Abstract
T cells expressing a CD19-specific chimeric antigen receptor (CAR19) are demonstrating remarkable efficacy in hematologic malignancies. Treatment is often associated with life-threatening cytokine release syndrome (CRS) which can be effectively treated with cytokine blockade using the antibodies, Siltuximab or Tocilizumab respectively targeting IL-6 or the IL-6 receptor. As IL-6 blockade is moving into the clinic for the treatment of CRS as well as IL-6-driven rheumatologic and malignant diseases, clinicians are utilizing serum cytokine panels more frequently to assess the effects of IL-6 inhibitors. It is paramount to ascertain whether levels obtained are accurate, especially as certain drugs may, in theory, affect quantification. We report the comparative quantification of IL-6 and sIL-6R using Luminex-based immunoassay kits from two vendors. Our results indicate good agreement of the commercial immunoassays in measurement of IL-6 but disagreement in quantitation of sIL-6R. We found that both Siltuximab and Tocilizumab can interfere with the measurement of their respective ligands using reagents from one vendor but not the second. This has significant implications for the analysis of IL-6 and sIL-6R pharmacokinetics analysis in Siltuximab or Tocilizumab-treated patients. We found that high levels of IL-6 can falsely reduce the measured levels of sIL-6R and high levels of sIL-6R can reduce levels of IL-6 when measured with some commercial assays. These data demonstrate the importance of assessing the impact of cytokine-blocking agents on accuracy of clinical biomarker assays in other diseases, as drugs targeting TNF-alpha, IL1B, and IL5 are being used more frequently in a large number of diseases.
Keywords: IL-6, sIL6-R, Tocilizumab, Siltuximab, CAR-T, Luminex
1. Introduction
CAR19 T cells have demonstrated great promise in clinical trials, with clinical response rates of over 90% in children with relapsed/refractory ALL(Maude et al., 2015; Maude et al., 2014a; June et al., 2014). Encouraging response rates have also been seen in CLL(Porter et al., 2015) and NHL(Kochenderfer et al., 2015). These response rates are associated with considerable in vivo CAR T cell proliferation and clearance of CD19+ cells. However, killing of tumor cells is accompanied by cytokine release syndrome (CRS) (Porter et al., 2015). CRS can be mild with flu-like symptoms including fever, nausea, chills or life-threatening and severe with shock and respiratory compromise, leading to multi-system organ failure (Winkler et al., 1999). Markedly elevated levels of interleukin-6 (IL-6) and other cytokines drive CRS. Timely monitoring of cytokine levels, especially IL-6 can be extremely helpful in understanding CRS (Maude et al., 2014b; Xu & Tang, 2014; Lee et al., 2014). We have recently described an algorithm for early prediction of severe CRS by monitoring serum cytokines (Teachey et al. Cancer Discovery, In Press).
IL-6 is a pro- and anti-inflammatory cytokine involved in various biologic processes. IL-6 has been associated with inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, vasculitis, and malignant and non-malignant lymphoproliferative disorders, including multiple myeloma and Castleman’s syndrome (LH & Rose-John, 2014; Rossi et al., 2015). IL-6 is a small secreted glycoprotein composed of 184 amino acids, which form four helixes with a molecular weight ranging from 21 to 30 kD depending on the cells of origin(Simpson et al., 1997). It is produced by a range of cells under different conditions, such as stromal cells, hematopoietic cells, epithelial cells, or muscle cells (Rossi et al., 2015; Thompson et al., 2012; Wolf et al., 2014). IL-6 concentrations are typically in the low picogram per ml range, in healthy individuals (Robak et al., 1998). However, IL-6 levels can elevate to nanograms per ml in patients with inflammation or infection, including sepsis (LH & Rose-John, 2014; Thompson et al., 2012; Robak et al., 1998; Oda et al., 2005).
Siltuximab, (CNTO 328, Sylvant) is a human-murine chimeric monoclonal antibody (MAb) against IL-6. It is a potent inhibitor of IL-6 with an estimated Kd of 1 pM (Zaki et al., 2004), and in 2014 was FDA-approved for the treatment of patients with multicentric Castleman’s disease. Tocilizumab (Atlizumab, Actemra) is a humanized monoclonal antibody against IL-6R, which binds to both soluble and membrane-bound IL-6Rs with a Kd of ~2.54 nM (Mihara et al., 2005). It blocks IL-6 induced signal transduction pathways through competitive inhibition of IL-6 binding to its receptors (Nishina et al., 2013), and has been approved in Japan for treatment of Castleman’s disease. Both Tocilizumab and Siltuximab have been used to treat CRS following CART19 therapy (Maude et al., 2014b; Grupp et al., 2013; Calabrese & Rose-John, 2014).
There are several commercially available sandwich Enzyme-Linked Immuno-Sorbant Assay (ELISA)-based immunoassays for IL-6 and sIL-6R. Bead-based immunoassays, especially Luminex bead-based assays, due to their multiplexing capability, have replaced many of the traditional immunoassays (Leng et al., 2008). However, the analyte-specific antibodies and the recombinant protein used for the standard curve in each assay largely determine the specificity, reproducibility and sensitivity in either traditional microplate based or current bead based immunoassays (Butler et al., 1986; Vignali, 2000). As a consequence, measurements of cytokine and receptor likely vary between vendors or even assay lots (Thompson et al., 2012; Ellington et al., 2010). We hypothesized that measurement of IL-6 or sIL-6R in serum could be altered by the presence of combinations of IL-6, sIL-6R, Tocilizumab, and/or Siltuximab (Fig. 1). IL-6 binds to the membrane-bound or soluble IL-6R with an affinity of around 1 nM (LH & Rose-John, 2014), thus IL-6 or sIL-6R can compete for binding with therapeutic or detection antibodies depending on the epitopes recognized by the antibodies.
