Abstract
Background and Purpose:
Soil is the main habitat of saprophytic and pathogenic fungi. Mucoromycotina constitutes a large group of soil fungi, with certain opportunistic members causing systemic infections in immunocompromised hosts. The majority of human and animal infections are caused by the members of the genera Rhizopus, Mucor, Rhizomucor, Lichtheimia (Absidia), Cunninghamella, and Mortierella. Accordingly, in the present study, we aimed to isolate and identify the main genera of the order Mucorales, using molecular assays and morphological features.
Materials and Methods:
In total, 340 soil samples were collected from seven public parks throughout the city and sidewalk gardens in 14 municipal districts in Isfahan, Iran. All the samples were cultured on the appropriate media, incubated at 27°C for 2- 4 days, and examined daily for visible fungal growth. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method was applied and macroscopic, microscopic, and physiological characteristics were assessed to identify fungal colonies.
Results:
400 pure colonies, belonging to the orders Mucorales and Mortierellales, including the genera Lichtheimia, Rhizopus, Rhizomucor, Mucor, Cunninghamella, and Mortierella, were identified. The genus Rhizopus (35.5%) was the most frequent isolate, followed by Mucor (32.25%) and Rhizomucor (27.5%).
Conclusion:
The results emphasize the importance of opportunistic fungi in public areas and indicate the risk of exposure for immunocompromised individuals.
Key Words: Mucorales, Mucor, Rhizopus, Lichtheimia, Rhizomucor, Mortierella, PCR-RFLP
Introduction
In the literature, no relationship has been established between the distribution of fungal species and environmental factors [1]. The amount and type of microorganisms in a particular section of soil are associated with several factors, such as sunlight, temperature, moisture, soil pH, nutrients, and reduction potential [2].
Fungi, as a group of microorganisms with a wide distribution in soil, play important roles in the soil ecosystem and soil-borne fungal diseases. Today, it is well established that soil can be a reservoir of most pathogenic and opportunistic fungi [3-6]. In fact, soil-borne pathogenic fungi may enter the human body via direct inoculation into wounds, direct soil ingestion, or indirect ingestion via contaminated food.
Fungal spores can be dispersed in different environments through dust or mud particles from soil disturbances and enter the respiratory tract [2, 6]. Mucorales is an order of mostly saprophytic fungi, growing on organic substances, such as food, dead plants, or animal waste materials in soil [7, 8]. These organisms may survive in the soil for a long time before infecting humans who are in contact with contaminated soil [9].
In 2007, in a taxonomic reclassification, Zygomycetes was abolished as a class, and zygomycosis is now mainly attributed to the order Mucorales of subphylum Mucoromycotina [10]. Therefore, the term “mucormycosis”, previously known as zygomycosis, is currently used for opportunistic infections caused by the order Mucorales [11].
Mucormycosis is the third most common invasive fungal infection, following aspergillosis and candidiasis [12]. The importance of mucormycosis has been highlighted in recent years as a consequence of the dramatic increase in the number of patients with predisposing factors [13-15]. In general, six families, including Cunninghamellaceae, Lichtheimiaceae, Mucoraceae, Saksenaeaceae, Syncephalastraceae, and Thamnidiaceae, have been described to be responsible for human infections [14].
With this background in mind, the main aim of this study was to investigate the diversity of fungi belonging to the subphylum Mucormycotina, particularly the order Mucorales, in the soil of different public places and sidewalk gardens, located in the populated areas of municipal districts of Isfahan, Iran, using molecular and morphological assays.
DNA-based molecular assays have been shown to be rapid and highly reliable tools, especially for rRNA genes. These assays have become widely accepted for phylogenetic identification of various fungi [8]. In the present study, polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method was applied to identify the important genera of the order Mucorales, as successfully performed in previous studies [16, 17].
Materials and Methods
During August 2014 to October 2015, a total of 340 soil samples were gathered from different sites, i.e., seven public parks and different sidewalk gardens located in 14 municipal districts of Isfahan, Iran. First, all the debris, present on the soil surface, was removed. Then, approximately 400 g of soil at a depth of 5-10 cm was collected from the surface layer and stored in sterile bags, using a sterile stainless steel spoon. All the samples were immediately transferred to the laboratory for the next processing stage.
