences following birth. In particular, the magnitude of delayed adult effects should be a function of accumulated exposures that trigger or inhibit the expression of frailty. The model with discrete frailty is appealing because it captures well the idea, implicit in Barker fetal programming theory, that individuals vulnerable to the impact of adverse early conditions on adult health and mortality are those who experience deprivations above a given threshold. The disadvantage of a discrete model is that it requires direct or indirect specification of the threshold—a quantity that is, for all purposes, difficult to either theorize about or empirically estimate.
Second, because it is the mortality implications of the frailty trait that matter, one can pose that δ has a changing impact on mortality as a result of exposures throughout various stages of the life course. In the more general form of the model, the trait can potentially have three effects on the force of mortality: before an early age Y0, after a critical adult age Y1, and in the age interval (Y0, Y1). In this article, we include only delayed effects past age Y1. (See Online Resource 1 for a more general formulation.)
Third, the fraction of individuals at adult ages who could express Barker frailty must increase whenever mortality declines. This can take place via three different mechanisms. The first operates by simply reducing selective pressure: individuals who would have died early in life in a higher-mortality regime can survive to adult ages in a more beneficial mortality regime, and some of them will be carriers of higher values of Barker frailty. The second mechanism increases the fraction of births that are carriers of Barker frailty and, therefore, augments the pool of individuals who can potentially express it, irrespective of mortality changes. This is possible when there are improvements associated with mortality decline—such as reduced maternal exposure to parasitic and infectious diseases, and better prenatal care—that translate into lower fetal and perinatal mortality rates. These changes can be captured by redefining the density of δ. A third mechanism induces a downward shift of Barker threshold (in the absence of changes in the initial density of δ) and could capture situations involving changes in epidemiological regimes or environmental conditions that place children at higher risk.
Model With Fixed Critical Ages and Mortality Decline
We assume that (1) the fraction of births at time t that can express Barker frailty is a fixed quantity, PB(0,t )= g, and (2) Barker effects are equivalent to a relative mortality risk equal to λB (λB > 1) applicable only at adult ages y > Y1.4 We also assume that the fraction of births in the cohort that does not carry Barker frailty, (1 − g), does not express Barker frailty, so their excess mortality risk at ages over Y1 is equal to λNB = 1. The first simplification is tantamount to fixing ex ante the complement of the distribution function for the random trait δ to be ψ(δ), and to assume δ0 such that ψ(δ0)= g = PB(0,t). The second simplification is equivalent to assuming that relative risks associated with Barker frailty are age-invariant at ages older than Y1 and set to R(δ, y) = λB for y > Y1 and R(δ, y) = 1 everywhere else.
Assume that the mortality regime undergoes a secular change with onset at t = 0 and that each birth cohort is exposed to a mortality level corresponding to the year when they were born. Thus, members of a birth cohort born t > 0 years after the onset of the secular decline experience throughout their lives mortality rates from the life table for year t. To define each birth cohort’s life table, we assume that there is a baseline mortality pattern characterized by mortality rates {μs(y)} and that the force of mortality for year t (“background” mortality) is μ(y, t) = k(t) × μs(y), where k(t) is a monotonically decreasing function of time (linear or exponential).5 Thus, the force of mortality at age y ≥ Y1 for a member of a cohort born in year t is given by
| (1) |
The expected value of excess mortality at age y and time t is
and the attributable mortality associated with Barker frailty is given by
where PB(y,t) is the fraction of the population who expresses Barker at age y and time t.6 Taking logs in Eq. (1) and then derivatives with respect to time, we get an expression for the rate of change over time of the aggregate mortality rates:
| (2) |
The term after the addition sign is always positive, and Eq. (2) will be less (in absolute value) than the average rate of background mortality decline. Furthermore, the quantity is dependent on changes in PB(y,t) that proceed faster in the initial stages of the mortality decline and are gradually spent later in the process. This expression illustrates the first singularity resulting from Barker frailty: namely, a distortion of the rate of mortality decline.
Discrete Barker frailty also flattens the slope of average adult mortality. In fact, the age derivative of the average force of mortality is exactly analogous to Eq. (2) but with the roles of k(t)
| (3) |
Because ∂PB(y,t)∂ y is always negative, the age-specific slope of average adult mortality will be smaller than the slope in the background mortality pattern because of Barker effects.7 The differences will be higher at ages closer to Y1 and at more advanced stages of the secular decline when PB(y,t) attains its maximum. In the limit, as t → ∞ or y → ∞, the slopes of background and average mortality will be identical. This expression illustrates the second singularity (affecting the age pattern of adult mortality) described earlier.