Fig. 1.
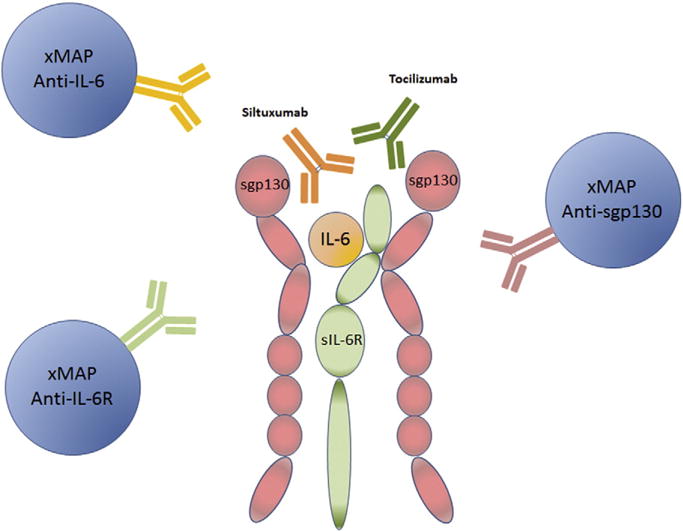
Potential interaction among IL-6, sIL-6R, sgp130, Siltuximab, Tocilizumab, and xMAP antibody sets to IL-6, IL-6R and sgp130.
In this study, we describe the results of a comparison of Luminex kits for human IL-6 and sIL-6R from EMD Millipore and Life Technologies to evaluate the effect of the presence of Siltuximab, sIL-6R, IL-6, and Tocilizumab on assay performance.
2. Materials and methods
2.1. Materials
IL-6 (PHC0064), sIL-6R (10398-H08H-25) recombinant proteins, Luminex singleplex kits for IL-6 (LHC0061) and sIL-6R (LHR0061) were purchased from Life Technologies (Grand Island, NY). Another set of Luminex singleplex kits for IL-6 (HCYTOMAG-60K-01) and sIL-6R (HSCRMAG-32K-01) were purchased from EMD Millipore (Darmstadt, Germany).
Siltuximab (Janssen) and Tocilizumab (Genentech) were obtained from Children’s Hospital of Philadelphia Pharmacy. Siltuximab was added to final concentrations of 20 μg/ml and 600 μg/ml in standard curves samples. These concentrations were chosen based on its reported serum Cmax of 332 μg/ml (JanssenBiotech. SYLVANT (siltuximab), n.d.). For similar reasons, concentrations of Tocilizumab of 20 μg/ml, 80 μg/ml, and 160 μg/ml were chosen based on reported serum Cmax of 88 μg/ml (FDA. ACTEMRA® (tocilizumab), n.d.).
The fixed concentration of IL-6 and sIL-6R added into sIL-6R and IL-6 standard curves respectively was 1.78 μg/ml. This was attained by dissolving 5 μg of the lyophilized protein in 1.4 ml of assay buffer solution and adding 50 μl of the resulting solution to the corresponding reaction wells to a final volume of 100 μl.
All serum samples used in these studies were obtained as part of ongoing clinical trials of CART19 as a treatment for ALL (NCT01626495), CLL (NCT01747486), or NHL (NCT02030834). The protocols were approved by the Institutional Review Board of the University of Pennsylvania, the U.S. Food and Drug Administration (FDA), and the Recombinant DNA Advisory Committee and were conducted under an FDA-approved Investigational New Drug Application. All patients gave informed consent in accordance with the Declaration of Helsinki.
2.2. Measurement of IL-6, sIL-6R and sgp130 concentrations in patient serum samples
Human cytokine magnetic 30-plex panel catalog number LHC6003M was purchased from Life Technologies (Carlsbad, CA). The following analytes are measured by the panel (IL-1RA, FGF-Basic, MCP-1, G-CSF, IFN-γ, IL-12, IL-13, IL-7, GM-CSF, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IFN-α, IL-15, IL-10, MIP-1α, IL-17, IL-8, EGF, HGF, VEGF, MIG, RANTES, Eotaxin, MIP-1β, IP-10, IL-2R).
Human soluble cytokine receptor magnetic bead 14-plex panel (catalog number HSCRMA32KPX14) was purchased from EMD Millipore (Darmstadt, Germany). The following analytes are in the panel (sCD30, sEGFR, sgp130, sIL-1RI, sIL-1RII, sIL-2Rα, sIL-4R, sIL-6R, sRAGE, sTNFRI, sTNFRII, sVEGF-R1, sVEGF-R2, sVEGF-R3).
Serum samples cryopreserved at −80 °C from day −1 or baseline to day 28 were thawed and analyzed according to the manufacturers’ protocols. Assay plates were measured using a FlexMAP 3D instrument (Luminex, Austin, TX), and data acquisition and analysis were done using xPONENT software (Luminex).
