Abstract
Objectives
This study sought to test the hypothesis that hyperpolarization-activated cyclic nucleotide–gated (HCN)–based biological pacing might be improved significantly by hyperpolarizing the action potential (AP) threshold via coexpression of the skeletal muscle sodium channel 1 (SkM1).
Background
Gene-based biological pacemakers display effective in vivo pacemaker function. However, approaches used to date have failed to manifest optimal pacemaker properties, defined as basal beating rates of 60 to 90 beats/min, a brisk autonomic response achieving maximal rates of 130 to 160 beats/min, and low to absent electronic backup pacing.
Methods
We implanted adenoviral SkM1, HCN2, or HCN2/SkM1 constructs into left bundle branches (LBB) or left ventricular (LV) epicardium of atrioventricular-blocked dogs.
Results
During stable peak gene expression on days 5 to 7, HCN2/SkM1 LBB-injected dogs showed highly stable in vivo pacemaker activity superior to SkM1 or HCN2 alone and superior to LV-implanted dogs with regard to beating rates (resting approximately 80 beats/min; maximum approximately 130 beats/min), no dependence on electronic backup pacing, and enhanced modulation of pacemaker function during circadian rhythm or epinephrine infusion. In vitro isolated LV of dogs overexpressing SkM1 manifested a significantly more negative AP threshold.
Conclusions
LBB-injected HCN2/SkM1 potentially provides a more clinically suitable biological pacemaker strategy than other reported constructs. This superiority is attributable to the more negative AP threshold and injection into the LBB.
Keywords: gene therapy, HCN channels, heart block, pacemakers, sodium channels
Electronic cardiac pacing provides effective treatment for heart block and/or sinus node dysfunction but has shortcomings, including inadequate autonomic modulation, limited battery life, lead fracture, and an association with potentially deleterious cardiac remodeling (1). In seeking better alternatives, we and others have explored diverse strategies to create biological pacemakers (1). These strategies have used spontaneously active cells (e.g., derivatives of embryonic stem cells or induced pluripotent stem cells) or pacemaker function–related genes delivered via cell platforms or viral vectors.
Recent efforts have focused on improving gene-based biological pacemakers. However, engineered hyperpolarization-activated cyclic nucleotide–gated (HCN) variants have provided improvements that are subtle (e.g., HCN2 E324A) (2) or excessive (e.g., HCN212) (3). Alternative strategies have included overexpressing calcium-stimulated adenylyl cyclase (AC1) (4) or the dominant negative inward rectifier channel Kir2.1AAA (5), and the combination strategies of HCN2/AC1 (4) or HCN2/Kir2.1AAA (5). Although these strategies represent substantial improvements, no strategy has achieved the pre-defined optimal outcomes of: 1) basal beating rates of 60 to 90 beats/min; 2) autonomic responsiveness resulting in rate increases to 130 to 160 beats/min; and 3) low to absent dependence on electronic backup pacing (4,5).
To achieve these optimal parameters, we coexpressed skeletal muscle sodium channel 1 (SkM1) with HCN channel 2 (HCN2). Our rationale was as follows: HCN channels generate inward current that drives the membrane toward threshold for the rapid inward sodium current (INa). To reach the threshold for INa, channel opening must be maximized. We have shown that the SkM1 sodium channel has a more depolarized inactivation versus voltage curve and more rapid recovery kinetics from inactivation than the cardiac sodium channel SCN5A (6,7). Thus, SkM1 is expected to provide greater availability of sodium channels during diastole, leading to a more negative threshold potential, improved pacemaker stability, and increased beating rates.
Methods
Experiments conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23, revised 1996) and were performed using protocols approved by the Columbia University Institutional Animal Care and Use Committee.
Intact canine studies
Adult mongrel dogs were prepared, anesthetized, and fitted with electronic pacemakers (VVI 35 beats/min) and underwent radiofrequency ablation of the atrioventricular node as described previously (2). One series of animals was injected in the left bundle branch (LBB) with the appropriate adenovirus construct to obtain the following groups: HCN2 (n = 12), including 7 previously reported HCN2/green fluorescent protein (GFP)-treated animals (8), 3 current HCN2/GFP-treated animals, and 2 animals injected with HCN2 plus empty vector; SkM1/GFP (designated SkM1; n = 6); and HCN2/SkM1 (n = 6). Outcomes in the HCN2/empty group were comparable to those with HCN2/GFP and were therefore combined into one group designated HCN2.
Left thoracotomies were performed on a second series of animals using previously described methods (6), and adenovirus constructs were injected into 3 left ventricular (LV) anterobasal epicardial sites to obtain the following groups: HCN2/GFP (n = 10; designated HCN2), SkM1/GFP (n = 7; designated SkM1), and HCN2/SkM1 (n = 6). Injections were in close proximity (approximately 4 mm) of one another. The injection site was marked with 2 sutures.
Further experimental details and statistical analysis details are in the Online Appendix.