Because theories of secular mortality decline assign importance to time trends of the rate of change of mortality, and theories of senescence focus on variation of the adult mortality slope and correlations with early mortality, it is important to describe two additional features: (1) the age-specific time trajectory of the reduction in the rate of mortality decline relative to the background rate of mortality decline; and (2) the time- and age-specific changes of the rate of increase in adult mortality rates relative to the adult mortality slope in the background mortality pattern. The pertinent indicators are defined in Palloni and Beltrán-Sánchez (2015); their graphic profiles are displayed in Online Resource 1, and we discuss their implications in the following section.
Model With Random Critical Ages and Mortality Decline
The simple model presented earlier contains a massive simplification: namely, that the critical age is fixed. However, most DOHaD theories imply, explicitly or not, that critical ages are themselves a function of individual experiences, individual traits, and features of the background epidemiological regime. That is, critical ages must have both a systematic and a random component. Because our knowledge about the operation of the three mechanisms for Barker effects is not robust enough, it is difficult to include in the model the factors that determine these critical ages. And, even if we did, it would be a daunting task to identify and properly specify the components of these ages because they are likely related to accumulation of adverse experiences throughout the life course.
Without precise knowledge of determinants of critical ages, we can assess only the influence that random critical ages have on the magnitude and patterns of effects of Barker frailty on adult mortality. This assessment is important because by doing so, we will at least be able to approximately gauge the role that these ages play in the overall impact of Barker frailty and effects on adult mortality patterns and trends.
We extend the model so that critical ages are now randomly determined (but devoid of a systematic component). For clarity and compactness of notation, we relabel the critical age as Z and assume that it has a well-defined density fZ(z) for all individuals who belong to the subgroup endowed with Barker frailty. A critical age is irrelevant for all those who cannot express Barker frailty. Further, assume that Z ≥ 40 so that Barker effects are nil below age 40. Elsewhere (Palloni and Beltrán-Sánchez 2015), we show that the expected value of the force of mortality at some age y ≥ 40 can be approximated by the following expression:
| (4) |
where ΦZ(y) is the distribution function of critical ages z evaluated at age y, and is the fraction of all survivors to age y who carry Barker frailty and whose critical ages satisfy z ≤ y.8 Equation (4) makes it plain that the introduction of random critical ages erodes the impact of Barker frailty because it is now associated with a smaller subset of individuals who survive to age y, : not all of those who could express Barker frailty, but only those whose critical ages are younger than age y. However, given that y → ∞, the fraction of the cohort with Barker frailty that does not influence mortality level (because their critical ages are higher than y) diminishes rapidly. As a consequence, the dilution of the mortality impact of Barker effects decreases and converges to what would have been observed had the critical age been fixed at age 40. The main result is that the rates of change of mortality rates and adult mortality slopes are now as follows:
These are expressions analogous to Eqs. (2) and (3). The difference between these and the previous equations is that the driver of the rates of change is , a quantity that is always strictly smaller than AMyt(λB). As a consequence, the effects of Barker frailty on the baseline rate of mortality decline and on the adult slope will be lower than if critical ages are fixed and equal to the lowest bound (e.g., at age 40).
In summary, when critical ages are random, the influence exerted by Barker frailty on both the rate of mortality decline and on the adult mortality slope is reduced. The magnitude of this reduction depends on the nature of the cumulative distribution function of Z: to the extent that the probability mass for Z is located at older ages, the impact of Barker frailty will be minor. The effects will be stronger and converge to those associated with a regime with fixed critical ages when the probability mass is located at younger adult ages.
Model With Fixed Critical Ages, Changing Size of Vulnerable Population and Mortality Decline An important shortcoming of the models presented earlier is the assumption that the relative size of the population that could express Barker frailty is fixed across birth cohorts. This simplification fails to translate with high fidelity the nature of Barker frailty as defined earlier. The most natural extension is to let PB (0,t)= g(t) be an increasing function of time. An increase in the fraction of births marked by Barker frailty over time could be the result of a change in the distribution of the latent Barker frailty trait δ or of a downward shift of Barker threshold. In either case, the extension is suited to capture scenarios where the size and/or heterogeneity of birth cohorts at risk of expressing Barker frailty increases as a result of new epidemiological regimes with better maternal health, lower fetal and perinatal mortality, and/or worse interaction effects of early environments and Barker frailty.