The samples were crushed in a mortar under sterile conditions and then homogenized. Afterwards, 5 g of each soil sample was suspended in 20 ml of sterile double-distilled water and shaken for 5 min to prepare a soil suspension [18, 19]. Approximately 0.5 ml of the suspension was poured in the bottom of a Petri dish. Then, cooled molten agar medium of Sabouraud dextrose agar (SDA) or potato dextrose agar (PDA), supplemented with chloramphenicol (50 mg/l), was added to the suspension and entirely mixed. All the samples were incubated at 27°C for 2-4 days and were observed daily in terms of fungal colony growth. The primary identification was performed on the basis of macroscopic and microscopic features. Definite identification of Rhizopus, Mucor, Lichtheimia, and Rhizomucor at the species level was performed, based on molecular analysis and morphological and physiological studies. Moreover, identification of Mortierella and Cunninghamella species was based on the morphological characteristics [20].
Macroscopic and microscopic features
Mucorales colonies are typically floccose and dense. These colonies rapidly fill the entire Petri dish with abundant intertwined aerial mycelium, resembling gray cotton candy. The hyphae are predominantly aseptate or very sparsely septate and wide. Features which are most useful for distinguishing Mucorales include the presence of rhizoids, shape of sporangium, length of sporangiophore, shape of columella, presence or absence of apophysis and collarette, and organization and branching of stolons [15, 21].
Molecular studies
Genomic DNA, pertaining to all pure Mucorales colonies, was extracted, using the phenol-chloroform method [22]. A mixture of specific sense primers (MucL1: 5’ TGATCTACGTGACATATTTCT 3’; AbsL1: 5’ TGA TCTACACGGCATCAAAT 3’; RpL1: 5’ TGATCTACGTGACAAATTCT 3’; and RmL1: 5’ TGATCTACGCGAGCGAACAA 3’) was used, corresponding to Mucor, Lichtheimia, Rhizopus, and Rhizomucor species, respectively. Also, an antisense degenerate primer (MR1: 5’ AGTAGTTTGTCTTCGGTCAA 3’) corresponding to Mucorales was applied for the amplification of the purified extracted DNA. In a previous study by Machouart and colleagues, these primers had been successfully applied for the detection of the abovementioned genera belonging to the order Mucorales [17].
The following temperature program was applied for the amplification of the extracted DNA: primary denaturation at 94°C for 3 min, 30 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 7 min [23]. The PCR products of Mucorales were digested individually with the selected restriction enzymes, as presented in Table 1.
Table 1.
Restriction enzymes, species specificity, and fragment size used in the present study
| Enzyme (restriction site) |
Specificity | Fragment size (bp) |
|---|---|---|
| PpuMI | Rhizomucor spp. (except R. variabilis) | 68+720 |
| XhoII | Rhizomucor pusillus | 215+573 |
| BmgBI | Rhizopus spp. (except R. stolonifer) | 593+235 |
| AseI | Rhizopus microsporus and R. azygosporus | 750+75 |
| CspCI | Rhizopus oryzae | 214+249+579+614 |
| AflII | Mucor spp. | 750+87 |
| XmnI | Mucor circinelloides, M. racemosus, M. ramosissimus, and M. plumbeus | 613+224 |
| Lichtheimia corymbifera and L. blakesleeana | 518+306 |
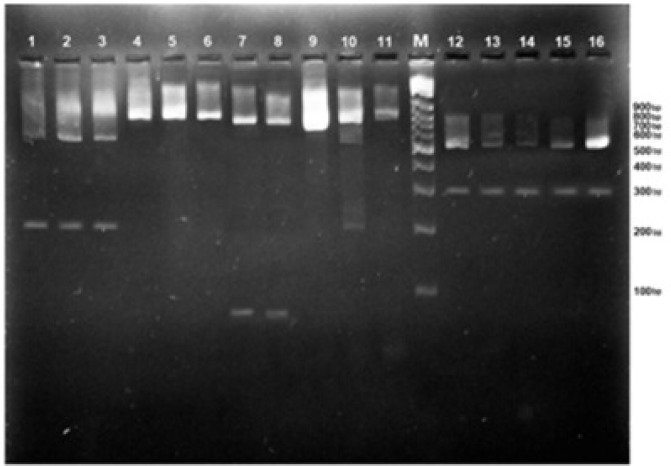
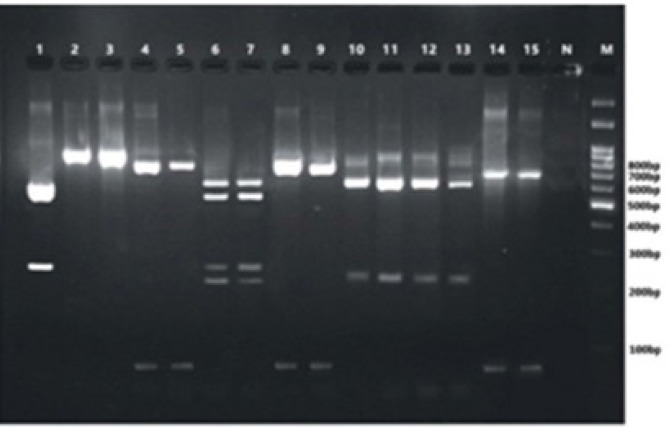
For PCR-RFLP analysis, a mixture was prepared, using the following components in a final volume of 25 µl: restriction enzyme (2 µl), enzyme buffer (2.5 µl), PCR product (10 µl), and distilled water (10.5 µl). The reaction mixture was incubated at 37°C for 2 h. The PCR-RFLP products were run on agarose gel electrophoresis (1.5% and 2%, respectively) in tris/borate/EDTA (TBE) buffer and photographed under ultraviolet transillumination (Figures 1 & 2).