Results
Intact animal studies
BASELINE FUNCTION
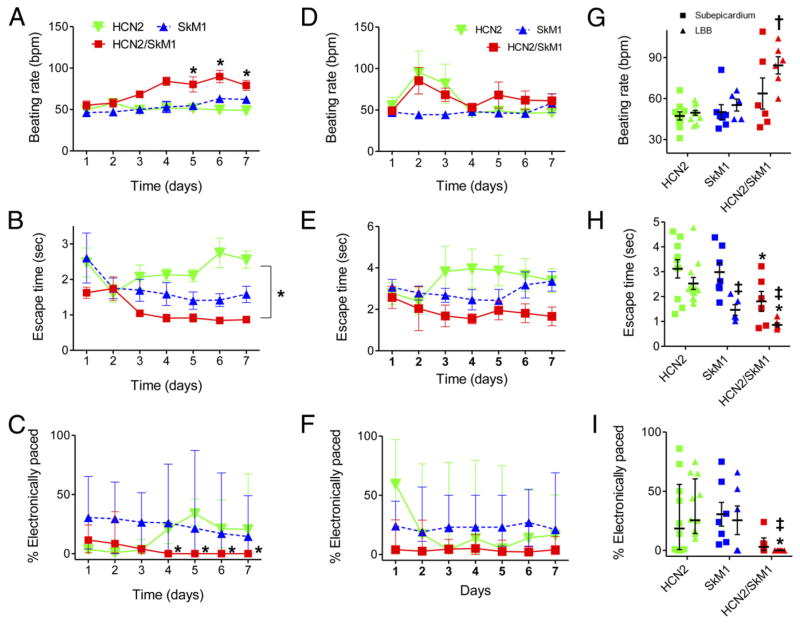
Biological pacing effectiveness was evaluated in light of baseline heart rates, escape times after overdrive pacing, and percentage time during which the backup electronic pacemaker drove the heart (Fig. 1). These parameters were compared in animals injected with biological pacemakers into the LBB or LV subepicardium. Electrocardiograms (ECGs) were recorded while animals rested quietly on a table (baseline beating rates). Over 7 days, biological pacemaker function in HCN2/SkM1 LBB-injected animals was superior (i.e., faster basal rates, shorter escape times, and lower percentage of electronically stimulated beats) to that of animals with HCN2 or SkM1 alone and was superior to that of animals with LV subepicardial injection of HCN2/SkM1. Typical baseline ECGs and escape times of LBB-injected animals are shown in Online Figure 1 and immunochemical staining of LBB revealing presence of HCN2 and SkM1 is shown in Online Figure 2.
Figure 1. HCN2/SkM1-Based Biological Pacemakers Exhibit Improved Function Over HCN2 and SkM1, With LBB Injection Providing Superior Outcomes to Left Ventricular Myocardial Injection.
(A to C) Summary data for left bundle block (LBB)–injected animals receiving hyperpolarization-activated cyclic nucleotide–gated channel 2 (HCN2; n = 12), skeletal muscle sodium channel 1 (SkM1; n = 5 in A and B, n = 6 in C), or HCN2/SkM1 (n = 6). (A) Baseline beating rates on days 3 to 7 were faster in HCN2/SkM1-injected animals than in animals injected with only HCN2 or SkM1 (*). (B) Mean escape times on days 4 to 7 were shorter in HCN2/SkM1-injected animals than HCN2-injected animals (*). (C) Median percentage of electronically stimulated beats calculated over 24-h periods were significantly lower in HCN2/SkM1-injected animals than HCN2-injected animals. On days 4 to 7, electronic backup pacing was eliminated in HCN2/SkM1-injected animals. (D to F) Summary data for subepicardially injected animals receiving HCN2 (n = 10), SkM1 (n = 7), or HCN2/SkM1 (n = 6). (D) Mean baseline beating rates. (E) Mean escape times. (F) Median percentage of electronically stimulated beats. (G to I) Summary data pooled for days 5 to 7. (G) Baseline beating rates in LBB-injected animals receiving HCN2/SkM1 were faster than in LBB-injected animals receiving either HCN2 or SkM1 (†). (H) Escape times in LBB-injected animals receiving SkM1 or HCN2/SkM1 were significantly shorter compared with those of the respective subepicardial injections (‡). Escape times of HCN2/SkM1-injected animals were significantly shorter than those of the respective HCN2 injections (*). (I) Median percentage of electronically stimulated beats was reduced to 0% in LBB-injected animals receiving HCN2/SkM1 and was significantly lower than in LBB-injected animals receiving HCN2 (*) or in subepicardially injected animals receiving HCN2/SkM1 (‡). Note that in panels C, F and I, error bars are presented as interquartile range. *†‡p < 0.05.
AUTONOMIC MODULATION
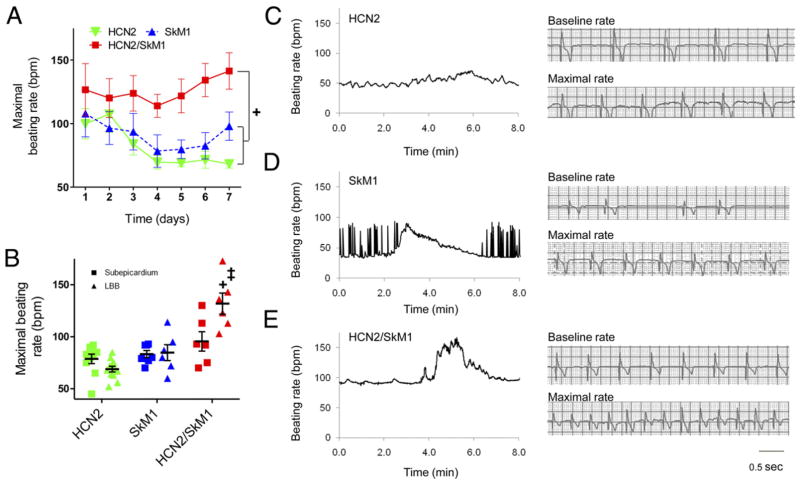
Sensitivity to autonomic modulation of pace-mapped rhythms was studied via 24-h ECG recordings. Faster beating rates were reached in HCN2/SkM1 LBB-injected animals than those injected with HCN2 or SkM1 (Fig. 2A). At 5 to 7 days, beating rates were significantly faster in animals that received HCN2/SkM1 into the LBB as compared with subepicardial injection (Fig. 2B). Typical recordings of maximal beating rates in LBB-injected animals are in Figures 2C, 2D, and 2E.