Suppose that the fraction of births that express Barker frailty is g(t), an increasing function of t. The average mortality rate at age y and time t is given by Eq. (1), but PB(y,t) is now a function of the time-dependent fraction of individuals who are vulnerable to Barker effects, h(t) = (1 − g(t))/g(t). The expression for PB(y,t) is
This is identical to the one defined earlier, with h(t) replacing h. The derivatives of μ̄(y,t) with respect to t and y are analogous to those for the case of time-invariant h with one significant difference—namely, PB(y,t) and AM1(λB) are now influenced by the decreasing time-varying odds, h(t), of being born free of Barker frailty. Because h(t) decreases over time, both the rate of change of average mortality and the slope of adult mortality will deviate more from the baseline parameters than in the case when h is fixed. Analogous expressions to those presented earlier hold for the case when critical ages are random. In all cases, one must replace PB(y,t) and its derivatives for and its derivatives.
Simulation of Mortality Regimes
To evaluate the magnitude of Barker effects and to gain insights on the relations described earlier, we simulate a series of cohorts undergoing a secular mortality decline and expose them to several variants of discrete Barker frailty, with fixed and random critical ages. The objective of the simulations is to shed light on the behavior of two functions: the age-specific rates of mortality change and the adult mortality slope across birth cohorts. The details of the simulation are presented in Online Resource 1.
Results
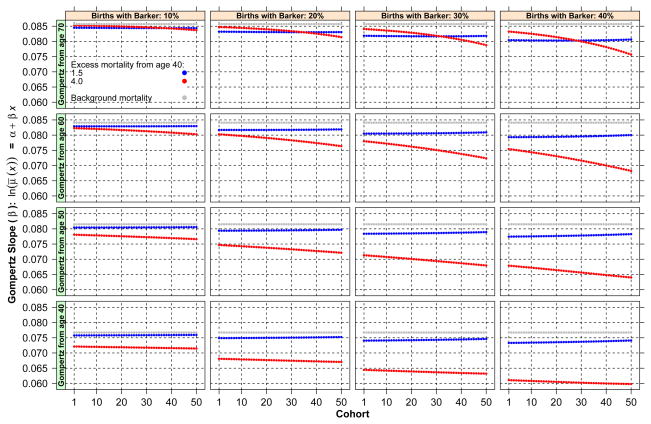
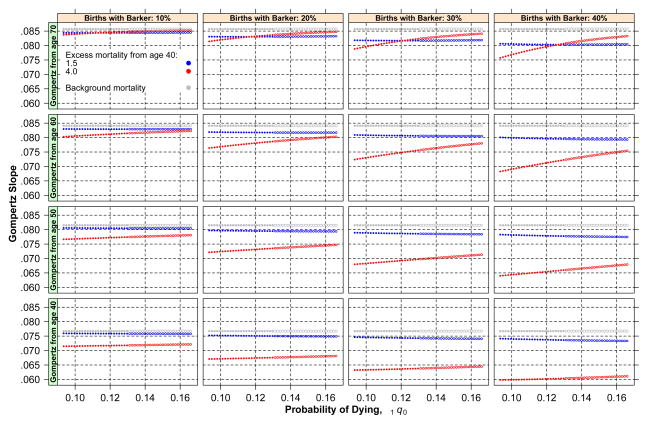
The most important results of the simulation with fixed critical age (at 40) are displayed in Figs. 1–4. Because in the case of random critical ages, the patterns of results are identical to those in the case of fixed critical ages, albeit with effects of smaller magnitude, we omit the corresponding figures. Figure 1 displays estimated slopes of adult mortality by birth cohort separately for populations with different levels of prevalence of Barker frailty (four panels, one for each population) and by lowest bound of age considered in the estimation of the adult mortality slope (40, 50, 60, and 70). In addition, each figure distinguishes three cases according to levels of Barker effects: 1.5 (blue), 4 (red), no excess or baseline (gray). (See the online version of the article to view the figures in color.) The first feature observed in the figure is that, as expected, the adult mortality slope declines with birth cohort in all cases. The magnitude of the difference between a cohort’s slope and the baseline slope varies but can attain values as high as 0.020, or 23 % error. Considering that the width of the range of plausible human adult mortality slopes is not larger than 0.04–0.08, the magnitude of the absolute error is substantial. The second feature is that deviations of each cohort’s slopes from the baseline increase for more recent cohorts. If the simulation had involved a larger number of cohorts, the deviations would have attained a maximum and then began to recede until becoming negligible. The third feature is that Barker excess matters a great deal. Indeed, the scenario with excess mortality equal to 1.5 generates a trajectory very close to the one that obtains if only standard frailty applies and produces much gentler deviations than the scenario with heavier adult mortality penalties. The final feature of Fig. 1 is that the population with a larger fraction of births susceptible to Barker effects presents the largest deviations of adult slopes from the baseline adult slope.
Fig. 1.
Estimated slopes of adult mortality by birth cohort for populations with different levels of prevalence of Barker frailty and by lowest bound of age considered in the estimation of the adult mortality slope (40, 50, 60, and 70)
Fig. 4.