Data quality was examined based on the following criteria. The standard curve for each analyte has a R2 value > 0.95 with or without minor fitting using xPONENT software. Non-extrapolated data from low bead counts that also have CV (coefficient of variation) >20% were flagged. For the 14-plex kit, the results for the two control samples included in the kit was required to be within the expected ranges provided by the manufacturer for >10 analytes. For the 30-plex kit, > the results for the in house control needed to be within the 95% of CI (confidence interval) derived from historical in house control data for >25 of the analytes. No further tests were done on samples with results out of range low (<OOR). Samples with results that were out of range high (>OOR) or greater than two times the standard curve maximum value (SC max) were re-tested at higher dilutions. Results that passed the above quality controls or retests were used in translational correlative studies.
2.3. IL-6 standard curve preparation and IL-6 measurement in the serum
The expected concentration values for IL-6 standard curve (STD) were 5000, 1666.7, 555.6, 185.2, 61.7, 20.6 and 6.9 pg/ml. To evaluate Tocilizumab, Siltuximab and sIL-6R effects on recombinant IL-6 standard curves and serum IL-6 measurement, defined levels of one of the reagents were added to each of the standard curve assays. Otherwise, the assay was carried out according to the manufacturer’s protocol.
2.4. sIL-6R standard curve preparation and sIL-6R measurement in the serum
The expected concentration values for sIL-6R STD were at 25,000, 8333.3, 2777.8, 925.9, 308.6, 102.9, and 34.3 pg/ml. To determine the effect of Tocilizumab, Siltuximab and IL-6 on recombinant sIL-6 standard curves and serum measurements, defined levels of one of the reagents were added to each of the standard curve assays. Otherwise, the assay was carried out according to the manufacturer’s protocol.
2.5. Data analysis
All data used are the mean of duplicated raw data. Deviations are expressed in %CV and used for error bars on the graphs. IL-6 values for serum 1 and 2, and sIL-6R values for serum 3 and 4 were analyzed as a 2-way analysis of variance, with main effects for treatment and serum, and their interaction. Analyses were done separately for Millipore and LifeTech kits. Values were normally distributed so no transformations were necessary. Other P-values (Supplemental tables) were obtained using Excel TTEST function (1 tail, two sample equal variance).
3. Results
3.1. Evaluation of data derived from IL-6 and sIL-6R Luminex immunoassays from different vendors
IL-6 standard curves from Millipore and LifeTech both started from 5000 pg/ml with seven 1:3 serial dilution points (see Materials and Methods). Both curves had almost identical measured concentrations to the expected concentrations, which indicated close to 100% recovery at all seven concentrations with minimal %CV in duplicates (Supplemental Fig. 1A). sIL-6R standard curves from Millipore and LifeTech both started from 25,000 pg/ml with seven 1:3 serial dilution points (see Materials and Methods). Again, both curves had almost 100% recovery at all seven concentrations with minimal %CV in duplicates (Supplemental Fig. 1B). Essentially, data obtained using IL-6 and sIL-6R immunoassay kits were indistinguishable based on the standard curves between the two vendors.
We then used IL-6 kits to measure IL-6 in human serum from two patients, hereafter called sera 1 and 2. As described in the supplemental material section, both contain high levels of IL-6. The two samples were then diluted such that the IL-6 levels would be in the middle region of the 7 to 5000 pg/ml IL-6 standard curve, (Supplemental Table 3). This adjustment was performed to avoid high measurement variability derived from extrapolated data. We found no significant difference in IL-6 quantification for either serum 1 or 2 when comparing the two kits (Fig. 2A). There was also no significant difference in IL-6 quantification when sera were analyzed using a LifeTech 30-Plex kit human cytokine panel: 495pg/ml (serum 1) and 1250pg/ml (serum 2). This kit includes the same IL-6 beads as in the IL-6 single plex kit from the same manufacturer. Diluted human sera 3 and 4 from ALL patients were used in sIL-6R testing after dilution such that the expected concentrations were in the middle range of sIL-6R standard curve 34 to 25,000 pg/ml (Supplemental Table 3). Contrary to IL-6, the sIL-6R results were dramatically different between the two vendors, and the difference was statistically significant with P < 0.05 (Fig. 2B). Both values from LifeTech kit were higher than those from Millipore. However, the values from Millipore sIL-6R kit agreed with the expected sIL-6R levels of 6291 pg/ml (serum 3) and 9598 pg/ml (serum 4) that were obtained using a Millipore soluble cytokine receptor 14-plex kit which contains the same sIL-6R beads as in the singleplex kit. To investigate the basis for this difference we measured the recombinant protein standard curves for the Millipore as an unknown sample using the LifeTech sIL-6R kit, and vice-versa. We found that the Millipore standard appeared higher than the nominal concentration when measured using the LifeTech kit, and the LifeTech standard appeared lower than the nominal concentration when measured using the Millipore kit (data not shown). This discrepancy matches the different sIL-6R values in patient samples reported by the two vendor’s kits. The results demonstrate that Luminex kits from different vendors can report different sIL-6R concentrations when used to measure the same serum samples.
Fig. 2.