Figure 2. HCN2/SkM1-Based Biological Pacemakers Injected Into the LBB Have Faster Maximal Beating Rates Than Those Based on HCN2 or SkM1.
(A) Maximal pace-mapped beating rates in LBB-injected animals. Maximal beating rates were faster in HCN2/SkM1 than HCN2 and SkM1 groups (+). (B) Summary data pooled for days 5 to 7. Maximal pace-mapped beating rates in LBB-injected animals were significantly faster in HCN2/SkM1 versus HCN2 or SkM1 (+). HCN2/SkM1. LBB-injected animals also had significantly faster maximal beating rates than respective subepicardially injected animals (‡; subepicardially injected animals: HCN2 n = 10, SkM1 n = 7, HCN2/SkM1 n = 6; LBB-injected animals: HCN2 n = 12, SkM1 n = 6, HCN2/SkM1 n = 6). (C to E) Left panels show beating rates for every beat during 8 min surrounding an episode of maximal pace-mapped beating rate recorded in LBB-injected animals. Right panels provide tracings of baseline and maximal beating rates of the recordings shown on the left. (C) Gradual warm up and cool down in an HCN2-injected animal. (D) Baseline bigeminy and stable maximal beating rate in an SkM1-injected animal. (E) Stable baseline beating and robust rate acceleration followed by cool down in an animal injected with HCN2/SkM1. Abbreviations as in Figure 1. +‡p < 0.05.
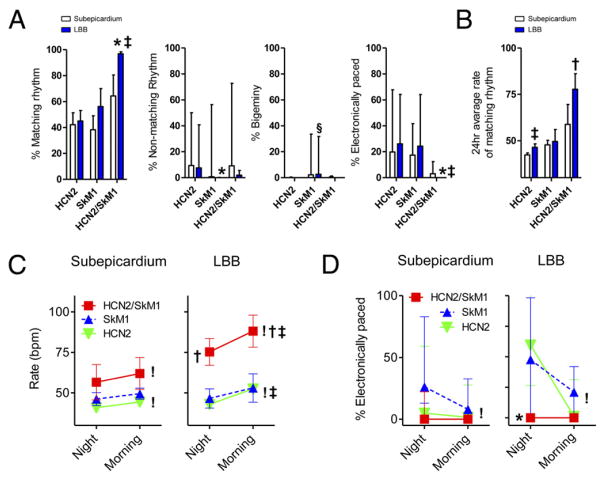
A detailed analysis of percentage pace-mapped rhythms and their autonomic modulation was performed on the ECG Holter recordings at 5 to 7 days. The percentage of matching pace-mapped beats was significantly higher in HCN2/SkM1 LBB-injected animals (>95% of all beats), requiring less pacemaker backup than the respective HCN2-and SkM1-injected groups (p < 0.05) (Fig. 3A). The percentage of matching beats in animals that received HCN2/SkM1 into subepicardium was lower (approximately 60%) and did not differ from that of HCN2 and SkM1 control groups. Animals injected with SkM1 alone either into the subepicardium or LBB showed persistent bigeminy or trigeminy in more than 10% of beats, whereas no such arrhythmias were detected in animals injected with HCN2 or HCN2/SkM1 (p < 0.05) (Fig. 3A). The percentage of electronically paced beats was reduced in the HCN2/SkM1-LBB group to 0% of all beats (p < 0.05 vs. respective HCN2 and SkM1 groups) (Fig. 3A). The 24-h average rate of pace-mapped rhythms is summarized in Figure 3B, showing a faster rate in HCN2/SkM1-LBB versus the HCN2-LBB and SkM1-LBB groups (p < 0.05). These results are consistent with the 5- to 7-day averages of baseline and maximal beating rates reported in Figure 2. Finally, animals that received HCN2 into the LBB exhibited faster 24-h average beating rates than animals that received HCN2 into the subepicardium (p < 0.05) (Fig. 3B).
Figure 3. Detailed Analysis of Rhythms and Their Circadian Modulation.