Cohort’s mortality rates in three adult age groups and its correspondent infant mortality. Open circles represent older cohorts (i.e., those born in the early stages of the mortality decline), and closed circles correspond to recent cohorts
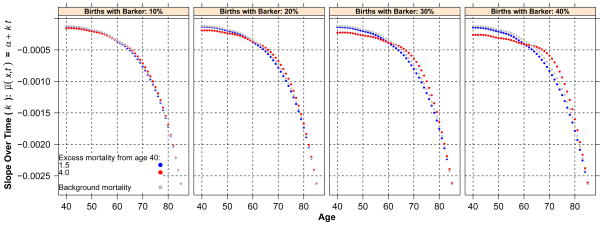
Figure 2 displays the average rate of mortality decline by adult age separately by levels of prevalence of Barker frailty. In each graph, we jointly plot the rates of decline estimated for the baseline scenario (gray), and two scenarios differing in the magnitude of excess mortality, 1.5 (blue) and 4 (red). By design, the absolute magnitude of the decline increases with age, but the concavity of the curves differs substantially across scenarios: when excess adult mortality is high, the rates are well below the baseline for all ages above 55–60 (and before age 90–95) and of lower absolute magnitude at ages below 55–60, although the differences here are trivial. The average relative difference between the most severe Barker regime and the baseline is as high as 50 % at age 72.5 when Barker effects are set to 4.
Fig. 2.
Average rate of mortality decline by adult age separately by levels of prevalence of Barker frailty
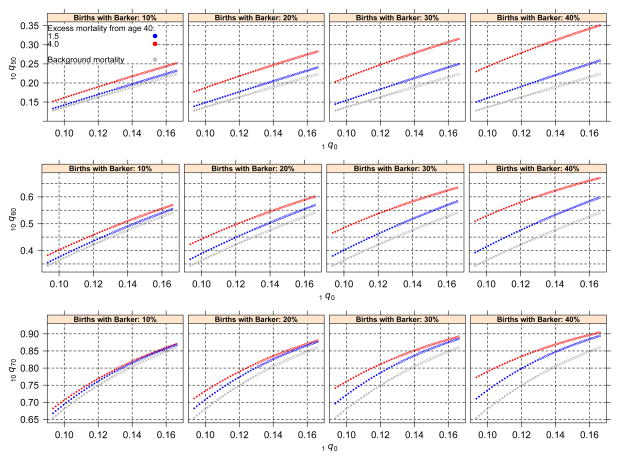
Figures 3 and 4 illustrate patterns of relations between a cohort’s early mortality and measures of adult mortality, and Tables 1 and 2 show correlation coefficients associated with these patterns. In each figure, open circles represent older cohorts (i.e., those born in the early stages of the mortality decline), and closed circles correspond to recent cohorts. Figure 3 plots the relation between the estimated Gompertz slope of a cohort and the level of infant mortality experienced by the same cohort. The figure includes four cases corresponding to different levels of prevalence of Barker frailty and four cases for different lower bounds of ages included in the estimation of a cohort’s slope. The key feature is that in all cases, the presence of Barker frailty induces a positive correlation where there is none (Table 1). The correlation is strongest when Barker effects are larger and when the range of ages included in the estimation of the slope is older. Researchers conventionally use age 50 or 60 as a lower bound. Even in these cases, however, the slope induced by Barker frailty is substantial; and the larger the magnitude of Barker effects, the steeper the slope becomes. Figure 4 plots a cohort’s mortality rates in three adult age groups and its corresponding infant mortality. The key feature is not the disparities of the curves’ levels—given that these depend on mortality levels associated with the cohorts—but rather the slope of the curves.9 In all cases, these slopes are flatter than the baseline, and they become flatter with increasingly larger magnitudes of Barker effects (Table 2). Figures 3 and 4 confirm the main implication of the main model: when declining mortality is driven by Barker frailty, any correlation between an observed cohort’s early and adult mortality will be deceiving because it will suggest patterns that are absent—the result of artifacts produced by Barker frailty.
Fig. 3.
Estimated Gompertz slope of a cohort and the level of infant mortality experienced by the same cohort. Open circles represent older cohorts (i.e., those born in the early stages of the mortality decline), and closed circles correspond to recent cohorts
Table 1.
