Abstract
Background and Purpose:
Pistacia atlantica, which belongs to the Anacardiaceae family, grows in the Zagrossian region of Iran. The aim of this study was to evaluate the antifungal properties of Pistacia atlantica and olive leaf extracts against different fungal species.
Materials and Methods:
In this study, we assessed the activities of olive leaf extracts and Pistacia atlantica leaf and fruit extracts against Candida species, including C. albicans, C. glabrata, C. tropicalis, and C. krusei. In addition, antifungal activities against three filamentous species, i.e., Aspergillus niger, Aspergillus flavus, and Aspergillus fumigates, were assessed, using the agar-well diffusion method.
Results:
The minimal inhibitory concentrations (MICs) values of fruit and leaf extracts from Pistacia atlantica ranged 6.25-12.5 mg ml-1 and 6.25-25 mg ml-1 against the tested Candida and Aspergillus species, respectively. The olive leaf extracts showed no activity against Candida species or Aspergillus flavus, while they exhibited antifungal potency against Aspergillus niger and Aspergillus fumigatus (MIC: 12.5-25 mg/ml). The MICs of the mixture of selected extracts ranged from 6.25 to 25 mg/ml.
Conclusion:
Based on the results, the ethanolic extracts of the selected plants exhibited antifungal potency against the tested fungi and could be used as natural antifungal agents.
Key Words: Antifungal, Fungi, Olive, Pistacia
Introduction
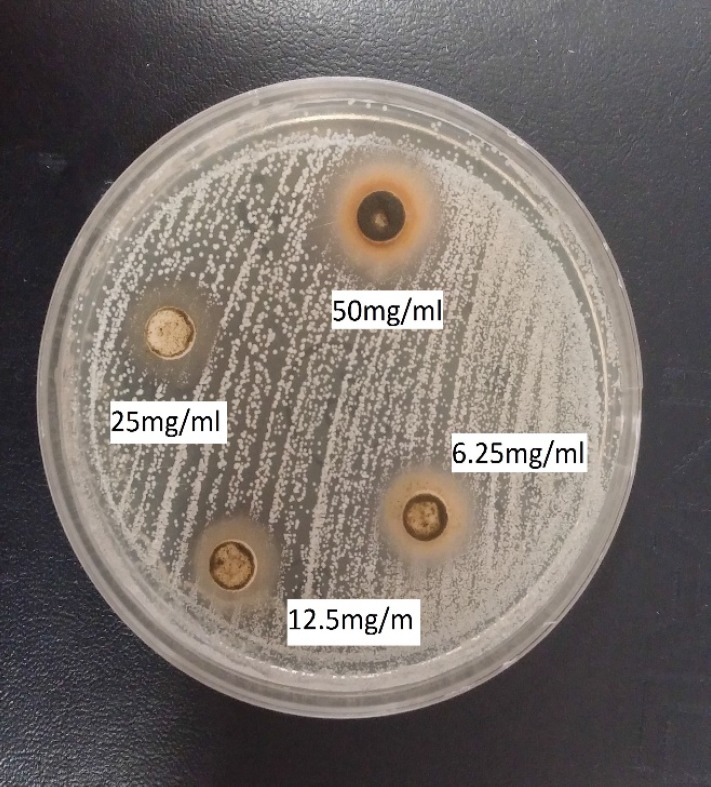
Pistacia atlantica or Bene from the Anacardiaceae family is a native fruit in Iran. This plant is the most economically important tree species, which grows in the Zagrossian region of Western Iran and is used in traditional medicine (Figure 1). The oil extracted from Pistacia atlantica is known as Bene hull oil [1].
Figure 1.
Anti-Candida activity assay of the ethanolic fruit extract of Pistacia atlantica against C. albicans using agar-well diffusion method (dilutions ranging from from 6.25 -50 mg ml-1; MIC = 6.25 mg ml-1
Among 11 Pistacia species, only three including Pistacia lentiscus var. Chia, Pistacia vera, and Pistacia atlantica grow in Iran. These plants are of high economical and pharma-ceutical importance and are regarded as the most important Pistacia species [2]. Some Pistacia species have been used in traditional medicine as tonic, aphrodisiac, antiseptic, and antihypertensive agents; moreover, these species have been applied for the treatment of dental, gastrointestinal, hepatic, urinary tract, and respiratory tract disorders.