(A) Summary data on percentage of pace-mapped beats (percentage matching rhythm), nonmatching beats, bigeminal beats, and electronically paced beats in all animals. Animals that showed <5% of matching beats or persistent bigeminy were excluded from subsequent analysis (B to D; subepicardially injected animals: HCN2 n = 9, SkM1 n = 6, HCN2/SkM1 n = 6; LBB-injected animals: HCN2 n = 11, SkM1 n = 4, HCN2/SkM1 n = 6). (B) 24-h average rate of matching beats. (C) Summary data on morning/night modulation of pace-mapped beating rates. (D) Dependence on electronic backup pacing during morning/night. Note that percentage nonmatching, percentage bigeminy, and percentage paced are presented as median and interquartile range. Abbreviations as in Figure 1. *p < 0.05 versus respective HCN2. †p < 0.05 versus respective HCN2 and SkM1. ‡p < 0.05 versus respective myocardium. §p < 0.05 versus respective HCN2 and HCN2/SkM1. !p < 0.05 for morning versus night.
To test whether the changes in beating rate and dependence on backup electronic pacing were consistent with what would be expected based on a normal circadian rhythm, we compared these parameters during 2 h of sleep (2:00 to 4:00 AM) with 2 h of feeding and activity (8:00 to 10:00 AM). Regardless of injection site, HCN2 and HCN2/SkM1 groups exhibited a significant rate acceleration of pace-mapped rhythms from morning to night (p < 0.05) (Fig. 3C). During sleep as well as during feeding and activity, pace-mapped rhythms were significantly faster in HCN2/SkM1 LBB-injected animals as compared with those in HCN2-LBB and SkM1-LBB groups (p < 0.05) (Fig. 3C). Furthermore, both HCN2-LBB and HCN2/SkM1-LBB groups exhibited faster beating rates in the morning than the respective subepicardially injected groups (p < 0.05) (Fig. 3C). The percentage of electronically paced beats during night and morning is summarized in Figure 3D. Subepicardially or LBB-injected animals that received HCN2 exhibited a lower percentage of electronic pacing in the morning than at night (p < 0.05) (Fig. 3D).
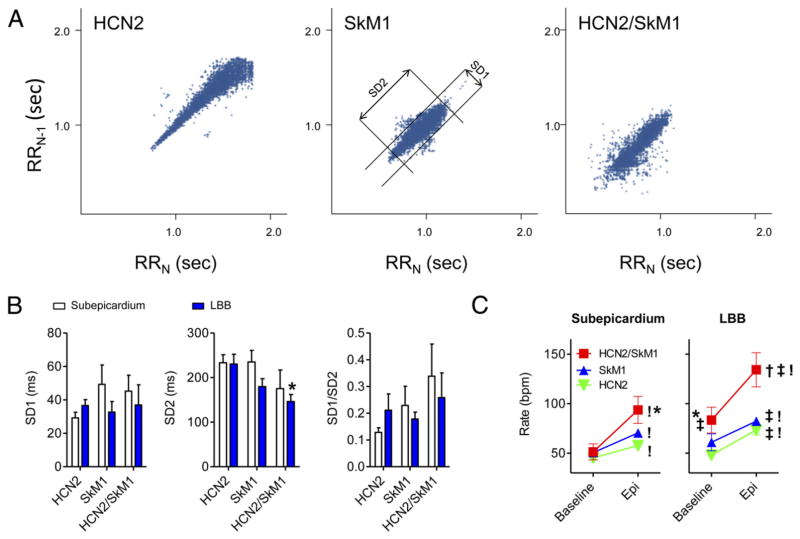
Poincaré plots of pace-mapped rhythms also demonstrated differences in autonomic modulation as analyzed by heart rate variability (HRV) among animals with the 3 gene constructs injected into the LBB (Fig. 4A). Quantitative analysis of SD parameters revealed that the level of parasympathetic modulation expressed by short-term variation of heart rates (SD1) was comparable among the 3 groups tested (Fig. 5B, left panel). Sympathetic modulation, expressed as long-term variation of heart rates (SD2), was significantly reduced (i.e., normalized) in the HCN2/SkM1-LBB group as compared with that of animals LBB-injected with HCN2 (p < 0.05) (Fig. 4B, middle panel). The parasympathetic-sympathetic balance (SD1:SD2 ratio) did not differ among the 3 groups (p > 0.05) (Fig. 5B, right panel). Among the subepicardially injected groups, no significant changes in SD1, SD2, and SD1/SD2 were found.
Figure 4. Detailed Analysis of Heart Rate Variability and Response to Epinephrine Infusion.
(A) Representative Poincaré plots of pace-mapped beats in 24-h Holter recordings of HCN2, SkM1, and HCN2/SkM1 LBB-injected animals. The middle panel (SkM1-injected animal) also defines SD of instantaneous RR-interval variability (SD1) and SD of long-term continuous RR-interval variability (SD2). (B) Summary data of SD1, SD2, and SD1:SD2. Animals that showed <5% of matching beats or persistent bigeminy were excluded from this analysis. Subepicardially injected animals: HCN2 n = 9, SkM1 n = 6, HCN2/SkM1 n = 6; LBB-injected animals: HCN2 n = 11, SkM1 n = 4, HCN2/SkM1 n = 6. (C) Summary data on the beating rates at baseline and during epinephrine infusion. Subepicardially injected animals: HCN2 n = 10, SkM1 n = 6, HCN2/SkM1 n = 6; LBB-injected animals: HCN2 n = 12, SkM1 n = 4, HCN2/SkM1 n = 6. Abbreviations as in Figure 1. *p < 0.05 versus respective HCN2. !p < 0.05 for baseline versus epinephrine. †p < 0.05 versus respective HCN2 and SkM1. ‡p < 0.05 versus respective subepicardium.