Estimated correlation coefficient for the relationship between Gompertz slope of a cohort and the level of infant mortality experienced by the same cohort
| Excess Mortality From Ages X = 40, 50, 60, and 70 | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Births With Barker: 10 % | Births With Barker: 20 % | Births With Barker: 30 % | Births With Barker: 40 % | |||||
|
| ||||||||
| 1.5 | 4.0 | 1.5 | 4.0 | 1.5 | 4.0 | 1.5 | 4.0 | |
| Age 40 | ||||||||
| Barker | −.99 | .91 | −.99 | .93 | −.99 | .93 | −.99 | .93 |
| Background | −.09 | −.09 | −.09 | −.09 | −.09 | −.09 | −.09 | −.09 |
| Age 50 | ||||||||
| Barker | −.97 | .95 | −.98 | .97 | −.99 | .97 | −.99 | .99 |
| Background | .28 | .28 | .28 | .28 | .28 | .28 | .28 | .28 |
| Age 60 | ||||||||
| Barker | −.84 | .97 | −.93 | .98 | −.96 | .98 | −.97 | .99 |
| Background | .11 | .11 | .11 | .11 | .11 | .11 | .11 | .11 |
| Age 70 | ||||||||
| Barker | .89 | .96 | .75 | .97 | .08 | .98 | −.71 | .98 |
| Background | .16 | .16 | .16 | .16 | .16 | .16 | .16 | .16 |
Note: Values are computed from Fig. 3.
Table 2.
Estimated correlation coefficient for the relationship between cohort’s mortality rates in three adult age groups and corresponding infant mortality
| Excess Mortality From Ages X = 40, 50, 60, and 70 | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Births With Barker: 10 % | Births With Barker: 20 % | Births With Barker: 30 % | Births With Barker: 40 % | |||||
|
| ||||||||
| 1.5 | 4.0 | 1.5 | 4.0 | 1.5 | 4.0 | 1.5 | 4.0 | |
| 10Q50 | ||||||||
| Barker | 1.000 | .999 | 1.000 | .999 | 1.000 | .999 | 1.000 | .999 |
| Background | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 10Q60 | ||||||||
| Barker | .999 | .998 | .999 | .998 | .999 | .997 | .999 | .996 |
| Background | .999 | .999 | .999 | .999 | .999 | .999 | .999 | .999 |
| 10Q70 | ||||||||
| Barker | .994 | .994 | .993 | .993 | .993 | .993 | .992 | .993 |
| Background | .994 | .994 | .994 | .994 | .994 | .994 | .994 | .994 |
Note: Values are computed from Fig. 4.
Summary, Discussion, and Extensions
We propose a model for mortality patterns with discrete Barker frailty and Barker effects. We show, mathematically and via simulations, that a mortality regime with declining mortality and discrete Barker frailty will experience a stronger decelerating force than when only standard frailty prevails. As mortality declines, the selection pressure of standard frailty weakens over time and works against a sustained rate of mortality decline. Barker frailty augments the force opposing mortality decline with excess mortality among those who, having been exposed to adverse early-life conditions, manifest Barker effects upon attaining adult ages. Our formulation reconciles standard frailty with Barker frailty as survivors to critical ages become a “newly born” cohort at that age that experiences mortality rates with standard frailty and mortality multiplier Rδ (rather than δ) (Aalen 1988; Steinsaltz and Wachter 2006; Vaupel and Missov 2014; Vaupel and Yashin 1987; Vaupel et al. 1979).
A regime of declining mortality with Barker frailty exhibits a distinct dynamic. First, although standard frailty leads to progressive attenuation of the slope of adult mortality rates at older ages, Barker frailty can produce unequal levels of attenuation over time.
Second, when background mortality declines, average adult mortality could decline more slowly than background mortality rates, remain steady, or even increase. Because of this, the dynamics of changes could be nonlinear and the manifestation of excess adult mortality implied by Barker frailty undermines its sustained operation in a mortality regime.
Barker regimes regulated by random critical ages produce more subdued consequences than those with fixed critical ages because in the latter case, all individuals who are susceptible to expressing Barker frailty do so. Instead, when critical ages vary, individuals susceptible to Barker frailty can express it only after attaining the critical age that they are endowed with—not before. As a consequence, the pool of individuals who can oppose resistance to mortality decline at ages under x, exerting a force equivalent to what would be experienced if excess mortality associated with Barker frailty were reduced.
The simulation confirms inferences derived formally. We single out two fundamental results with important implications for interpretation of empirical patterns and extant mortality theories:
Rates of change of mortality rates over time or the nature of secular mortality decline: Any secular mortality decline regime with standard frailty will experience deceleration of the rate of mortality decline. If, in addition, Barker frailty and effects are nontrivial, the rate of deceleration will be augmented and particularly so at older ages. The force of the deceleration will be age- and time-dependent: the rate of mortality decline will oscillate over time, and the size of the oscillations will vary with age. The magnitude of the deceleration will attain a maximum at ages closer to the mean of the critical ages and somewhere in the middle of the period of secular decline. The implication of this result for empirical research is important: in particular, observed oscillations of the rate of change of the force of mortality over time at various ages cannot be mechanically interpreted as outcomes of shifts in the determinants of mortality decline because they can be completely accounted for by the dynamic of Barker frailty.
