Abstract
Background and Purpose:
Superficial mycotic infections have been only poorly described in koalas and there are no reliable mycologically confirmed data regarding clinical isolation of dermatophytes in this animal. We report an 11-year-old female koala, kept in a zoo in Tokyo, Japan, and presenting with hyperkeratotic lesions and scaly plaques on forepaw claws and pads reminiscent of fungal infection.
Case Report:
Direct microscopy of the scrapings was indicative of a dermatophyte infection. By culture and subsequent repeated subculturing of clinical specimens on Sabouraud dextrose agar, Mycobiotic agar, and potato dextrose agar, two distinct strains with different colony morphotypes (designed as types I and II) were identified. Macroscopic and microscopic characteristics of the strains were suggestive of three different species, i.e. Microsporum canis, M. gypseum, and M. fulvum. However, partial sequencing of internal transcribed spacer (ITS) region of rDNA, translation elongation factor-1α (Tef-1α), and beta-tubulin (BT2) genes confirmed the identity of both isolates as M. gypseum. The animal was treated with a continuous terbinafine regimen (250 mg/kg) once daily for 12 weeks.
Conclusion:
To the best of our knowledge, the present report is the first confirmed case of dermatophytosis in a koala. The genetics underlying a variety of phenotypic traits in most classical dermatophyte species are unknown, and further studies are needed to understand this phenomenon.
Key Words: Dermatophytosis, Koala, Microsporum gypseum
Introduction
Dermatophytosis is one of the most familiar fungal conditions to veterinary and medical dermatologists. It represents a spectrum of cosmopolitan zoonotic infections, affecting keratinized tissues (i.e., skin, nail, and their appendages) in humans and other animals. This condition is caused by a large group of keratinophilic fungi of the genera, Trichophyton, Microsporum, and Epidermophyton [1, 2]. Despite being less frequently reported, M. gypseum is known as the most prevalent geophilic species, causing dermatophytosis in humans and many domestic and wild animals [3-5].
The koala is an iconic Australian animal that there are limited numbers of the animal in aboriginal countries zoos [6]. It was nationally listed as a ‘vulnerable’ species under the Threatened Species Conservation Act 1995 [7, 8]. Given this issue, a variety of disciplines such as detection, identification, and treatment of infectious agents, including dermatophytes, has an important role in efforts to conserve this endangered animal. Generally, superficial mycoses have been only poorly studied in koalas and as far as we know there are no reliable mycological or molecular data regarding the isolation and identification of dermatophytes in this animal. Here, we report on the isolation, phenotypic and genotypic identification of a dermatophytosis in a captivated koala.
Case Report
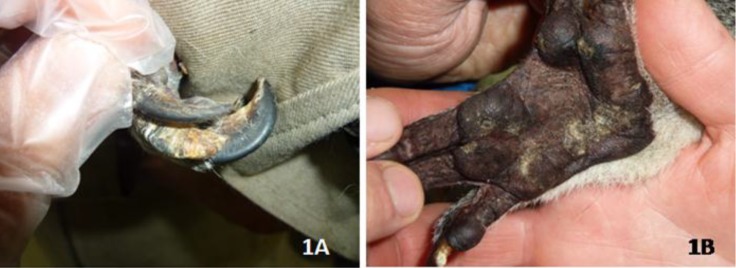
Inspection of an 11-year-old female Queensland koala (Phascolarctos cinereus), kept in a zoological park, Tokyo, Japan, revealed hyperkeratotic lesions on the third and fourth claws of right forepaw, along with white-yellowish, non-erythematous, and scaly plaques on forepaw pads, and hyperkeratosis and parakeratosis of the affected areas (Figures 1A & 1B).
Figure 1.