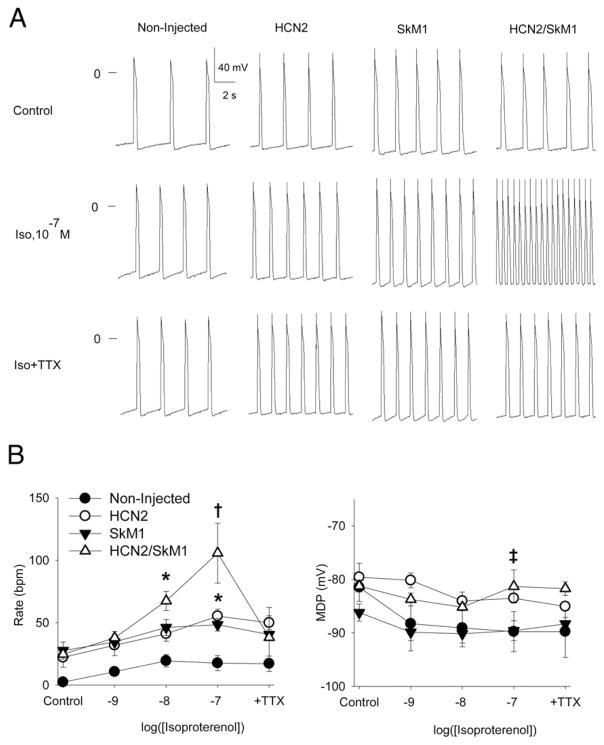
Figure 5. Tissue Bath Experiments Confirm Critical Role for SkM1 in Determining Beating Rates of HCN2/SkM1-Injected Preparations.
(A) Representative microelectrode traces of LBB preparations from HCN2 (n = 5), SkM1 (n = 6), and HCN2/SkM1 (n = 6) injected animals and noninjected zones (n = 6) under control conditions, after graded application of isoproterenol and after isoproterenol (Iso) 0.1 μM plus tetrodotoxin (TTX) 0.1 μM. (B) Summary data for beating rates (left panel) and maximum diastolic potential (right panel). Abbreviations as in Figure 1. *p < 0.05 versus noninjected. †p < 0.05 versus HCN2, SkM1, and noninjected. ‡p < 0.05 for the combined groups with HCN2 (HCN2 and HCN2/SkM1) versus the combined groups without HCN2 (SkM1 and noninjected).
On the final study day, all animals showed a significant rate acceleration upon intravenous epinephrine administration (1.0 μg/kg/min; p < 0.05) (Fig. 4C). Furthermore, during epinephrine infusion, animals subepicardially injected with HCN2/SkM1 exhibited faster beating rates than the respective HCN2 group (p < 0.05). Similarly, during baseline and during epinephrine infusion, HCN2/SkM1 LBB-injected animals showed significantly faster beating rates than their respective HCN2 or SkM1 groups (p < 0.05). Finally, in HCN2/SkM1 LBB-injected animals, beating rates in baseline and epinephrine groups were significantly faster than in subepicardially injected animals (p < 0.05).
Isolated tissue studies
Figures 5A and 5B provide representative examples and summary data from isolated tissue experiments conducted on LBB from HCN2-, SkM1- and HCN2/SkM1-injected animals. In normal Tyrode solution, beating rates did not differ among groups. However, when isoproterenol was added, HCN2/SkM1-treated preparations beat faster than the others (p < 0.05). With isoproterenol 0.1 μM superfusion maintained, we added tetrodotoxin 0.1 μM, which selectively blocks SkM1 current (7). Tetrodotoxin significantly slowed the HCN2/SkM1-injected preparations, bringing their beating rates into the same range as the HCN2-injected preparations (Fig. 5B). This is consistent with a major contribution of SkM1 to the beating rates in the presence of isoproterenol. During superfusion with isoproterenol 0.1 μM, maximum diastolic potential was significantly more depolarized in HCN2-overexpressing tissue than in tissue that did not overexpress HCN2 (p < 0.05) (Fig. 5B).
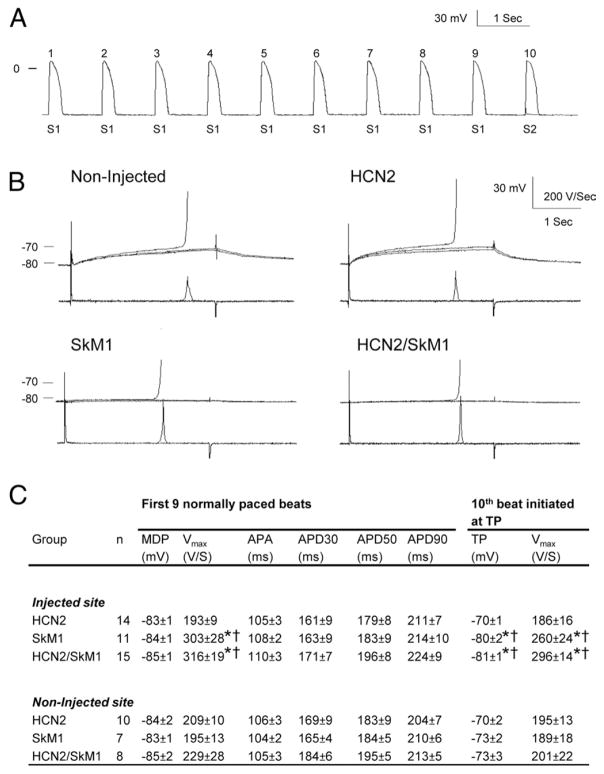
To test whether threshold potential shifts negatively in the presence of SkM1, we conducted experiments on dogs in which viral constructs were injected into myocardium. Figures 6A, 6B, and 6C provide typical tracings and summary data. Data acquired from the first 9 action potentials (APs) per cycle that were stimulated normally confirmed the functional presence of SkM1 in the SkM1 and HCN2/SkM1 groups (Fig. 6C). Specifically, as in previous reports (6,8), SkM1 overexpression induced an increase in maximal upstroke velocity in the SkM1 and HCN2/SkM1 groups compared with those in the respective noninjected controls and the HCN2-injected group (p < 0.05). The 10th AP was generated with a current pulse that was varied to identify the threshold potential for AP initiation. Threshold was reached at more negative voltages in SkM1- and HCN2/SkM1-injected preparations than in noninjected and HCN2-injected controls (p < 0.05).