Based on scientific evidence, various parts of Pistacia species exhibit significant pharmacolo-gical activities, i.e., antioxidant, antimicrobial, antiviral, anti-tumor, anticholinesterase, anti-inflammatory, antihyperlipidemic, antinocicep-tive, antidiabetic, antiatherosclerotic, and hepato-protective activities [3]. According to a recent literature review on studies focusing on the effects of P. atlantica species on bacteria, the extracts were shown to inhibit E. coli growth (MIC: 163 μg/ml). However, the hydroalcoholic extracts of Pistacia atlantica did not exert any effects on H. pylori. The findings indicated the inhibitory effects of hydroalcoholic extracts on the tested bacteria, with the exception of H. pylori. On the other hand, phenol/chloroform extraction could have inhibitory effects on H. pylori [4].
The fruit, oil, and leaf extracts of Olea europaea L. (commonly known as olive) are regarded as important sources of nutrition, applied in traditional and modern medicine. Olive compounds are an essential part of Mediterranean diets, with polyphenols constituting a major part of these compounds [5].
Investigation of polyphenols in olive leaves is of great significance, owing to their positive effects on health, induced by their antihyper-tensive, antidiabetic, anticarcinogenic, anti-athero-sclerotic, anti-inflammatory, and antimicrobial activities [6]. Therefore, the main objective of the present study was to investigate the antifungal activity of extracts from P. atlantica and olive leaf against Candida species and filamentous fungi, such as Aspergillus niger, Aspergillus flavus, and Aspergillus fumigatus.
Material and Methods
In this study, P. atlantica leaves and fruits were collected from the Zagros Mountains, while olive leaves were gathered from North of Iran. The leaves and fruits of the plants were dried in shade and crushed to fine powder.
Preparation of the extracts
The crude ethanolic extracts were dried at room temperature for alcohol evaporation. The dried extracts were stored in sterile bottles at -20°C for further analysis.
Microorganisms and inoculum preparation
The fungal isolates assessed in this study included C. albicans, C. tropicalis, C. glabrata, and C. krusei from the oral cavity, Aspergillus niger and Aspergillus flavus isolated from environmental samples, and Aspergillus fumigatus from the clinical fungus ball sample. All the selected microorganisms were obtained from the Department of Medical Mycology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
The stock fungi were subcultured on Sabouraud dextrose agar (SDA, Merck, Germany) and incubated overnight at 37°C. A few subcultured colonies were diluted in sterile normal saline to obtain 106 colony forming units (CFU/ml) with a turbidity of 0.5 McFarland [7].
Screening for antimicrobial properties
Aliquots (1000 mg) of dried plant extracts were dissolved in 2 ml of 100% dimethyl sulfoxide (DMSO; Sigma-Aldrich, USA), and the final concentration of each plant extract was adjusted to 500 mg/ml. The serial two-fold dilutions of plant extracts were prepared at a concentration range of 3.12-50 mg/ml.
The minimum inhibitory concentrations (MICs) of ethanolic extracts were screened, using the agar-well diffusion method. The aliquot (0.1 ml) of the prepared inoculum (106 CFU/ml) from the tested organism was cultured on SDA medium in Petri dishes and was uniformly dispersed, using a sterile spreader.
Afterwards, the wells (7 mm in diameter) were punched onto the SDA medium, using a sterile borer. Each well was filled with 0.1 ml of serial dilutions of the tested plant extracts [7]. Sterile DMSO was used as the negative control, while amphotericin B (antibiotic discs containing 30 meg.) and clotrimazole (10 μg per disc) were used as the positive controls. The plates were incubated at 37°C for 24 h. Moreover, the inhibition zones around the wells were measured in millimeter.
Results
Table 1 summarizes the antifungal properties of olive leaves and P. atlantica leaf and the fruit and leaf extracts by agar-well diffusion method against the selected fungal species. The olive leaf extracts were ineffective against Candida spp. and Aspergillus flavus, whereas they showed positive inhibitory effects on Aspergillus niger and Aspergillus fumigatus (MIC range: 12.5-25 mg/ml).
Table 1.