Figure 1.
Agarose gel electrophoresis of 18S rRNA PCR products of different species of Mucorales after restriction digestion with XhoII and CspsI. Lanes 1, 2, & 3: digestion with XhoII (Rhizomucor pusillus); Lanes 4, 5, & 6: undigested PCR products; Lanes 7 & 8: digestion with AseI (Rhizopus microsporus or R. azygosporus); Lanes 9, 10, & 11: undigested PCR products; Lanes 12, 13, 14, 15, & 16: digestion with AcII (Lichtheimia corymbifera or L. blakesleeana); Lane M: 100 bp molecular-size marker
Figure 2.
Agarose gel electrophoresis of 18S rRNA PCR products of different species of Mucorales after restriction digestion with BmgBI (Lanes 1, 2, & 3), AfIII (Lanes 4, 5, 8, & 9), CspsI (Lanes 6 & 7), XmnI (Lanes 10, 11, 12, & 13), and PpumI (Lanes 14 & 15); Lane 1: Rhizopus spp. (except R. stolonifer); Lanes 2 & 3: undigested PCR products; Lanes 4, 5, 8, & 9: Mucor spp.; Lanes 6 & 7: Rhizopus arrhizus; Lanes 10, 11, 12, & 13: M. circinelloides, or M. racemosus, or M. ramosissimus, or M. plumbeus; Lanes 14 & 15: Rhizomucor spp.; Lane N: negative control; Lane M: 100 bp molecular-size marker
Results
A total of 393 and seven pure colonies, belonging to the orders Mucorales and Mortierellales, were obtained, respectively. Rhizopus was the most frequently detected species with a frequency rate of 35.5%, followed by Mucor (32.25%), Rhizomucor (27.5%), Lichtheimia (2.5%), Mortierella (1.75%), and Cunninghamella (0.5%) (Table 2).
Table 2.
Distribution of Mucorales and Mortierellales in the soil of different public parks and municipal districts
| Genus | Species | No. (%) | Total (%) |
|---|---|---|---|
| Mucor | M. Circinelloides | 3 (0.75%) | 129 (32.25%) |
| M. Racemosus | 4 (1%) | ||
| M. Plumbeus | 2 (0.5%) | ||
| Mucor spp. | 120 (30%) | ||
| Rhizomucor | R. Pusillus | 6 (1.5%) | 110 (27.5%) |
| Rhizomucor spp. | 104 (26%) | ||
| Rhizopus | R. Arrhizus | 10 (2.5%) | 142 (35.5%) |
| R. Stolonifer | 12 (3%) | ||
| Rhizopus spp. | 120 (30%) | ||
| Lichtheimia | L. Corymbifera | 2 (0.5%) | 10 (2.5%) |
| Lichtheimia (Absidia) spp. | 8 (2%) | ||
| Cunninghamella | C. bertholletiae | 2 (0.5%) | 2 (0.5%) |
| Mortierella | M. Wolfii | 1 (0.25%) | 7 (1.75%) |
| Mortierella spp. | 6 (1.5%) | ||
| Total (%) | 400 (100%) | 400 (100%) |
As presented in Table 2, R. stolonifer with a frequency of 3% was the predominant species, followed by R. arrhizus and R. pusillus with frequency rates of 2.5% and 1.5%, respectively; the lowest frequency rate was attributed to M. wolfii (0.25%). M. racemosus with a frequency rate of 1% was the predominant isolate among the identified species of the genus Mucor. Also, two C. bertholletiae isolates were identified in pure Mucorales cultures.