Figure 6. SkM1 Overexpression Shifts TP Negatively.
Action potential (AP) parameters and threshold potential (TP) were registered from left ventricular subepicardial preparations isolated from HCN2-, SkM1-, or HCN2/SkM1-injected and noninjected regions. Preparations were paced at a cycle length of 1,000 ms with 2-ms current pulses at double threshold amplitude (S1). A 30-ms test current pulse (S2) of variable amplitude was substituted for every 10th regular pulse. (A) Typical train of 9 APs initiated with 2-ms 2× threshold S1 current pulses followed by a 10th AP initiated by a 30-ms suprathreshold current pulse (S2). (B) Fast-sweep recordings of typical tracings of 30-ms subthreshold and suprathreshold current pulses in noninjected, HCN2-, SkM1-, and HCN2/SkM1-injected preparations. (C) Summary data on AP parameters and TP measurements. APA = action potential amplitude; APD30, APD50, APD90 = AP duration to 30%, 50%, and 90% repolarization, respectively; MDP = maximum diastolic potential; Vmax = maximum upstroke velocity; other abbreviations as in Figure 1. TP was measured for just above threshold current amplitude. *p < 0.05 versus respective HCN2. †p < 0.05 versus respective noninjected.
Discussion
Injecting the pacemaker gene HCN2 together with SkM1 into the LBB has provided a construct that compares favorably with other biological pacemaker strategies reported to date. With the HCN2/SkM1 biological pacemaker, rhythms were generated in more than 95% of the beats, in a canine model with a cardiac rhythm status comparable to that of patients requiring ventricular demand pacing. Baseline beating rates were well within a target range of 60 to 90 beats/min and demonstrated brisk autonomic responsiveness as evidenced by the significant response to the epinephrine infusion and the high level of 24-h HRV. The next step in developing HCN2/SkM1-based biological pacemakers will be to move to a delivery system that generates long-term function. Such a system may be provided by lentiviral vectors or by HCN2/SkM1-overexpressing human mesenchymal stem cells (hMSCs).
SkM1/HCN2 pacemaker function in relation to other approaches
Outcomes for baseline and maximal rates of LBB-implanted HCN2/SkM1-based biological pacemakers compared favorably with results reported for AC1 and various HCN isoforms, mutants, and gene combinations. Rates with AC1 (4), wild-type HCN2, and genetically engineered HCN2-E324A, were consistently slower than with HCN2/SkM1 (2). Although the HCN2/Kir2.1AAA strategy generated robust pacemaker activity at relatively rapid baseline beating rates (90 to 95 beats/min), dependence on electronic backup pacing was not eliminated (5). Further concerns of this strategy include the prolongation of repolarization induced by Kir2.1AAA (9) and the unknown degree of autonomic modulation. Although the AC1 strategy shows promise with respect to the high efficiency of pacemaker function (>95% of the beats originated from the injection site), physiological beating rates (approximately 60 beats/min), and high sensitivity to parasympathetic modulation, it also manifested relatively slow maximal beating rates and did not eliminate electronic backup pacing (4). Moreover, the AC1 strategy elevated cAMP levels and impacts on calcium handling in cells (10–12), presenting the potential for unwanted side effects such as triggered activity and calcium overload. Although we did not see such side-effects (4), they remain concerns. In contrast, the HCN2/SkM1 gene combination induced baseline and maximal beating rates with ranges that we had targeted as optimal for a biological pacemaker. We had previously shown as well that the calcium overload one might fear with a sodium channel construct was not an issue here (6). Finally, favorable pacemaker function as manifested by short escape times and low to absent dependence on electronic backup pacing was also characteristic of HCN2/SkM1 LBB-injected animals.
Autonomic modulation of biological pacemaker function
Autonomic modulation of pacing rates is a potential key advantage of biological over electronic pacing (8). The extent of autonomic modulation that may be obtained via a biological approach likely depends on the gene construct or the cells used. To facilitate the comparison among the various biological pacemaker strategies, we analyzed several measures of autonomic modulation. First, the average baseline beating rate in the HCN2/SkM1-LBB group was relatively rapid (approximately 80 beats/min) (Fig. 1), and the animals maintained robust rate acceleration, reaching average maximal rates of approximately 130 beats/min (Fig. 2). Furthermore, maximal beating rates always remained within the physiological range, never exceeding 180 beats/min. This outcome is superior to the slower maximal beating rates reported here for HCN2 or SkM1 (Fig. 2) and elsewhere for AC1 (4) in LBB-injected animals, superior to results with injection of HCN2/SkM1 into subepicardium (Fig. 2), and superior to the very rapid maximal rates reported for animals in which the chimera HCN212 and the combination of HCN2/AC1 were both injected into LBB (3,4).