Minimum inhibitory concentrations (MICs) (mg/ml)a of the ethanolic extracts of Pistacia atlantica and olive leaf for Candida and Aspergillus species
| Extracts |
C.
albicans |
C.
tropicalis |
C.
glabrata |
C.
krusei |
A.
niger |
A.
flavus |
A.
fumigatus |
|---|---|---|---|---|---|---|---|
| Pistacia atlantica fruit | 12.5 | 25 | 6.25 | 6.25 | NI | 12.5 | 6.25 |
| Pistacia atlantica leaf | 25 | NI | 6.25 | 6.25 | 25 | 25 | 12.5 |
| Olive leaf | NI | NI | NI | NI | 25 | NI | 12.5 |
|
bMixure of the three extracts |
25 | 25 | 6.25 | 12.5 | 12.5 | 25 | 6.25 |
Values represent the mean of three replicates; NI: No inhibition; C.: Candida; A.: Aspergillus,
Leaf and fruit extracts of Pistacia atlantica and leaf extract of Olive
The MICs of P. atlantica fruit and leaf extracts ranged 6.25-12.5 mg ml-1 and 6.25-25 mg ml-1 against the tested Candida and Aspergillus species, respectively. The MICs of the mixture of P. atlantica leaf and fruit extracts and olive leaf extracts were within the range of 0.625-25 mg/ml against the tested microorganisms (Figure 1, Table 1 and 2).
Table 2.
Inhibition zone (mm)a of Pistacia atlantica and olive leaf against Candida and Aspergillus species
| Extracts |
C.
albicans |
C.
tropicalis |
C.
glabrata |
C.
krusei |
A.
niger |
A.
flavus |
A.
fumigatus |
|---|---|---|---|---|---|---|---|
| Pistacia atlantica fruit | 15 | 15 | 20 | 14 | N.I | 14 | 14 |
| Pistacia atlantica leaf | 15 | N.I | 14 | 13 | 12 | 16 | 12 |
| Olive leaf | N.I | N.I | N.I | N.I | 13 | N.I | 11 |
|
bMixture of the three extracts |
12 | 14 | 13 | 16 | 12 | 13 | 12 |
| Positive control | |||||||
| Fluconazole | 26 | 27 | 17 | 27 | - | - | - |
| Amphotericin B | - | - | - | - | 27 | 16 | 21 |
Amphotricin B (30 meg/disc) and clotrimazole (10 μg/disc) were used as the positive controls.
Values represent the mean of three replicates; NI: No inhibition
Leaf and fruit extracts of Pistacia atlantica and olive leaf extract
Discussion
In the current study, the ethanolic leaf and fruit extracts of P. atlantica and olive leaf extracts exhibited strong antifungal effects. The present findings were in accordance with previous research. In this regard, Hosseini et al. revealed that P. atlantica resin extracts could exhibit antibacterial activities against Streptococcus mutans and might be useful for oral hygiene during the treatment of dental injuries [8].
In a previous study by Masherghi et al., Rosmarinus officinalis extracts showed greater efficiency than P. atlantica extracts in inhibiting E. coli [9]. Moreover, Torabi and colleagues concluded that the essential oils of Eucalyptus kingsmillii and Eucalyptus salubris could exert less significant inhibitory effects on E. coli, compared to P. atlantica extracts [10].
Furthermore, Azizian et al. investigated the effects of P. atlantica on bacteria, including E. coli, Pseudomonas aeruginosa, Staphylococcus aureus, and H. pylori and made a comparison with conventional antibiotics. In the mentioned study, MICs of P. atlantica extracts were 163 μg/ml, 104.16 μg/ml, and 204.67 μg/ml for E. coli, Pseudomonas aeruginosa, and Staphylococcus aureus, respectively, while the extracts showed no significant antifungal activity against H. pylori. These findings demonstrated the inhibitory effects of these herbal extracts, although no antifungal activity against H. pylori was detected [11].
Paraschos et al. reported the antimicrobial properties of mastic water extract, which is the essential oil obtained from the resin of Pistacia
lentiscus var. Chia, against E. coli, Staphylo-coccus aureus, and Candida species. Overall, linalool and a-terpineol constituted the strongest antimicrobial compounds in this plant [11].
In a literature review, the antifungal activity of dichloromethane extracts from olive tree (Olea Cuspidata and Olea Glandulifera Linn.) against C. albicans and Aspergillus niger was investigated. The findings revealed maximum antifungal activity of plant extracts against C. albicans (inhibition zone of 26 mm) and Aspergillus niger with an inhibition zone of 22 mm [13].
Moreover, in the mentioned study, analysis of antifungal activity showed more significant effects against fungi at higher concentrations of dichloromethane extracts. Although individual phenolic compounds in olive extracts could show strong in vitro activities, the antioxidant and antimicrobial activities of combined phenolics were similar or more effective than individual phenolic compounds [13].