According to the findings, 51.81% of the isolates from the soil samples of public parks belonged to the genus Rhizopus, followed by Rhizomucor with a frequency rate of 28.31%, while in the soil samples from municipal districts, the genus Mucor (45.30%) was the most frequent isolate, followed by Rhizomucor (26.92%) (Tables 3 & 4).
Table 3.
Distribution of Mucorales and Mortierellales in the soil of different public parks
| Genus | Park No. Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | No. (%) | Total (% ) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mucor | M. circinelloides | - | - | - | 1 | - | 1 | - | 2 (1.20) | 23(13.86) |
| M. racemosus | 1 | - | - | - | - | - | - | 1 (0.60) | ||
| Mucor spp. | 2 | 9 | 1 | 2 | 1 | 3 | 2 | 20 (12.06) | ||
| Rhizomucor | R. pusillus | - | 1 | - | - | 1 | - | - | 2 (1.20) | 47(28.31) |
| Rhizomucor spp. | 12 | 20 | 3 | 3 | 4 | - | 3 | 45 (27.11) | ||
| Rhizopus | R. oryzae | 1 | 1 | 1 | - | 1 | 1 | 5 (3.01) | 86(51.81) | |
| R. stolonifer | 1 | 2 | 1 | - | 3 | 2 | 9 (5.42) | |||
| Rhizopus spp. | 20 | 1 | 2 | 17 | - | 30 | 2 | 72 (43.38) | ||
| Lichtheimia | L. corymbifera | 1 | - | - | - | - | - | - | 1 (0.60) | 5 (3.01) |
| Absidia spp. | 2 | - | - | - | 1 | 1 | - | 4 (2.41) | ||
| Cunninghamella | C. bertholletiae | 1 | - | - | - | - | - | - | 1 (0.60) | 1(0.60) |
| Mortierella | Mortierella spp. | 1 | 1 | 1 | - | - | 1 | - | 4 (2.41) | 4(2.41) |
| Total (%) | 42 (25.30) |
34 (20.48) |
9 (5.42) |
24 (14.46) |
7 (4.22) |
40 (24.10) |
10 (6.02) |
166 (100) |
166(100) |
Table 4.
Distribution of Mucorales and Mortierellales in the soil of different municipal districts
| Genus | District No. Species |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | No. (%) | Total (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mucor | M. circinelloides | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | 1 (0.43) | 106 (45.30) |
| M. racemosus | 1 | - | - | - | - | - | 1 | - | - | - | - | - | 1 | - | 3 (1.28) | ||
| M. plumbeus | - | - | 2 | - | - | - | - | - | - | - | - | - | - | - | 2 (0.85) | ||
| Mucor spp. | 5 | 13 | 22 | 5 | - | 4 | 21 | 2 | 6 | 3 | 7 | 3 | 2 | 7 | 100 (42.74) | ||
| Rhizomucor | R. pusillus | - | 2 | - | - | - | - | - | - | - | - | - | 1 | - | 1 | 4 (1.71) | 63 (26.92) |
| Rhizomucor spp. | 7 | 8 | 10 | 2 | 1 | 1 | 3 | 10 | 5 | 2 | 1 | 3 | 5 | 1 | 59 (25.21) | ||
| Rhizopus | R. oryzae | - | - | - | - | 1 | - | - | 1 | - | 1 | - | - | 1 | 1 | 5 (2.14) | 56 (23.93) |
| R. stolonifer | - | - | - | 1 | - | 1 | - | - | - | - | - | 1 | - | - | 3 (1.28) | ||
| Rhizopus spp. | 4 | 5 | 9 | 6 | - | 4 | 12 | - | 1 | - | 3 | 2 | - | 2 | 48 (20.51) | ||
| Lichtheimia | L. corymbifera | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 1 (0.43) | 5 (2.14) |
| Absidia spp. | 3 | - | - | - | - | - | - | - | - | - | - | 1 | - | - | 4 (1.71) | ||
| Cunninghamella | C. bertholletiae | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | 1 (0.43) | 1(0.43) |
| Mortierella | M. wolfii | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 1 (0.43) | 3 (1.28) |
| Mortierella spp. | - | - | - | - | - | - | - | - | 1 | 1 | - | - | - | - | 2 (0.85) | ||
| Total (%) |
20 (8.55) |
28 (11.96) |
43 (18.38) |
14 (5.98) |
3 (1.2) |
11 (4.7) |
37 (15.81) |
13 (5.56) |
13 (5.56) |
7 (2.99) |
11 (4.70) |
11 (4.70) |
9 (3.85) |
14 (5.98) |
234 (100) |
234 (100) |
Discussion
Mucorales are known as thermotolerant moulds, widely found on organic substrates and soil. Temperature of 27°C and high humidity are the optimal environmental conditions, required for the growth and sporulation of Mucorales on these substrates. According to the literature, most Mucorales, as thermophilic species, have been isolated from composting plant materials [9, 24]. In addition, the outbreak of rhinocerebral or pulmonary mucormycosis has been associated with the inhalation of sporangiospores in dust due to excavation, construction, or contaminated air-conditioning filters [9].