Second, we investigated the average beating rates comparing a period of rest (2:00 to 4:00 AM) with one of physical activity and feeding (8:00 to 10:00 AM). We found beating rates in accordance with those expected with a normal response to circadian modulation (Figs. 3C and 3D). We also found the circadian response in the HCN2/SkM1-LBB group to be superior to that in animals with LBB gene transfer of HCN2 or SkM1 and myocardial gene transfer of HCN2/SkM1.
Finally, we investigated sensitivity to parasympathetic and sympathetic modulation via analysis of HRV and infusion of epinephrine. The significant reduction in SD2 in the comparison of HCN2/SkM1-LBB with HCN2-LBB (Fig. 4B, middle panel) might suggest reduced sensitivity to sympathetic modulation in the former. However, this is unlikely given the strong in vitro (Fig. 5) and in vivo (Fig. 4C) responses to isoproterenol and epinephrine, respectively, which indicated more profound sensitivity to sympathetic stimuli in HCN2/SkM1-LBB than HCN2-LBB preparations. It should be noted that in the HCN2-LBB group, accelerations (likely induced by sympathetic stimuli) and decelerations (likely resulting from reduced biological pacemaker function) were frequently observed at rest, when beating rates in the HCN2/SkM1 group were relatively stable. Therefore, it appears likely that sympathetic stimulation during rest in the HCN2/SkM1-LBB group was below the level of that in the HCN2-LBB group, although the 24-h average beating rates in the HCN2/SkM1-LBB group were higher (Fig. 3B). These data indicated that LBB-injected animals that received HCN2 likely manifested increased sympathetic activity during rest as a result of their slower beating rates. The lower average values found for SD2 in the HCN2/SkM1-LBB group therefore indicated reduced activity of the sympathetic system during rest rather than reduced sensitivity to sympathetic modulation (13).
Mechanisms underlying pacemaker function based on HCN2/SkM1 gene transfer
The microelectrode experiments on myocardial bundles obtained from subepicardially injected animals demonstrated the effect of SkM1 to move the threshold potential to more negative voltages (Fig. 6). This observation is significant because shifting the threshold in this direction would result in AP initiation earlier during phase 4 depolarization of automatic fibers. Although this change in AP threshold likely is a major mechanism by which SkM1 improves pacemaker function, other mechanisms should be considered. For example, SkM1 may help to reduce current-to-load mismatch that is potentially present at the interface between transduced and adjacent nontransduced myocardium. The cardiac sodium channel Nav1.5 is similarly thought to contribute to pacemaker impulse propagation in the sinoatrial node periphery (14,15).
Based on our original hypothesis that SkM1 would improve HCN2-based pacemaker function, we expected induction of some degree of pacemaker function originating from the injection site of SkM1 adenovirus. Yet, we also recorded persistent bigeminal rhythms originating from the injection site (Fig. 3A). The timing of these extra beats at coupling intervals of 300 to 700 ms and their dependence on slow baseline heart rates (Fig. 3D) is consistent with either early afterdepolarizations (16) or re-entry (17). That SkM1-associated bigeminy is not attributable to an SkM1 action on repolarization was shown in our earlier studies (6,7). Moreover, with the SkM1/HCN2 combination, we saw no bigeminy or other instances of proarrhythmia.
Clinical applicability
We consider clinical applicability with the caveat that the standard for the field is electronic pacing, with its considerable strengths and shortcomings that have been described in detail (1). Biological pacing is being explored by us and by others as a possible adjunct to/replacement for electronic pacing. However, a great deal remains to be done before clinical testing is in order. Given that framework, what can be said about the approach described here? Gene transfer of HCN2/SkM1 generated robust pacemaker function at beating rates close to physiologically desirable levels. The range of function obtained in the short-term setting of the present study compares favorably to that seen with demand electronic pacing of the ventricle. However, for clinical implementation, the level of function that can be generated stably over much longer terms will be crucial to the success of such an approach. To this end, one logical next step is the use of the HCN2 and SkM1 genes in combination with a long-term viral expression vector such as the lentiviral vector (18). In contrast, adeno-associated viral vectors cannot support genes the size of SkM1 without further modifications (19).
In addition, we previously reported the use of MSCs for the delivery of HCN2 current to myocardium and fabrication of a cell-based biological pacemaker that functioned stably over 6 weeks (20). In a different study, we also showed that the SkM1 current can be efficiently delivered to myocardium via the MSC platform (21). Hence, the MSC platform offers an alternative means of gene delivery. However, MSCs show a tendency to migrate from the injection site, causing a loss of pacemaker function over time. For this reason, ongoing efforts are focused on the encapsulation of MSCs, which, if successful, would generate an attractive delivery vehicle for HCN2 and SkM1 ion channels.