The adult mortality slope or the rate of senescence: Any secular mortality decline regime with standard frailty will experience deceleration of the adult mortality slope or rate of senescence. If, in addition, Barker frailty and effects are strong, the rate of increase of mortality with age at adult ages will decrease and do so with varying strength at different ages and times during the mortality decline. The implication is that in mortality regimes with Barker frailty and Barker effects, standard interpretations of time trends of the age-dependent rates of adult mortality increase could be incorrect because they may be influenced by the changing composition of cohorts by Barker frailty.
Importantly, Barker effects will not be observable in all countries, and their impact will depend on whether the populations meet the conditions defined in the earlier section Conditions of Observability. Thus, the impact of Barker effects on adult life expectancy is more likely to be observed in countries that experienced mortality declines that were at least partially sustained by the diffusion of medical technology rather than by amelioration of standards of living. Preliminary findings from Latin American countries, for example, have suggested that forgone gains in life expectancy at age 60 associated with Barker effects may be as high as 20 % of the projected values over a period of 30 to 50 years (Palloni and Souza 2013). The changing composition of cohorts by early exposures represents a powerful force that could drag down or halt short-run progress in life expectancy at older ages. The methods developed here facilitate the study of these type of effects with simple, parsimonious models.
The impact of Barker effects on health and mortality is similar to—yet distinct from—pleiotropic effects and mutation accumulation, which are two views about mechanisms of senescence that involve complex interactions between early and late life through the operation of deleterious alleles with differential impact on early and late life (Charlesworth 2001; Finch and Kirkwood 2000; Medawar 1952; Silvertown 2013; Williams 1957). The imprints on human mortality patterns left by these mechanisms has been modeled recently using more general mathematical models than the simple ones that we use here (Wachter et al. 2013, 2014). However, the dynamic of Barker effects has important peculiarities that should be reflected in any formal representation. First, unlike the dynamic inherent in pleiotropy and mutation accumulation, Barker effects can have potent deleterious effects early in life and thus reduce or suppress altogether their manifestation late in life. Although we did not model these directly (but see the general formulation in Online Resource 1), any general model for Barker effects should at least be flexible enough to integrate them. Second, the expression and size of effects due to DOHaD, Barker mechanisms, and particularly PAR are strongly modulated by the interaction between individual propensities, Barker frailty, and environmental (not genetic) conditions encountered in early life. Third, observation of Barker effects is tightly linked with the nature of mortality decline to which susceptible cohort members are exposed. These complex interactions with environments and demographic regimes are not part of the current models for pleiotropic or mutation accumulation effects.
The framework proposed in this article can be extended in several directions. First, to show its utility as a tool to interpret real phenomena, one can translate selected relations embedded in the model(s) into predicted outcomes and design feasible empirical tests involving population data to verify such predictions. Second, the models are flexible enough and admit a number of generalizations. Thus, one could establish a better connection between each of the variants of developmental origins theories and the formal models that represent effects. For example, the nature of critical ages is likely to be different when PAR mechanisms are dominant than when contraction of early infections has more powerful effects. Suitable functional forms to represent critical ages could be deduced from predictions derived from the theories themselves rather than imposed ex ante, as we did here. Similarly, the magnitude of excess adult mortality implied by Barker frailty should be specified in accordance to the types of chronic illnesses known or suspected to be influenced by adverse effects of early conditions. Excess adult mortality is almost certainly not age-invariant, and its level and age patterns might be tightly linked to the sources of delayed effects.
Furthermore, as suggested by researchers working on senescence (Finch 2007), adverse early conditions may affect the rate of senescence. If so, our models should impose random effects on the slope of adult mortality, not just on its level as is normally assumed (Beltrán-Sánchez et al. 2012). This finding adds a new layer of complexity because the adult mortality slope will be altered both as an artifact of standard and Barker frailty and as a result of direct effects of adverse early conditions and mortality decline.
Finally, a burgeoning literature (Bateson and Gluckman 2011; Gluckman and Hanson 2006; Kuzawa and Eisenberg 2014) shows that the expression of poor early conditions may implicate germ cells—and, if so, the risk of adult manifestation of early conditions is passed on from one generation to the next. The models proposed here ignore this aspect, but there is no inherent reason why they could not be extended to incorporate such relations through application of generalized stable population models.