In the present study, olive leaf extracts showed no significant activity against Candida species, while they exhibited antifungal potency against Aspergillus niger; this finding was in agreement with previous research [12]. Also, several reports have demonstrated the antimicrobial properties of olive leaf extracts, considering their high phenolic content [13, 14]. In addition, in a previous study, combined phenolics in olive leaf extracts showed similar or better antioxidant and antimicrobial activities than individual phenolic compounds [6].
Faiza et al. revealed strong antimicrobial activity in olive leaf extracts [15]. Also, in a previous study, the antimicrobial properties of P. lentiscus and P. atlantica extracts against eight bacteria, five molds, and yeasts were revealed. The mentioned study reported that Klebsiella pneumoneae and Escherichia coli were not sensitive to the ethanolic extracts of P. lentiscus and P. atlantica. However, Staphylococcus aureus, Salmonella typhimurium, and C. albicans strains were sensitive to these extracts; these findings were in line with the present study.
In the aforementioned study, the ethanolic extracts of P. lentiscus showed inhibitory activities against all the tested strains, except for Rhizopus stolonifer and Aspergillus flavus. It should be noted that the flavonic extracts of P. lentiscus exhibited antifungal activities against yeasts and molds, while showing no antibacterial activity [16].
Recent findings are not in agreement with the results of the present study. Paraschos et al. reported the antimicrobial properties of mastic water extract (essential oil of Pistacia lentiscus var. chia L. resin) against E. coli, Staphylococcus aureus, and Candida species. Linalool and α-terpineol were reported as the strongest antimicrobial compounds in this plant [11]. These findings were in agreement with previous literature. Also, in the present research, the ethanolic leaf and fruit extracts of P. atlantica showed antifungal activities against all the tested strains, except for C. tropicalis and Aspergillus niger.
So far, several members of the genus Pistacia have been chemically investigated. These species are mainly characterized by the presence of flavonoid glycosides and flavonoids, which are hydroxylated phenolic substances. According to in vitro studies, phenolic compounds, triterpenoids, and terpenes are effective antimicrobial substances against a wide array of microorganisms; also, it should be noted that two major essential volatile oil constituents, i.e., α-pinene and α-terpineol, possess interesting antibacterial properties [17].
In a previous study, Gokmen et al. screened the antimicrobial activity of Olea europaea (olive) leaf extracts against Gram-positive and Gram-negative bacteria. A similarity was detected between the inhibitory zones of olive leaf extracts and commercial antibiotics such as gentamicin against Salmonella typhimurium, Proteus vulgaris, and Pseudomonas aeruginosa.
In the aforementioned study, the MICs of olive leaf extracts against Listeria mono-cytogenes, E. coli, Enterobacter sakazakii, and Pseudomonas aeruginosa were ≥ 32 mg/ml. On the other hand, the MIC values against other bacteria (i.e., Bacillus cereus, Staphylococcus aureus, Enterococcus faecalis, Proteus vulgaris, E. coli, and Salmonella typhimurium) were ≥ 16 mg/ml. These findings revealed that olive leaf extracts could be used in medicine, pharmaceutical industries, and food products as natural antimicrobial food additives [18].
Conclusion
As the findings revealed, olive leaf extracts and P. atlantica leaf and fruit extracts showed considerable antifungal activities; also, the mixture of the extracts exhibited the same antifungal potency. According to previous research, the strong antifungal activity of olive and Pistacia species could be probably attributed to the phenolic and triterpenoid compounds of these plants [6,7,17]. Therefore, these plants could be used as natural antifungal agents to overcome resistance to antibiotics for the treatment of infectious diseases.
Acknowledgments
The authors would like to thank the Department of Medical Mycology at Ahvaz Jundishapur University of Medical Sciences for providing laboratory facilities.
Authors’ Contributions
Z.Sh. and M.Z. obtained the specimens and performed all the tests. B.S. wrote, designed, reviewed, and edited the article, and S.YN. prepared and authenticated the medicinal plants.
Conflicts of Interest:
The authors declare no conflicts of interest regarding the publication of this paper.
Financial disclosure
The authors declare no financial interests related to the materials of the study.