Conclusions
When HCN2/SkM1 was administered to the LBB, pacemaker function was facilitated by the slow depolarizing HCN2 current and the hyperpolarized AP threshold generated by SkM1. This dual gene therapy provided both highly efficient pacing and a brisk autonomic response to degrees that appear superior to those of previously developed gene- or cell-based approaches.
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service National Heart, Lung, and Blood Institute grants HL-094410 and HL-12738 and by the American Heart Association. Gerard Boink received grant support from the Netherlands Foundation for Cardiovascular Excellence, the Netherlands Heart Foundation, the Dr. Saal van Zwanenberg Foundation, and the Interuniversity Cardiology Institute of the Netherlands. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- AC1
adenylyl cyclase 1
- AP
action potential
- HCN2
hyperpolarization-activated cyclic nucleotide–gated channel 2
- hMSC
human mesenchymal stem cell
- HRV
heart rate variability
- Kir2.1AAA
dominant negative inward rectifier channel
- LBB
left bundle branch
- LV
left ventricle/ventricular
- SkM1
skeletal muscle sodium channel 1
Footnotes
For an expanded Methods section, and supplemental figures and references, please see the online version of this article.
References
- 1.Rosen MR, Robinson RB, Brink PR, Cohen IS. The road to biological pacing. Nat Rev Cardiol. 2011;8:656–66. doi: 10.1038/nrcardio.2011.120. [DOI] [PubMed] [Google Scholar]
- 2.Bucchi A, Plotnikov AN, Shlapakova I, et al. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation. 2006;114:992–9. doi: 10.1161/CIRCULATIONAHA.106.617613. [DOI] [PubMed] [Google Scholar]
- 3.Plotnikov AN, Bucchi A, Shlapakova I, et al. HCN212-channel biological pacemakers manifesting ventricular tachyarrhythmias are responsive to treatment with If blockade. Heart Rhythm. 2008;5:282–8. doi: 10.1016/j.hrthm.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boink GJJ, Nearing BD, Shlapakova IN, et al. Ca2+-stimulated adenylyl cyclase AC1 generates efficient biological pacing as single gene therapy and in combination with HCN2. Circulation. 2012;126:528–36. doi: 10.1161/CIRCULATIONAHA.111.083584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cingolani E, Yee K, Shehata M, Chugh SS, Marbán E, Cho HC. Biological pacemaker created by percutaneous gene delivery via venous catheters in a porcine model of complete heart block. Heart Rhythm. 2012;9:1310–8. doi: 10.1016/j.hrthm.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Lau DH, Clausen C, Sosunov EA, et al. Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia: an in silico, in vivo, in vitro study. Circulation. 2009;119:19–27. doi: 10.1161/CIRCULATIONAHA.108.809301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Protas L, Dun W, Jia Z, et al. Expression of skeletal but not cardiac Na+ channel isoform preserves normal conduction in a depolarized cardiac syncytium. Cardiovasc Res. 2009;81:528–35. doi: 10.1093/cvr/cvn290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlapakova IN, Nearing BD, Lau DH, et al. Biological pacemakers in canines exhibit positive chronotropic response to emotional arousal. Heart Rhythm. 2010;7:1835–40. doi: 10.1016/j.hrthm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Miake J, Marbán E, Nuss HB. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J Clin Invest. 2003;111:1529–36. doi: 10.1172/JCI17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sinoatrial node cells and modulates the If pacemaker current. J Physiol. 2007;582:1195–203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younes A, Lyashkov AE, Graham D, et al. Ca2+-stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem. 2008;283:14461–8. doi: 10.1074/jbc.M707540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagan KA, Mahey R, Cooper DM. Functional co-localization of transfected Ca2+-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J Biol Chem. 1996;271:12438–44. doi: 10.1074/jbc.271.21.12438. [DOI] [PubMed] [Google Scholar]
- 13.Huikuri HV, Seppanen T, Koistinen MJ, et al. Abnormalities in beat-to-beat dynamics of heart rate before the spontaneous onset of life-threatening ventricular tachyarrhythmias in patients with prior myocardial infarction. Circulation. 1996;93:1836–44. doi: 10.1161/01.cir.93.10.1836. [DOI] [PubMed] [Google Scholar]
- 14.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–32. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 15.Verkerk AO, Wilders R, van Borren MM, Tan HL. Is sodium current present in human sinoatrial node cells? Int J Biol Sci. 2009;5:201–4. doi: 10.7150/ijbs.5.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J. 1995;68:949–64. doi: 10.1016/S0006-3495(95)80271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudy Y. Reentry: insights from theoretical simulations in a fixed pathway. J Cardiovasc Electrophysiol. 1995;6:294–312. doi: 10.1111/j.1540-8167.1995.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 18.Niwano K, Arai M, Koitabashi N, et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16:1026–32. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Sun W, Wang B, Xiao X, Liu XQ. Protein trans-splicing as a means for viral vector-mediated in vivo gene therapy. Hum Gene Ther. 2008;19:958–64. doi: 10.1089/hum.2008.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plotnikov AN, Shlapakova I, Szabolcs MJ, et al. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–13. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 21.Boink GJJ, Lu J, Driessen HE, et al. Effect of SkM1 sodium channels delivered via a cell platform on cardiac conduction and arrhythmia induction. Circ Arrhythm Electrophysiol. 2012;5:831–40. doi: 10.1161/CIRCEP.111.969907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.