Today, mucormycosis is identified as an emergent disease, owing to the increasing incidence and mortality over the past two decades [25]. The diagnosis and management of mucormycosis remain challenging tasks and no clinical or radiological signs specific for mucormycosis have been identified. Moreover, standardized serological or antigen detection tests are not currently available [26].
Researchers have attempted to compare the identification of Mucorales fungi, using phenotypic and molecular methods [11]. In the present study, all the phenotypic features of Mucorales were adapted in the molecular analysis for the definite identification of fungi. Different regions of rRNA operon have been frequent targets for the detection of Zygomycetes. Also, different fungal primers from 18S or ITS regions of rRNA gene have been used for PCR amplification [27]. In our study, primers selected from the 18S region were used to detect major genera of the order Mucorales.
Species involved in mucormycosis belong to the order Mucorales of subphylum Mucoromycotina. In addition, Mucoraceae is the most important family, comprising of Rhizopus, Mucor, and Lichtheimia as the most common species and Rhizomucor, Mortierella, Saksenaea, Syncephalastrum, Cunninghamella, and Apophysomyces as less common agents of mucormycosis [25].
In the present study, Rhizopus, Mucor, and Rhizomucor were the dominant genera with frequency rates of 35.5%, 32.25%, and 27.5%, respectively (Table 2). These organisms are ubiquitous saprophytes in nature, rarely infecting organisms with an intact immune system [28]. Members of the genus Rhizopus are the most common isolates, recovered from clinical mucormycosis samples. Additionally, members of the genus Mucor are second to Rhizopus in terms of frequency [12].
R. arrhizus (oryzae) is the most common cause of mucormycosis. Other less frequent etiological agents include L. corymbifera, A. elegans, C. bertholletiae, R. pusillus, and S. vasiformis [9]. In the present study, R. arrhizus (oryzae), R. pusillus, L. corymbifera, and C. bertholletiae were isolated from the soil samples of public parks and municipal districts, with the genus Rhizopus showing the highest frequency (Tables 3 & 4).
R. arrhizus was previously diagnosed as the causative agent of rhinocerebral mucormycosis in a man with diabetes mellitus in Isfahan, Iran. Accordingly, it was concluded that debilitated individuals are predisposed to mucormycosis [29]. Also, R. pusillus is the main etiological agent for infection in immunocompromised hosts. In the literature, 22 cases of R. pusillus infection were reported before 2013 [30]. However, Rhizopus species, which are responsible for almost 80% of infections, remain as the major cause of most mucormycosis cases. Also, a considerable number of mucormycosis cases have been associated with M. circinelloides [31].
In the present study, M. circinelloides and M. racemosus were isolated from the soil samples gathered from public parks and municipal districts, while M. plumbeus was only isolated from two municipal districts (Tables 2, 3, & 4). Although M. plumbeus is extensively used for research purposes in biotransformation of natural products, no cases of mycosis have been so far linked to this species [31].
In the present study, different Lichtheimia species including L. corymbifera were isolated from the soil samples of parks and municipal districts (Tables 2, 3, & 4). In general, the genus Lichtheimia (Absidia) consists of fungal species, which are ubiquitous soil inhabitants and important causative agents of mucormycosis in humans and animals [32]. In Europe, members of the genus Lichtheimia are the second or third most important cause of mucormycosis [33].