Supplementary Material
Footnotes
Evidence from animal studies has confirmed several tenets of DOHaD and Barker theories (Bateson and Gluckman 2011), but empirical evidence from human populations is less favorable, inconsistent, and generally identifies effects of small magnitudes (Bengtsson and Lindström 2000; Lundborg 2008; Madsen et al. 2010; Painter et al. 2005; Roseboom et al. 2001). However, there is scarce, if any, evidence drawn from countries that experience the types of mortality decline and environmental and ecological conditions that we highlight here as conditions for observability of Barker effects.
What follows is particular case of a more general model for Barker frailty. See Online Resource 1.
The difference between standard and Barker frailty traits is that the former is fixed at birth whereas, at least conceptually, the latter could be shaped by early postnatal experiences. None of the mechanisms that we describe at the outset can be entirely captured by a trait fixed at birth. Thus, the distribution at birth of δ must reflect both the at birth-distribution and prospective changes that result from subsequent heterogeneous individual experiences. It is thus a trait with a more complex genesis that cannot be well specified unless we know the impact of postbirth experiences on the at-birth trait or propensity. To circumvent this conceptual problem, one can think of the distribution of δ at birth as what would be observed at a very early age beyond which additional experiences do not affect the underlying quantity in the absence of mortality up to that age. In this case, the distribution of δ as the outcome of the mixing of the distribution of fixed-at-birth susceptibility and the (unconditional on survival) probability of subsequent exposures that promote delayed manifestations. Thus, δ acts as a net propensity, induced by in utero deprivation or subsequent exposures to express delayed manifestations.
The assumption that Barker frailty leads to mortality excess only at adult ages downplays the consequence of Barker frailty in a secular mortality decline because the proportional gains in survival to older ages implied by an arbitrary rate of background mortality decline should be higher when there is no excess mortality at younger ages. As a consequence, our model understates the effects of Barker frailty.
This simplified functional form for mortality decline avoids cumbersome algebra but leads to no loss of precision or generality.
The expressions for PB(y, t) and its derivatives with respect to time t and age y are shown in Online Resource 1.
By construction, is age- and time-invariant. The implication of this expression seems to have gone unnoticed in the literature (but see Vaupel and Missov (2014) for an analogous expression for continuous frailty). Even in the absence of Barker effects and with an age-invariant (y) at adult ages (as in a Gompertz baseline adult mortality pattern), the age derivative of the βs average mortality pattern cannot be constant (across ages or across time when there is a mortality decline). The regime of frailty assumed here will always induce an age-dependent slope smaller than the standard slope. This has important consequences for the study of old-age mortality in that the standard interpretation of an empirical slope estimated after fitting—for example, a Gompertz function to a cohort’s adult mortality rates—is probably always incorrect. As suggested by the expression, such estimate contains an age- and time-dependent downward bias. To avoid this bias, one needs to estimate a Gompertz model controlling for both age and the age-and time-varying negative term in the expression. To our knowledge, this has never been done in empirical studies. Elsewhere, we show that Barker effects and mortality decline will always induce a negative correlation between the levels of child mortality experienced by a cohort and the cohort’s adult mortality slope (Palloni and Beltrán-Sánchez 2016).
An expression for is in Online Resource 1.
Linear correlations shown in Table 2 clearly underestimate the actual differences in slopes between Barker and background mortality due to the nonlinear pattern, but they still show flatter curves under the Barker regime.
References
- Aalen OO. Heterogeneity in survival analysis. Statistics in Medicine. 1988;7:1121–1137. doi: 10.1002/sim.4780071105. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies, and health in later life. 2. Edinburgh, Scotland; New York, NY: Churchill Livingstone; 1998. [Google Scholar]
- Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: Implications for research and public health. Environmental Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Gluckman P. Plasticity, robustness, development and evolution. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- Beltrán-Sánchez H, Crimmins EM, Finch CE. Early cohort mortality predicts the rate of aging in the cohort: A historical analysis. Journal of Developmental Origins of Health and Disease. 2012;3:380–386. doi: 10.1017/S2040174412000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson T, Lindström M. Childhood misery and disease in later life: The effects on mortality in old age of hazards experienced in early life, southern Sweden, 1760–1894. Population Studies. 2000;54:263–277. doi: 10.1080/713779096. [DOI] [PubMed] [Google Scholar]