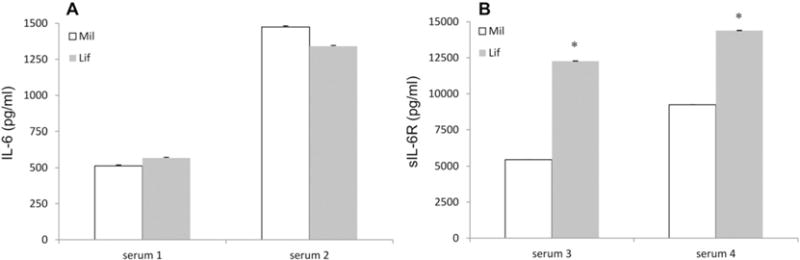
IL-6 (A) and sIL-6R measurements on two serum samples using Millipore kit (open bar) and LifeTech kit (gray bar). Error bars are %CV from replicates. * shows the statistical difference between the measured values obtained with the two assay kits.
3.2. Effect of Siltuximab, sIL-6R and Tocilizumab on the performance of recombinant IL-6 in standard curve and quantification of serum IL-6
We evaluated the effect of Siltuximab, sIL-6R and Tocilizumab on the performance of the IL-6 standard curve and quantification of IL-6 in serum samples using kits from both Millipore and LifeTech. In theory, only Siltuximab and sIL-6R should bind to IL-6 and thus have potential for either steric interference with binding of the assay detection antibody, or possibly inducing a conformational change that reduces binding. Tocilizumab will not bind to IL-6, and was used as a non-specific control in this context.
The results obtained using the Millipore IL-6 kit showed that the addition of 20 μg/ml of Siltuximab reduced the measured vs. the expected IL-6 concentrations by −51% to −70% over the entire range of the standard curve (Fig. 3A, Supplemental Table 1). Higher concentration (600 μg/ml) Siltuximab had limited additional effect, reducing the expected concentration by −56% to −74% (Fig. 3A, Supplemental Table 1A). 1.78 μg/ml of sIL-6R also reduced the measured IL-6 concentrations ranging from −23% to −47% from the highest to the lowest concentration points (Fig. 3A, Supplemental Table 1A). Reductions of measured IL-6 concentrations by Siltuximab and sIL-6R all reached statistical significance for each of the seven points on the standard curves. In contrast, Tocilizumab under the same assay conditions showed no significant effect on the measured IL-6 concentrations. These data suggest that the reduction effects are specific to Siltuximab and sIL6R and are not the result of overloading the assays with high concentrations of antibody/protein. Importantly, the degree of reduction from Siltuximab and sIL-6R on the measured IL-6 concentration was not correlated to the IL-6 concentration being measured, indicating that the competitor was saturating within this range of IL-6 concentrations.
Fig. 3.
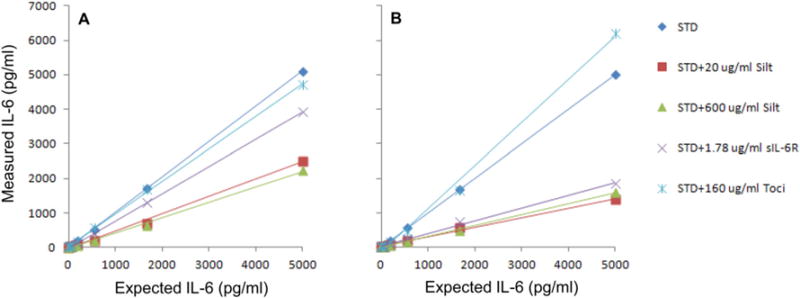
Siltuximab, sIL-6R and Tocilizumab effect on IL-6 standard curves generated using Millipore kit (A) and Life Technologies kit (B).
The equivalent experiments using the LifeTech IL-6 kit gave similar inhibitory effect of Siltuximab and sIL-6R on measured IL-6 concentrations, although the effect was even more dramatic (Fig. 3B). In this case, the percent reduction correlated with the concentrations of IL-6, ranging from −72% to −30% for addition of 20 μg/ml Siltuximab, and −63% to −25% for addition of sIL-6R from the highest to the lowest concentrations of IL-6 standard curve. Addition of 600 μg/ml Siltuximab did not increase inhibition compared to 20 μg/ml (Fig. 3B, Supplemental Table 1B). Furthermore, 160 μg/ml Tocilizumab essentially had no significant effect on the measured IL-6 concentrations except that it appeared to significantly increase the measured amount of IL-6 at the highest expected 5000 pg/ml points by 24% with P-value of 0.0048 (Fig. 3B, Supplemental Table 1B). The addition of sgp130 to 300 ng/ml had no effect on the measurement of IL-6 using either kit, whether or not sIL-6R was also present (data not shown).
The IL-6 immunoassay Luminex kits from Millipore and LifeTech performed similarly in quantifying natural endogenous IL-6 concentrations in serum samples. The maximal dose of 600 μg/ml Siltuximab and 1.78 μg/ml sIL-6R reduced the measured IL-6 concentrations by about −60% and −25% correspondingly in both serum samples measured by both Millipore and LifeTech kits. The reductions all reached statistical significance based on P-values (Fig. 4). Again, the reduction was Siltuximab and sIL-6R specific as 160 μg/ml Tocilizumab showed no significant effects on the measured IL-6 concentrations from both kits (Fig. 4).