Malek et al. [34] isolated 17 different fungal genera from the soil of parks in Gorgan, Iran. Overall, 36% of the isolates were related to Zygomycetes, and the genus Mucor was the most frequent isolate among other Zygomycetes genera. In the present study, the genus Rhizopus (51.81%) was the most frequent isolate detected in soil, followed by Rhizomucor (28.31%), Mucor (13.86%), Lichtheimia (Absidia) (3.01%), Mortierella (2.41%), and Cunninghamella (0.60%), respectively (Table 3). The discrepancy between the findings can be attributed to the differences in temperature, humidity, organic content of soil, and plant diversity in each area.
The Mortierellaceae species can be distinguished from Mucoraceae with respect to their very delicate features. In these species, sporangia are small and contain few or no columella; moreover, the mycelium is dichotomously branched. Considering these delicate features, species belonging to this family can be classified in a family other than Mucoraceae [21].
Isolation of the genus Mortierella from soil samples has been studied in the literature [35]. In the present study, different Mortierella species including M. wolfii were isolated from the soil of parks and municipal districts with frequency rates of 2.41% and 1.28%, respectively (Tables 3 & 4). According to previous research, M. wolfii is probably the only pathogenic species and an important etiological agent of bovine mycotic abortion, pneumonia, and systemic mycosis [21].
Yazdanparast et al. [36] reported the isolation of some saprophytic and keratinophilic fungi from the soil samples of parks and municipal districts of Tehran, Iran. They isolated the genera Cunninghamella, Mucor, and Rhizopus from the soil samples of several parks. Based on the findings, the isolation rate of Cunninghamella was higher than other genera.
So far, several researchers have isolated different fungi from various types of soil. In this regard, Agamirian et al. investigated the prevalence of fungi in soil of Qazvin, Iran and reported 14 genera including Rhizopus and Mucor [6]. Although the present study focused on Mucorales, genera belonging to other classes of fungi (e.g., Fusarium, Cladosporium, Aspergillus, Penicillium, Trichophyton mentagrophytes, Chrysosporium, and Scopulariopsis) were also isolated.
In a previous study, Hedayati et al. (2004) isolated Mucor and Rhizopus species from the soil samples of potted plants in hospitals of Sari, Iran. Other isolated genera included Acromunium, Penicillium, Cladosporium, Paecilomyces, Chrysosporium, Alternaria, Aspergillus, Verticillium, Geotrichum, and yeasts [37]. The obtained findings were almost in agreement with the results of the present study.
Conclusion
In conclusion, Mucoromycotina representatives are recognized as ubiquitous organisms in the soil all over the world. These organisms are majorly found in clinical samples and are used in food fermentation [38]. Therefore, knowledge about the pathogenic ecology of Mucorales is essential to the understanding of the epidemiology of mucormycosis in high-risk patients.
Acknowledgements
We would like to thank the biotechnology and mycology laboratory of Islamic Azad University of Isfahan for the technical assistance and valuable help.
Authors’ contributions
A.Z. carried out the practical and laboratory examinations of the study. M.Z. participated in the study design, conducted practical and laboratory examinations, and drafted the manuscript. M.B. and J.H. participated in the study design and helped draft the manuscript.
Conflicts of Interest:
There was no conflict of interest in the present study.
Financial disclosure
There was no financial interest related to the materials of the manuscript.