References
- 1.Farhoosh R, Sharif A. Bene hull oil as a highly stable and antioxidative vegetable oil. Eur J Lipid Sci Technol. 2009;111:1259–65. [Google Scholar]
- 2.Karimi HR, Zamani Z, Ebadi A, Fathi MR. Morphological diversity of Pistacia species in Iran. Gen Resour Crop Evol. 2009;56(4):561–71. [Google Scholar]
- 3.Bozorgi M, Memariani Z, Mobli M, Salehi Surmaghi MH, Shams-Ardekani MR, Rahimi R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): a review of their traditional uses, phytochemistry, and pharmacology. Sci World J. 2013;2013 doi: 10.1155/2013/219815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azizian MI, Pakzad I, Azizian R, Azizi Jalilian F, Taherikalani M, Sadeghifard N, et al. Antibacterial effect of Hydro-extract of Pistacia atlantica on Bacteria in In vitro. Biomed Pharmacol J. 2013;6(2):133–6. [Google Scholar]
- 5.Taamalli A, Arraez-Roman D, Zarrouk M, Valverde J, Segura- Carretero A, Fernandez-Gutierrez A. The occurrence and bioactivity of polyphenols in Tunisian olive products and by-products: a review. J Food Sci. 2012;77(4):R83–92. doi: 10.1111/j.1750-3841.2011.02599.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee OH, Lee BY. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour Technol. 2010;101(10):3751–4. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Sadeghi-Nejad B, Azish M. In vitro antibacterial and antifungal effect of some medicinal plants. Afr J Microbiol Res. 2013;7(29):3802–6. [Google Scholar]
- 8.Hosseini F, Adlgostar A, Sharifnia F. Antibacterial activity of Pistacia atlantica extracts on Streptococcus mutans biofilm. Int Res J Biol Sci. 2013;2(2):1–7. [Google Scholar]
- 9.Mashreghi M, Momtazi F. Comparison of antibacterial effect of various concentrations of Rosmarinus officinalis, Hypericum perforatum and Carthamus tinctorius on the growth phases of Esherichia coli O157. J Rafsanjan Univ Med Sci. 2012;11(2):103–14. [Google Scholar]
- 10.Torabi SB, SadeghzadehL Investigation of components and antimicrobial effect of 10 Eucalyptus species on Micrococcus loteusand Escherichia coli. J Med Arom Plant. 2011;27(3):440–9. [Google Scholar]
- 11.Azizian MI, Pakzad I, Azizian R, Azizi Jalilian F, Taherikalani M, Sadeghifard N, et al. Antibacterial Effect of Hydro-extract of Pistacia atlantica on Bacteria in In vitro. Biomed Pharmacol J. 2013;6(2):133–6. [Google Scholar]
- 12.Paraschos S, Magiatis P, Gousia P, Economou V, Sakkas H, Papadopoulou C, et al. Chemical investigation and antimicrobial properties of mastic water and its major constituents. Food Chem. 2011;129(3):907–11. doi: 10.1016/j.foodchem.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 13.Majgaine P, Verma DL. Antifungal activity of olive tree and gladulifera Linn. IOSR J Pharm. 2013;3(5):20–23. [Google Scholar]
- 14.Aytul KK. Antimicrobial and antioxidant activities of olive leaf extract and its food applications. [Masters Thesis] Turkey: Graduate School of Engineering and Sciences of Izmir Institute of Technology; 2010. [Google Scholar]
- 15.Sudjana AN, D'Orazio C, Ryan V, Rasool N, Ng J, Islam N, et al. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int J Antimicrob Agents. 2009;33(5):461–3. doi: 10.1016/j.ijantimicag.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Faiza I, Wahiba K, Nassira G, Chahrazed B, Atik BF. Antibacterial and antifungal activities of olive (Olea europaea L.) from Algeria. J Microbiol Biotech Res. 2011;1(2):69–73. [Google Scholar]
- 17.Benhammou N, Bekkaraand FA, Panovska TK. Antioxidant and antimicrobial activities of the Pistacia lentiscusand Pistacia atlantica extracts. Afr J Pharm Pharmacol. 2008;2(2):22–8. [Google Scholar]
- 18.Paraschos S, Magiatis P, Gousia P, Economou V, Sakkas H, Papadopoulou C, et al. Chemical investigation and antimicrobial properties of mastic water and its major constituents. Food Chem. 2011;129(3):907–11. doi: 10.1016/j.foodchem.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 19.Tohidi M, Khayami M, Nejati V, Meftahizade H. Evaluation of antibacterial activity and wound healing of Pistacia atlantica and Pistacia khinjuk. J Med Plants Res. 2011;5(17):4310–4. [Google Scholar]
- 20.Gokmen M, Kara R, Akkaya L, Torlak E, Onen A. Evaluation of antimicrobial activity in olive (Olea europaea) leaf extract. Am J Microbiol. 2014;5(2):37–40. [Google Scholar]