- Charlesworth B. Patterns of age-specific means and genetic variances of mortality rates predicted by the mutation-accumulation theory of ageing. Journal of Theoretical Biology. 2001;210:47–65. doi: 10.1006/jtbi.2001.2296. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, … Pepys MB. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: Implications for obesity and diabetes. Clinical Chemistry. 2013;59:617–628. doi: 10.1373/clinchem.2012.187617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo IT, Preston SH. Effects of early-life conditions on adult mortality: A review. Population Index. 1992;58:186–212. [PubMed] [Google Scholar]
- Finch CE. The biology of human longevity: Inflammation, nutrition, and aging in the evolution of life spans. 1. Burlington, MA: Academic Press; 2007. [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Finch CE, Kirkwood TBL. Chance, development and aging. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Fong IW. Emerging relations between infectious diseases and coronary artery disease and atherosclerosis. Canadian Medical Association Journal. 2000;163:49–56. [PMC free article] [PubMed] [Google Scholar]
- Forsdahl A. Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? British Journal of Preventive & Social Medicine. 1977;31:91–95. doi: 10.1136/jech.31.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdahl A. Living conditions in childhood and subsequent development of risk factors for arteriosclerotic heart disease. The cardiovascular survey in Finnmark 1974–75. Journal of Epidemiology and Community Health. 1978;32:34–37. doi: 10.1136/jech.32.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, … Triplett EW. Toward defining the autoimmune microbiome for Type 1 diabetes. ISME Journal. 2011;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Developmental origins of health and disease. Cambridge, UK; New York, NY: Cambridge University Press; 2006. [Google Scholar]
- Kuzawa CW, Eisenberg DTA. The long reach of history: Intergenerational and transgenerational pathways to plasticity in human longevity. In: Weinstein M, Lane M, editors. Sociality, hierarchy, health: Comparative biodemography. Washington DC: National Research Council Press; 2014. pp. 65–94. [Google Scholar]
- Langley-Evans SC. Fetal nutrition and adult disease: Programming of chronic disease through fetal exposure to undernutrition. Oxfordshire, UK; Cambridge, MA: CABI Publishing; 2004. (Frontiers in Nutritional Science Series, No. 2) [Google Scholar]
- Lundborg P. The health returns to education—What can we learn from twins? Bonn, Germany: Institute for the Study of Labor; 2008. (IZA Discussion Paper No. 3399) [Google Scholar]
- Madsen M, Andersen A-MN, Christensen K, Andersen PK, Osler M. Does educational status impact adult mortality in Denmark? A twin approach. American Journal of Epidemiology. 2010;172:225–234. doi: 10.1093/aje/kwq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Kuzawa CW. Fetal programming of immune function: The early origins of immunity in Filipino adolescents. In: Langley-Evans SC, editor. Fetal nutrition and adult disease: Programming of chronic disease through fetal exposure to undernutrition. Oxfordshire, UK; Cambridge, MA: CABI Publishing; 2004. pp. 311–332. [Google Scholar]
- Medawar P. An unsolved problem in biology. London, UK: Lewis; 1952. [Google Scholar]
- Painter RC, Roseboom TJ, Bossuyt PM, Osmond C, Barker DJ, Bleker O. Adult mortality at age 57 after prenatal exposure to the Dutch famine. European Journal of Epidemiology. 2005;20:673–676. doi: 10.1007/s10654-005-7921-0. [DOI] [PubMed] [Google Scholar]
- Palloni A, Beltrán-Sánchez H. Dynamics of adult mortality regimes with delayed effects. 2015 Manuscript submitted for publication. [Google Scholar]
- Palloni A, Beltrán-Sánchez H. Demographic consequences of Barker frailty. In: Schoen R, editor. Dynamic demographic analysis. Cham, Switzerland: Springer International Publishing; 2016. pp. 147–176. [Google Scholar]
- Palloni A, Souza L. The fragility of the future and the tug of the past: Longevity in Latin America and the Caribbean. Demographic Research. 2013;29:543–578. doi: 10.4054/DemRes.2013.29.21. (article 21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom TJ, Van Der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: An overview. Molecular and Cellular Endocrinology. 2001;185:93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- Silvertown J. The long and the short of it. Chicago, IL: University of Chicago Press; 2013. [Google Scholar]
- Steinsaltz DR, Wachter KW. Understanding mortality rate deceleration and heterogeneity. Mathematical Population Studies. 2006;13:19–37. [Google Scholar]
- Vaupel JW, Manton KG, Stallard E. Impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- Vaupel JW, Missov T. Unobserved population heterogeneity: A review of formal relationships. Demographic Research. 2014;31:659–686. doi: 10.4054/DemRes.2014.31.22. (article 22) [DOI] [Google Scholar]
- Vaupel JW, Yashin AI. Repeated resuscitation: How lifesaving alters life tables. Demography. 1987;24:123–135. [PubMed] [Google Scholar]
- Wachter KW, Evans SN, Steinsaltz DR. The age-specific force of natural selection and biodemographic walls of death. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10141–10146. doi: 10.1073/pnas.1306656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter KW, Steinsaltz DR, Evans SN. Evolutionary shaping of demographic schedules. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10846–10853. doi: 10.1073/pnas.1400841111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.