References
- 1.Edel-Hermann V, Gautheron N, Mounier A, Steinberg C. Fusarium diversity in soil using a specific molecular approach and a cultural approach. J Microbiol Methods. 2015;111:64–71. doi: 10.1016/j.mimet.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Baumgardner DJ. Soil-related bacterial and fungal infections. J Am Board Fam Med. 2012;25(5):734–44. doi: 10.3122/jabfm.2012.05.110226. [DOI] [PubMed] [Google Scholar]
- 3.Moallaei H, Zaini F, Pihet M, Mahmoudi M, Hashemi J. Isolation of keratinophilic fungi from soil samples of forests and farm yards. Iran J Public Health. 2006;35(4):62–9. [Google Scholar]
- 4.Suhail M, Irum F, Jatt T, Korejo F, Abro H. Aspergillus mycoflora isolated from soil of Korti Barrage Sandy, Pakistan. Pak J Bot. 2007;39(3):981–4. [Google Scholar]
- 5.Zarrin M, Haghgoo R. Survey of keratinophilic fungi from soils in Ahvaz, Iran. Jundishapur J Microbiol. 2011;4(3):191–4. [Google Scholar]
- 6.Aghamirian MR, Ghiasian SA. The prevalence of fungi in soil of Qazvin, Iran. Jundishapur J Microbial. 2012;6(1):76–9. [Google Scholar]
- 7.Kim MJ, Park PW, Ahn JY, Kim KH, Seo JY, Jeong JH, et al. Fatal pulmonary mucormycosis caused by Rhizopus microsporus in a patient with diabetes. Ann Lab Med. 2014;34(1):76–9. doi: 10.3343/alm.2014.34.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagao K, Ota T, Tanikawa A, Takae Y, Mori T, Udagawa S, et al. Genetic identification and detection of human pathogenic Rhizopus species, a major mucormycosis agent, by multiplex PCR based on internal transcribed spacer region of rRNA gene. J Dermatol Sci. 2005;39(1):23–31. doi: 10.1016/j.jdermsci.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect. 2009;15(Suppl 5):2–9. doi: 10.1111/j.1469-0691.2009.02972.x. [DOI] [PubMed] [Google Scholar]
- 10.Neto FM, Camargo PC, Costa AN, Teixeira RH, Carraro RM, Afonso JE Jr, et al. Fungal infection by Mucorales order in lung transplantation: 4 case reports. Transplant Proc. 2014;46(6):1849–51. doi: 10.1016/j.transproceed.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Lee HJ, Kim YK, Ki CS, Huh HJ, Lee NY. Identification of mucorales from clinical specimens: a 4-year experience in a single institution. Ann Lab Med. 2016;36(1):60–3. doi: 10.3343/alm.2016.36.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bala K, Chander J, Handa U, Punia RS, Attri AK. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol. 2015;53(3):248–57. doi: 10.1093/mmy/myu086. [DOI] [PubMed] [Google Scholar]
- 13.Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, et al. A higher-level phylogenic classification of the fungi. Mycol Res. 2007;111(Pt 5):509–47. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Iwen PC, Thapa I, Bastola D. Review of methods for the identification of Zygomycetes with an emphasis on advances in molecular diagnostics. Lab Med. 2011;42(5):260–6. [Google Scholar]
- 15.Lackner M, Caramalho R, Lass-Florl C. Laboratory diagnosis of mucormycosis: current states and future perspectives. Future Microbiol. 2014;9(5):683–95. doi: 10.2217/fmb.14.23. [DOI] [PubMed] [Google Scholar]
- 16.Larche J, Machouart M, Burton K, Collumb J, Biava MF, Gerard A, et al. Diagnosis of cutaneous mucormycosis due to Rhizopus microsporus by an innovative PCR-restriction fragment-length polymorphism method. Clin Infect Dis. 2005;41(9):1362–5. doi: 10.1086/497078. [DOI] [PubMed] [Google Scholar]
- 17.Machouart M, Larche J, Burton K, Collomb J, Maurer P, Cintrat A, et al. Genetic identification of main opportunistic Mucorales by PCR-restriction fragment length polymorphism. J Clin Microbiol. 2006;44(3):805–10. doi: 10.1128/JCM.44.3.805-810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamed MS, Abdel-Motaleb HY, Mobarak FA. Management of rhino-orbital mcormycosis. Saudi Med J. 2015;36(7):865–8. doi: 10.15537/smj.2015.7.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaddeyya G, Niharika PS, Bharathi P, Kumar PR. Isolation and identification of soil mycoflora in different crop fields at Salur Mandal. Adv Appl Sci Res. 2012;3(4):2020–6. [Google Scholar]
- 20.Ziaee A, Zia M, Bayat M, Hashemi J. Occurrence of important mucormycosis agents in the soil of populous areas of Isfahan and their pathogenicity in immunocompromised patients. J Pure Appl Microbiol. 2016;10(1):81–8. [Google Scholar]