Hyperkeratotic lesions and scaly plaques on the claws (1A) and pad (1B) of the right forepaw of the koala
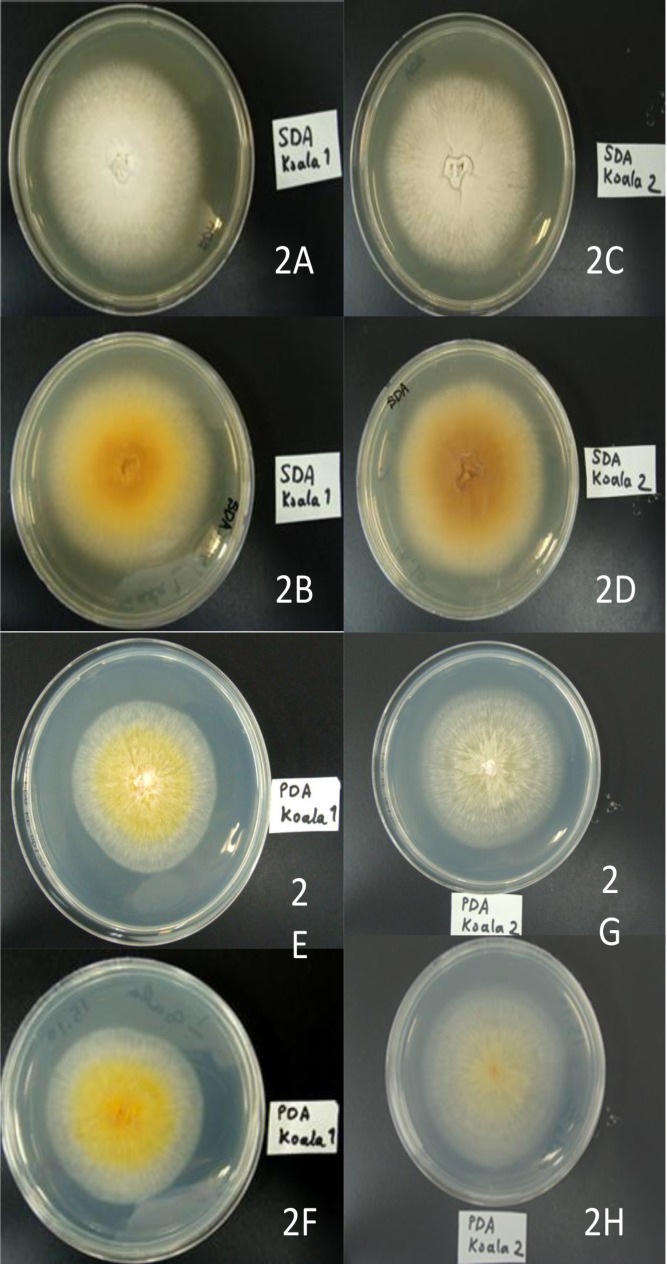
The animal was gently restrained, the affected claws and active border of the lesions of the paw were scraped off with a sterile scalpel blade, and the sample was submitted to our laboratory in Teikyo University Institute of Medical Mycology (TIMM), Tokyo, Japan, to be screened for potential fungal infection. A part of the scrapings was treated with 20% potassium hydroxide (KOH) and subjected to direct microscopy. On microscopy of a wet-mount preparation, observation of short, irregular, narrow, hyaline hyphae suggested a dermatophytic infection. The remaining sample was inoculated onto both Sabouraud dextrose agar (SDA) and Mycobiotic agar (Difco Laboratories., Detroit, MI, USA) plates, at separated points, and incubated at 28°C. Cultures became positive within a few days, and after 10 days, two distinct colonies with different morphology (designed as types I and II) were observed at the inoculated points. In order to purify the colonies and to observe the appearance in greater detail, original colonies of each morphological type were subcultured on SDA, Mycobiotic agar and potato dextrose agar (PDA) (Difco Laboratories, Detroit, MI, USA) plates and incubated at 28 °C for two weeks. During subculturing, colonies with macro-morphology similar to the wild-type isolates were formed. On SDA, the type-I colony appeared flat, outspread, slightly granular and white-colored in the center while being characterized by buff thalli in the margin, and a brownish-yellow reverse pigment was seen (Figures 2A, 2B). On PDA, the colony developed as flat, radiating, fluffy in texture, bright yellow to yellowish-orange and slightly folded in center, and buff to colorless with a fringe of submerged growth in the advancing border which made it resemble M. canis. The reverse pigment was also light yellowish (Figures 2E & 2F).
Figure 2.
The surface and reverse appearance of M. gypseum colonies (type I and II) grown on Sabouraud dextrose agar (2A, 2B, 2C, 2D) and potato dextrose agar (2E, 2F, 2G, 2H) after one week
On SDA, the type-II colony was uniformly buff-colored, raised in the center, and flat along the periphery edge with limited radial groves. It had a velvety to suede-like texture and a brownish reverse pigment (Figures 2C & 2D). On PDA, the colony consistently appeared discolored-yellow to colorless, radiating, and flat, with fluffy thalli and a pale yellow reverse pigment (Figures 2G & 2H).