Fig. 4.
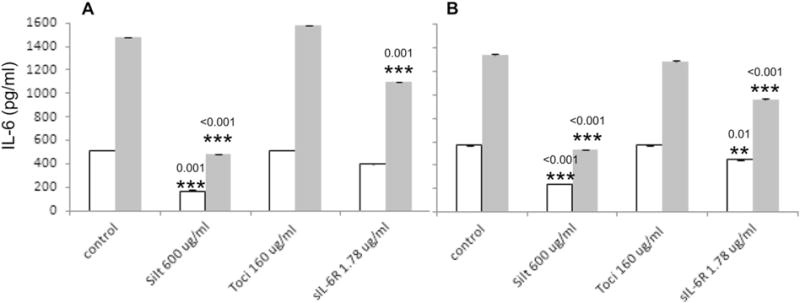
Siltuximab, Tocilizumab and sIL-6R effect on endogenous IL-6 concentration measurement in serum samples generated using Millipore (A) and LifeTech (B) kits. * shows the statistical difference in comparison to the corresponding control. Open box/bar — serum 1; gray box/bar — serum 2.
3.3. Tocilizumab, IL-6, and Siltuximab effect on the measurement of recombinant sIL-6R in standard curves and quantification of serum sIL-6R
We examined the effect of Tocilizumab, IL-6, and Siltuximab on the performance of sIL-6R standard curves and quantification of sIL-6R in serum samples using kits from both Millipore and LifeTech. In theory, only Tocilizumab and IL-6 should bind to sIL-6R and potentially interfere with measurement. Siltuximab will not bind to sIL-6R, and was used as a non-specific control.
There was a small but statistically significant reduction of the sIL-6R concentrations in the presence of 20 μg/ml Tocilizumab when measured using the Millipore kit, ranging from 5% to 25% from the highest to the lowest expected sIL-6R concentrations (Fig. 5A). 20 μg/ml Tocilizumab evidently reached maximal dose in reduction as 80 μg/ml Tocilizumab only marginally reduced the measured sIL-6R concentrations compared to 20 μg/ml. The reduction at every point of the standard curve except the highest concentration reached statistical significance. IL-6 at a concentration of 1.78 μg/ml exerted some reduction effect on the measured sIL-6R concentrations ranging from 12% to 7%. As expected, 600 μg/ml Siltuximab had no significant effect on the measured sIL-6R concentrations (Fig. 5A, Supplemental Table 2A).
Fig. 5.
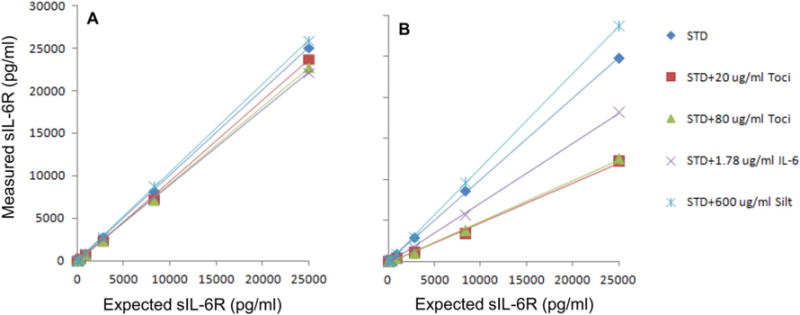
Tocilizumab, IL-6 and Siltuximab effect on sIL-6R standard curves generated using Millipore kit (A) and LifeTech kits (B).
Equivalent data was generated using LifeTech sIL-6R immunoassay kit. The specific reduction on the measured sIL-6R concentration was much more obvious than that from the Millipore sIL-6R immunoassay kit (Fig. 5B). In this study, 20 μg/ml Tocilizumab induced 51% to 70% reduction of the measured sIL-6R concentrations as compared to expected. The effect of IL-6 addition to measured sIL-6R levels was also very dramatic with a clear trend ranging from 27% to 64% reduction. 600 μg/ml Siltuximab exhibited no significant effect on measured sIL-6R concentrations (Fig. 5B, Supplemental Table 2B). Table 1 shows a summary of the effects of adding antibody, IL-6 or sIL-6R on measurement of recombinant protein standard curves for each of the four commercial Luminex assay kits. The addition of sgp130 to 300 ng/ml had no effect on the measurement of sIL-6R using either kit, whether or not IL-6 was also present (data not shown).
Table 1.
Summary of effects of adding antibody, IL-6 or sIL-6R on measurement of protein standard curves for each of four commercial Luminex assay kits.
| Assay | Antibody/cytokine/receptor added
|
|||
|---|---|---|---|---|
| Tocilizumab | Siltuximab | IL-6 | sIL-6R | |
| LifeTech IL-6 | No effect | Reduced | N/A | Reduced |
| Millipore IL-6 | No effect | Reduced | N/A | Slightly reduced |
| LifeTech sIL-6R | Reduced | No effect | Reduced | N/A |
| Millipore sIL-6R | Little/no effect | No effect | Little/no effect | N/A |
The results from measurement of serum sIL-6R levels were unexpected. First, as described earlier (Fig 2) two test serum samples (serum 3 and serum 4) both contained statistically different sIL-6R concentrations when comparing measurements made using Millipore and LifeTech kits (Figs. 2B and 6, control samples). Secondly, neither the addition of Tocilizumab nor IL-6 reduced the measured sIL-6R concentrations regardless which assay kit was used (Fig. 6). Based on the concentrations of sIL-6R reported in the serum by the LifeTech kit and the degree of inhibition by Tocilizumab and IL-6 on recombinant standards in this concentration range, we would have expected to see inhibition of detection of serum sIL-6. Notably, serum 4 was from a patient treated with Tocilizumab 5 days previously (see Supplemental Table 3).