- 21.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirhendi SH, Kordbacheh P, Kazemi B, Samiei S, Pezeshki M, Khorramizadeh MR. A PCR-RFLP method to identification of the important opportunistic fungi: Candida species, Cryptococcus neoformans, Aspergillus famigatus and Fusarium solani. Iran J Pub Health. 2001;30(3-4):103–6. [Google Scholar]
- 23.Ziaee A, Zia M, Bayat M, Hashemi J. Molecular identification of Mucor and Lichtheimia species in pure cultures of Zygomycetes. Jundishapur J Microbiol. 2016;9(4):e35237. doi: 10.5812/jjm.35237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (Zygomycosis) Clin Infect Dis. 2012;54(Suppl 1):S55–60. doi: 10.1093/cid/cir868. [DOI] [PubMed] [Google Scholar]
- 25.Bonifaz A, Triado-Sanchez A, Calderon L, Ponce RM. Cutaneous mucormycosis: mycological, clinical and therapeutic aspects. Curr Fungal Infect Rep. 2015;9(4):229–37. [Google Scholar]
- 26.Dannaoui E, Schwarz P, Slany M, Loeffler J, Jorde AT, Cuenca-Estrella M, et al. Molecular detection and identification of Zygomycetes species from paraffin-embedded tissues in murine model of disseminated Zygomycosis: a collaborative European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) evaluation. J Clin Microbiol. 2010;48(6):2043–6. doi: 10.1128/JCM.02319-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasai M, Harrington SM, Francesconi A, Petraitis V, Petraitiene R, Beveridge MG, et al. Detection of a molecular biomarker for Zygomycetes by quantitative PCR assays of plasma, bronchoalveolar lavage, and lung tissue in rabbit model of experimental pulmonary Zygomycosis. J Clin Microbiol. 2008;46(11):3690–702. doi: 10.1128/JCM.00917-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paramythiotou E, Frantzeskaki F, Flevari A, Armaganidis A, Dimopoulos G. Invasive fungal infections in the ICU: how to approach, how to treat. Molecules. 2014;19(1):1085–119. doi: 10.3390/molecules19011085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammadi R, Nazari M, Sayedayn SM, Ehteram H. A successful treatment of rhinocerebral mucormycosis due to Rhizopus oryzae. J Res Med Sci. 2014;19(1):72–4. [PMC free article] [PubMed] [Google Scholar]
- 30.Bala K, Chander J, Handa U, Punia RS, Raj R, Aggarwal S. Isolation and identification of Rhizomucor pusillus from rhinofacial mucormycosis in a diabetic patient. JJM Case Rep. 2014;1(3):1–4. [Google Scholar]
- 31.Granja LF, Pinto L, Almeida CA, Alviano DS, Da Silva MH, Ejzemberg R, et al. Spores of Mucor ramosissimus, Mucor plumbeus and Mucor circinelloides and their ability to activate human complement system in vitro. Med Mycol. 2010;48(2):278–84. doi: 10.3109/13693780903096669. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann K, Discher S, Voigt K. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp form a coherent group, Mycocladiaceae fam nov. Mycol Res. 2007;111(Pt 10):1169–83. doi: 10.1016/j.mycres.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz V, Jacobsen ID. Mucormycoses caused by Lichtheimia species. Mycoses. 2014;57(Suppl 3):73–8. doi: 10.1111/myc.12239. [DOI] [PubMed] [Google Scholar]
- 34.Malek E, Moosazadeh M, Hanafi P, Nejat ZA, Amini A, Mohammadi R, et al. Isolation of keratinophilic fungi and aerobic actinomycetes from park soils in Gorgan, North of Iran. Jundishapur J Microbiol. 2013;6(10):e11250. [Google Scholar]
- 35.Ellegaard-Jensen L, Aamand J, Kragelund BB, Johnsen AH, Rosendahl S. Strains of the soil fungus Mortierella show different degradation potentials for the phenylurea herbicide diuron. Biodegradation. 2013;24(6):765–74. doi: 10.1007/s10532-013-9624-7. [DOI] [PubMed] [Google Scholar]
- 36.Yazdanparast SA, Dargahi H, Shahrokhi S, Farahani RH. Isolation and investigation of keratinophilic fungi from municipality districts of Tehran. Thrita. 2013;2(3):2–5. [Google Scholar]
- 37.Hedayati MT, Mohseni-Bandpi A, Moradi S. A survey on the pathogenic fungi in soil samples of potted plants from Sari hospitals, Iran. J Hosp Infect. 2004;58(1):59–62. doi: 10.1016/j.jhin.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Muszewska A, Pawlowska J, Krzysciak P. Biology, systemics, and clinical manifestations of Zygomycota infections. Eur J Clin Microbiol Infect Dis. 2014;33(8):1273–87. doi: 10.1007/s10096-014-2076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]