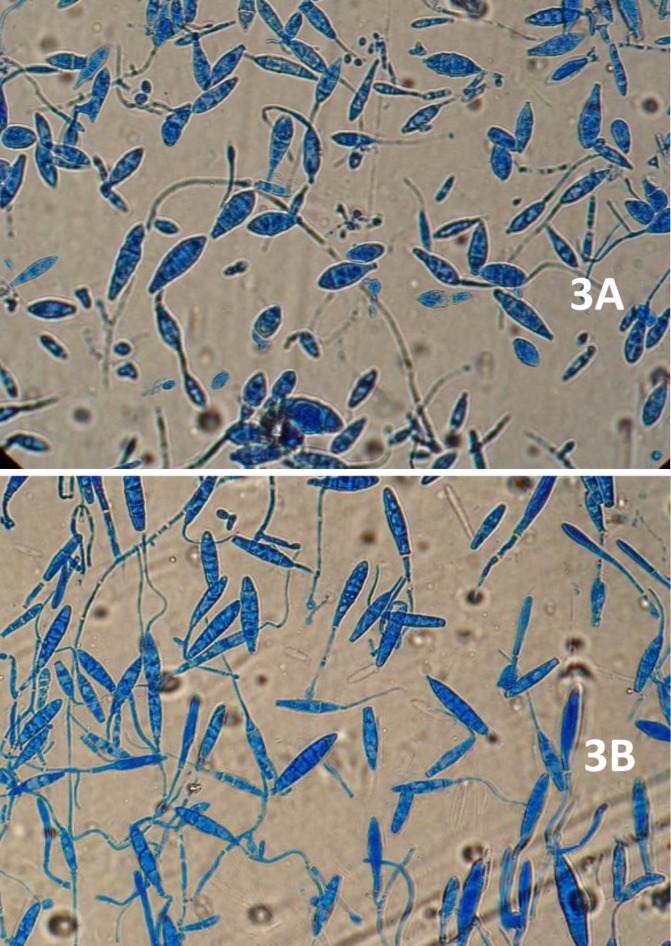
The two strains also differed in terms of microscopic configuration on both media. The teased mount preparation of morphotype-I colony was significant for numerous three- to five-celled, fusiform to cucumber-shaped macroconidia, as well as some round to clavate microconidia, characteristic of M. gypseum. Also, the distal end of most macroconidia was slightly oval to rounded, while the proximal end was almost truncate (Figure 3A).
Figure 3.
Microscopic examination of type-I (3A) and type-II (3B) colonies grown on Sabouraud dextrose agar (SDA
The microscopy of morphotype-II revealed abundant three- to six-celled, ellipsoidal, elongate, and bullet-shaped macroconidia, along with a limited number of pyriform to clavate microconidia, resembling those of M. fulvum (Figure 3B). The subculturing of both colony types was repeated twice under the same incubation conditions. The characteristics described above reappeared and could therefore be confirmed.
Cultures were fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) for 2 h at 4 °C. After being washed twice with the buffer, specimens were post-fixed with 1% osmium tetroxide in cacodylate buffer (pH 7.4) for 2 h at 4 °C. Next, the samples were dehydrated with graded acetone, freeze-dried in t-butyl alcohol, and coated with osmium tetroxide using an osmium coater, OPC 60A (Filgen, Nagoya, Japan), as described previously [9]. Colonies were examined using a JEOL JSM-7500F scanning electron microscope (JSM-7500F, JEOL, Japan), operated at 1kV. The type–I colony was characterized by abundant thick, spindle-shaped macroconidia with a rough surface (Figure 4A). Meanwhile, the type–II colony showed slender- and elliptic-shaped macroconidia with a rough surface (Figure 4B).
Figure 4.
Colonies were examined using a JEOL JSM-7500F scanning electron microscope (JSM-7500F, JEOL, Japan) at 1kV. The type–I colony was characterized by abundant, thick, and spindle-shaped macroconidia with a rough surface (4A). On the other hand, the type–II colony showed slender- and elliptic-shaped macroconidia with a rough surface (4B
To confirm the diagnosis of dermatophytosis and additionally to determine if the etiologic agents represented the same species/strain, the two isolates were subjected to PCR and sequencing by the following primer sets: The universal fungal ITS1/ITS4 primers [10] were used to amplify the DNA fragment covering the internal transcribed spacer regions (ITS1 and ITS2) and the flanking 5.8S subunit of the rRNA gene (rDNA); the pan-dermatophyte primers EF-DermF/EF-DermR [11] were used to amplify translation elongation factor1-α (Tef-1α), and the universal fungal primers T1 [12] and Bt2b [13] were used to amplify the beta tubulin (BT2) gene. Interestingly, analysis of all three loci by pairwise sequence alignment revealed that the two strains were identical (100% similarity). The ITS sequence was submitted to the open access validated CBS database for dermatophytes (www.cbs.knaw.nl/dermatophytes) and it showed 100% similarity to sequence JN134132, M. gypseum IFM 5292.