Fig. 6.
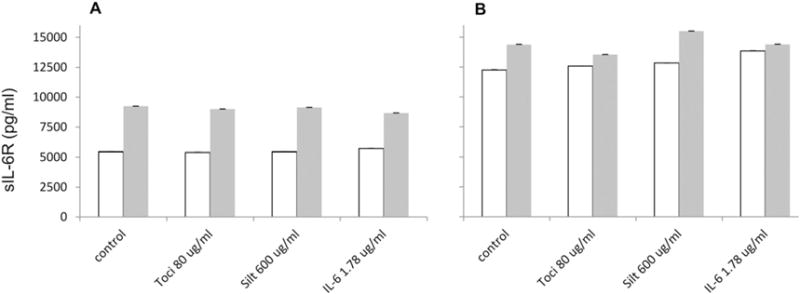
Tocilizumab, Siltuximab and IL-6 effect on endogenous sIL-6R concentration measurement in serum samples generated using Millipore (A) and LifeTech (B) kits. Open box/bar — serum 3; gray box/bar — serum 4.
3.4. Correlation of IL-6, sIL-6R, sgp130 with CART19, CRS and inflammatory markers
Fig. 7 shows plots of relevant information from an adult ALL patient (NCT01029366; UPCC04409-23) infused with CART19. Plots of absolute IL-6, sIL-6R and sgp130 levels, and fold changes from baseline serum day −1 prior to CART19 infusion are aligned with plots of the corresponding body temperature changes and ferritin levels. The patient experienced Grade 4 CRS with a rise at day 6 post-infusion of body temperature to 40.5 °C and elevated serum ferritin (over 10,000 ng/ml) and IL-6 (over 600 pg/ml, 120-fold increased from baseline). The level of serum sgp130 was not altered and sIL-6 receptor was modestly (~5-fold) increased. Tocilizumab therapy was administered at day 4 (indicated by a dark triangle) and was followed by resolution of fever, and a rapid drop in IL-6. Both sIL-6R and ferritin increased before dropping during the following 25 days. Similar trends were seen in other patients on this study [Teachey et al. Cancer Discovery, In Press]. Examining such data, it is impossible to differentiate an effect of endogenous Tocilizumab from treatment on sIL-6R measurement from a reduction in sIL-6R levels due to Tocilizumab or other therapy.
Fig. 7.
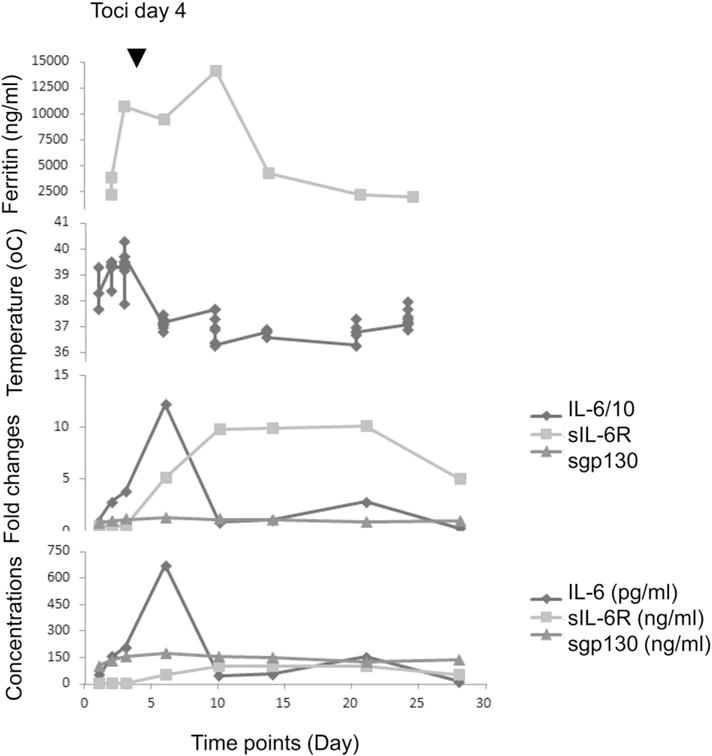
IL-6, sIL-6R and sgp130 concentrations and fold changes, ferritin, temperature time course for UPCC04409–23. IL-6 fold-change is shown 10 fold lower than actual. IL-6 concentration is in pg/ml, while sIL-6R and sgp130 are in ng/ml.