The minimal inhibitory concentrations (MICs) of the isolates towards seven antifungal agents were tested according to the Clinical and Laboratory Standard Institute (CLSI) broth microdilution (BMD) methods for filamentous fungi [14]. The MICs of amorolfine, terbinafine, itraconazole, bifonazole, ketoconazole, butenafine and micafungin were 0.25, 0.004, 1, 8, 4, 0.06, and 0.12 μg/mL for the colony type-I, and 0.25, 0.06, 1, 8, 4, 0.12, and 0.12 μg/mL for the colony type-II, respectively. The koala was treated with terbinafine, 250 mg/kg once daily for 12 weeks.
Discussion
The case presented here is peculiar in two aspects. It is the first mycologically confirmed report of dermatophytosis caused by M. gypseum in a koala. Also, gross- and micro-morphometrics of the two colony types, isolated from the same clinical specimen, differed substantially, and none of them bore resemblance to the typical morphology of M. gypseum. While the morphological features of the isolates were indicative of one or more of three different species, M. canis, M. gypseum and M. fulvum, molecular analysis confirmed the identity of both colony types as M. gypseum.
The decline in wildlife biodiversity coupled to economic incentives motivates many zoological parks to expand their conservative activities for increasing the survival chance of some endangered species [15-17]. The Koala bear, a species native to Australia only, is one of those threatened species whose health conditions, whether in captivity or free-ranging, is ethically, ecologically, and economically a major concern for conservationists and zoo directors; therefore, they should regularly be monitored for any infectious threats [17]. Dermatophytosis, which is among the few fungal communicable infections, might be one of these threats. Due to low host specificity, the infection may potentially have a vast impact on zoo animal health; hence, affected or asymptomatic animals can play as infectious reservoirs for other animals or personnel who are directly in contact with them [18]. M. gypseum has been reported as the causal agent of dermatophytosis in animals. It is usually transmitted from soil and can be secondarily transmitted by animals to humans [1]. In animals, dermatophytic lesions due to M. gypseum are most often localized in nails, pinnae, and paws [19]. This species has been cited as the etiologic agent of infection in many domestic and wild animals such as rabbit, horses, camels, pigs, wild ruminants [3], cats, dogs [20] and wild felids (ocelot) [5]. To date, dermatophytosis has been reported in two koalas held in Taronga Zoo, Sydney, Australia, solely based on veterinary histopathologic records and not mycological findings [21]. To our knowledge, this is the first molecularly proven case of dermatomycosis in a koala from which a dermatophyte species was isolated. Conventionally, verifying the presence of a dermatophyte in clinical specimens relies on the observation of hyaline hyphae and arthroconidia in KOH wet-mount or stained histological preparations; nonetheless, an additional culture in a semi-selective medium is usually required to specify the etiologic agent [1, 22]. In the current study, routine direct microscopy proved the dermatophytic infection in the koala, while the results of subsequent mycological analyses were ambiguous, casting doubt upon the identification of the fungal agent. Dermatophyte species, including M. gypseum, are known to exhibit variable and overlapping characteristics and undergo pleomorphism, the phenomenon in which a dermatophyte spontaneously loses its typical pigmentation and conidiation [1]. In clinical laboratories, such pleomorphic strains make challenges correct identification and may easily be discarded as contaminating agents.
The geophilic dermatophyte M. gypseum is the imperfect state of two teleomorphic species; Arthroderma gypseum and A. incurvatum, which are morphologically distinguishable from each other and from another dermatophyte, M. fulvum [1, 23, 24]. Generally, mature colonies of M. gypseum on Sabouraud’s glucose agar are flat, spreading, and powdery to grainy in texture, and cinnamon-buff to tawny-buff in color, usually a yellow-brown or, rarely, reddish pigment is produced on the reverse. Many strains develop a white fuzzy flocculent of mycelia in the center and, less commonly, some isolates extend a narrow white exterior margin [1, 20, 25]. However, the strains in this study developed colonies more buff in texture than powdery or granular. There is no evidence in the scientific literature of the production of a yellow pigment by M. gypseum, as developed by our strains. These macro-morphologies (Figure 2) are controversial and more suggestive of M. canis than M. gypseum. The micro-morphologic characteristics of our isolates were also arguable and shared between M. gypseum Figure 3A) and M. fulvum (Figure 3B) [25]. This phenomenon is confusing, but informatively clarified another unknown facets of dermatophytes that can convolute the identification. Importantly, we did not confine diagnostic analysis to morphology-based experiments only, and so the identity of the infectious isolate was determined as M. gypseum by ITS-sequencing, the golden diagnostic standard [23]. Further sequencing of two novel genetic markers for dermatophytes, BT2 and Tef-1α [11, 26] along with the results of antifungal susceptibility testing indicated that the two isolates belonged to the same strain of M. gypseum which concurrently exhibited two very different morphotypes. Generally, the genetic background of phenotypic variation in most classical dermatophyte species remains unknown and more studies are needed to understand this occurrence.