4. Discussion
IL-6 exerts its biological functions via two major pathways: classic signaling and trans-signaling pathways. In the classic signaling pathway, IL-6 binds to the IL-6 receptor (IL-6R) on hepatocytes and some leukocytes. The IL-6_IL-6R complex further recruits the ubiquitously expressed membrane-bound or soluble gp130 (sgp130), triggering the dimerization of gp130 and intracellular signaling. In the trans-signaling pathway IL-6 interacts with soluble IL-6R (sIL-6R) to form the IL-6_sIL-6R complex, which can bind to gp130 on any cell and initiate intracellular signaling (LH & Rose-John, 2014; Wolf et al., 2014) without a requirement for the stimulated cell to express IL-6R. sIL-6R is either produced as a soluble form from an alternatively spliced mRNA, or is released from the aforementioned cell types via post-translational modification and is normally present in blood at ~50 ng/ml and sgp130 at ~400 ng/ml (Robak et al., 1998). Since these soluble receptors are present in the circulation at much higher levels than IL-6 (1-5 pg/ml (Robak et al., 1998)), sIL-6R and sgp130 constitute a buffer for IL-6 in blood and mediate IL-6 inflammation (Scheller & Rose-John, 2012).
Over the past decade, cytokine-directed therapy has increasingly been used to treat a number of diseases. A large number of drugs that inhibit cytokines are in clinical use, including agents that inhibit TNF-alpha, IL1B, IL-5, and IL-6. In parallel, measurement of serum cytokines in clinical laboratories is becoming more commonplace both in diagnostic medicine and to guide therapy. Thus, it is paramount to ascertain whether levels obtained are accurate, especially as certain drugs may, in theory, affect quantification. Little data exist on the direct effects of drugs on the ex vivo quantification of cytokine levels. For example, after a patient is treated with a TNF-alpha receptor inhibitor such as etanercept, do TNF-alpha levels decrease because less TNF-alpha is produced, more is bound in sera, or simply because the drug affects the assay results without actually changing the in vivo serum levels of the cytokine-blocker? Our group and others are currently using IL-6 pathway inhibitors, including Tocilizumab and Siltuximab, to treat CRS after T-cell engaging therapies. Moreover, IL-6 pathway inhibitors are approved to treat other conditions including Castleman’s disease and juvenile idiopathic arthritis. Tocilizumab is currently being studied in clinical trials in patients with a large number of diseases, including rheumatoid arthritis, non-infectious uveitis, graft-versus host disease, hematophagocytic syndrome, schizophrenia, dermatomyositis, and Grave’s disease. Moreover, numerous case reports and case series describe successful off-label use of IL-6 pathway inhibitors. As more success stories emerge, their use will likely continue to grow.
The doses of these drugs that are used are derived from studies in different diseases, and different conditions may require different doses or schedules for optimal activity. Accordingly, many of these trials are measuring serum levels of IL-6 and IL-6R with the hope of finding a dose–efficacy relationship using serum levels as a biomarker to predict the best schedule and dose intensity. Understanding how the drugs affect the measured levels is key to using cytokines as an accurate biomarker. Moreover, it is important to understand if results vary based on other factors including the type of assay and the manufacturer of the assay.
The in vitro reconstruction experiments described in this study of IL-6 and sIL-6R quantification do provide useful information on how these assays may be affected by known parameters such as Tocilizumab, Siltuximab, endogenous levels of Il-6 and IL-6R and the choice of assay vendor.
The data in this report illustrates that it is insufficient to evaluate immunoassay kits or antibodies to a given analyte in the same assay format but from different vendors based only on the standard curves. Variations in assay performance can be revealed when such test reagents are used to measure the endogenous analytes in serum. The differences between immunoassays can also only become apparent when testing for analytes in the presence of other factors that also interact with the analytes in question. An example discussed here is IL-6 measurement in the presence of Silt/sIL-6R. Factors contributing to variability in IL-6 and sIL-6R measurements can include: (1) the choice of antibody and the different epitopes recognized by antibodies from different vendors; (2) the antigenic character and physical properties of IL-6 and sIL-6R. The receptor has almost twice the number of amino residues of IL-6; and (3) IL-6 and sIL-6R proteins used as quantitation standards may be derived from different sources, and consequently vary in purity and have different post-translational modifications and may be folded differently. The IL-6 and sIL-6R used in the standard curves or added as competitors in these studies were all recombinant proteins. The character of the standards and the antibody sets used in Millipore and LifeTech kits are considered proprietary information by the vendors. Given all these considerations, it is not remarkable that there is variation in different measured concentrations for IL-6, sIL-6R or other analytes in the samples tested on the same format assays but from different vendors. As a general principle, biomarker concentrations cannot be compared unless they are generated in the same immunoassays from the same vendor, preferably in the same assay. However, in our experience, results obtained using kits from the same vendor and same manufactured lot will often be highly reproducible.
A side-by-side quantification of recombinant and endogenous IL-6 and sIL-6R using Luminex based immunoassay kits from two major vendors Millipore and Life Technologies has been performed in this study. Our results clearly demonstrate that immunoassays for the same biomarker, in this case IL-6 and sIL-6R, in the same format, such as Luminex bead based assay, from different vendors can yield different concentrations on the same samples. The presence of Siltuximab/sIL-6R and Tocilizumab/IL-6 at therapeutic concentrations will further affect the measured IL-6 and sIL-6R concentrations respectively, reducing the apparent concentrations for both recombinant and endogenous IL-6 and recombinant sIL-6R. Importantly, assay kits from different vendors can differ in sensitivity to this interference.
Supplementary Material
Acknowledgments
Financial support was provided by a grant from Novartis (to Dr. June), by grants from the National Institutes of Health (R01CA165206, to Dr. June; R01CA193776, to Dr. Teachey; K23GM110496 to Dr. Weiss), a Marshall A. Lichtman Specialized Center of Research Program award from the Leukemia and Lymphoma Society to Dr. June, the Jeffrey Jay Weinberg Memorial Foundation, and the Children’s Hospital of Philadelphia Hematologic Malignancy Research Fund, a Stand Up to Cancer—St. Baldrick’s Pediatric Dream Team translational research grant (SU2C-AACR-DT1113), St. Baldrick’s Foundation Scholar Awards (to Dr. Maude), and a Research Scholar Grant from the American Cancer Society (RSG-14-022-01-CDD, to Dr. Teachey).
We thank Natalka Kengle for the assistance with the Luminex cytokine assays, and Jeffrey Finklestein, Farzana Nazimuddin, Chelsie Bartoszek, Tatiana Mikheeva, Marina Bogush, and Patrick Landis for the sample processing. This study was supported by research funding from Novartis.
Footnotes
Disclosure of conflicts of interest
Carl June, Stephan Grupp, David Teachey, Simon Lacey, J. Joseph Melenhorst, Noelle Frey, Shannon Maude, Fang Chen, Edward Pequignot, and David Porter received research funding from Novartis. Shannon Maude performs consultancy for Novartis. Carl June and David Porter have patents and royalties, and Stephan Grupp has a patent in the field of cell and gene therapies that are managed in accordance with University of Pennsylvania policy and oversight.
Authorship contributions
FC designed and conducted the experiments and prepared the manuscript. DTT reviewed the data and assisted in the writing. EP performed statistical analyses. NF, DP, SLM and SAG conducted the clinical trials of CTL019 and edited the manuscript. CHJ was the sponsor of the trials and advised on experimental design. JJM contributed to experimental plan design and edited the manuscript. SFL designed the experiments, and wrote and edited the manuscript.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jim.2016.03.005.
References
- Butler JE, Spradling JE, Suter M, Dierks SE, Heyermann H, Peterman JH. The immunochemistry of sandwich ELISAs–I. The binding characteristics of immunoglobulins to monoclonal and polyclonal capture antibodies adsorbed on plastic and their detection by symmetrical and asymmetrical antibody-enzyme conjugates. Mol Immunol. 1986;23(9):971–982. doi: 10.1016/0161-5890(86)90128-8. [DOI] [PubMed] [Google Scholar]
- Calabrese LH, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014;10(12):720–727. doi: 10.1038/nrrheum.2014.127. [DOI] [PubMed] [Google Scholar]
- Ellington AA, Kullo IJ, Bailey KR, Klee GG. Antibody-based protein multiplex platforms: technical and operational challenges. Clin Chem. 2010;56(2):186–193. doi: 10.1373/clinchem.2009.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. ACTEMRA® (tocilizumab) http://wwwaccessdatafdagov/drugsatfda_docs/label/2010/125276lblpdf.
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JanssenBiotech. SYLVANT (siltuximab) http://wwwjanssenbiotechcom/sites/default/files/pdf/SYLVANTPrescribingInformationpdf.
- June CH, Maus MV, Plesa G, et al. Engineered T cells for cancer therapy. Cancer Immunol Immunother. 2014;63(9):969–975. doi: 10.1007/s00262-014-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63(8):879–884. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LH Calabrese, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014;10(12):720–727. doi: 10.1038/nrrheum.2014.127. [DOI] [PubMed] [Google Scholar]
- Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014b;20(2):119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014a;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Kasutani K, Okazaki M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5(12):1731–1740. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nishina N, Kikuchi J, Hashizume M, Yoshimoto K, Kameda H, Takeuchi T. Baseline levels of soluble interleukin-6 receptor predict clinical remission in patients with rheumatoid arthritis treated with tocilizumab: implications for molecular targeted therapy. Ann Rheum Dis. 2013;73(5):945–947. doi: 10.1136/annrheumdis-2013-204137. [DOI] [PubMed] [Google Scholar]
- Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamua M. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine. 2005;29(4):169–175. doi: 10.1016/j.cyto.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Porter DL, Hwang WT, Frey V, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;2(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robak T, Gladalska A, Stepien H, Robak E. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediat Inflamm. 1998;7(5):347–353. doi: 10.1080/09629359890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21(6):1248–1257. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- Scheller J, Rose-John S. The interleukin 6 pathway and atherosclerosis. Lancet. 2012;380(9839):338. doi: 10.1016/S0140-6736(12)61246-X. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Hammacher A, Smith DK, Matthews JM, Ward LD. Interleukin-6: structure–function relationships. Protein Sci. 1997;6(5):929–955. doi: 10.1002/pro.5560060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Huffman KM, Kraus WE, Kraus VB. Critical appraisal of four IL-6 immunoassays. PLoS One. 2012;7(2):e30659. doi: 10.1371/journal.pone.0030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243(1–2):243–255. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8) Blood. 1999;94(7):2217–2224. [PubMed] [Google Scholar]
- Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70(1):11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Tang YM. Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Lett. 2014;343(2):172–178. doi: 10.1016/j.canlet.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Zaki MH, Nemeth JA, Trikha M. CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer. 2004;111(4):592–595. doi: 10.1002/ijc.20270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