In conclusion, this study highlights the importance of dermatophytosis in koalas and reports M. gypseum as a causative agents. Additionally, our study reinforced some of the caveats in the diagnosis of dermatophytes, namely the multi-formity of phenotypic traits in dermatophytes, inconsistency of morphological characters, and efficacy of genomic information for reliable species delineation.
Acknowledgments
This work was financially supported by Teikyo University, Tokyo, Japan. We thank all personnel in Teikyo University Institute of Medical Mycology (TIMM). A part of this study was presented at 58th Annual Meeting of Japanese Society for Medical Mycology, 1-2 November 2014, Tokyo, Japan.
Author’s contribution
H.M. has performed the mycological and molecular experiments, Y. N. has prepared the electron microscopic photos, A.R.M. has written the main parts of the paper, K.S. has helped to collect clinical data and K.M. has supervised all steps in the study.
Conflicts of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1.Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995;8(2):240–59. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay RJ, Merz WG. Topley & Wilson’s microbiology and microbial infections. Medical mycology. Arnold: Hodder Headline; 2005. [Google Scholar]
- 3.Chermette R, Ferreiro L, Guillot J. Dermatophytoses in animals. Mycopathologia. 2008;166(5-6):385–405. doi: 10.1007/s11046-008-9102-7. [DOI] [PubMed] [Google Scholar]
- 4.Ranganathan S, Balajee SA, Raja SM. A survey of dermatophytosis in animals in Madras, India. Mycopathologia. 1997;140(3):137–40. [PubMed] [Google Scholar]
- 5.Rotstein DS, Thomas R, Helmick K, Citino SB, Taylor SK, Dunbar MR. Dermatophyte infections in free-ranging Florida panthers (Felis concolor coryi) J Zoo Wildl Med. 1999;30(2):281–4. [PubMed] [Google Scholar]
- 6.Sherwin WB, Timms P, Wilcken J, Houlden B. Analysis and conservation implications of koala genetics. Conserv Biol. 2000;14(3):639–49. [Google Scholar]
- 7.Wales NS. Threatened species conservation act 1995. Queensland, Australia: Government Printer; 1995. [Google Scholar]
- 8.Hrdina F, Gordon G. The koala and possum trade in Queensland, 1906-1936. Australian Zool . 2004;32(4):543–85. [Google Scholar]
- 9.Taguchi Y, Hasumi Y, Abe S, Nishiyama Y. The effect of cinnamaldehyde on the growth and the morphology of Candida albicans. Med Mol Morphol. 2013;46(1):8–13. doi: 10.1007/s00795-012-0001-0. [DOI] [PubMed] [Google Scholar]
- 10.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols. 1990;18(1):315–22. [Google Scholar]
- 11.Rezaei-Matehkolaei A, Makimura K, de Hoog GS, Shidfar MR, Satoh K, Najafzadeh MJ, et al. Multilocus differentiation of the related dermatophytes Microsporum canis, Microsporum ferrugineum and Microsporum audouinii. J Med Microbiol. 2012;61(Pt 1):57–63. doi: 10.1099/jmm.0.036541-0. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungusfusariumare nonorthologous. Mol Phylogenet Evol. 1997;7(1):103–16. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- 13.Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61(4):1323–30. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, CLSI M38-A. 2nd ed. Wayne PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 15.Rabb GB. The changing roles of zoological parks in conserving biological diversity. Am Zool. 1994;34(1):159–64. [Google Scholar]
- 16.Deem S. Role of the zoo veterinarian in the conservation of captive and free-ranging wildlife. Int Zoo Yearbook. 2007;41(1):3–11. [Google Scholar]
- 17.Hundloe TJ, Hamilton C. Koalas and tourism: an economic evaluation. Australia: Australian Institute; 1997. [Google Scholar]
- 18.Cafarchia C, Weigl S, Figueredo LA, Otranto D. Molecular identification and phylogenesis of dermatophytes isolated from rabbit farms and rabbit farm workers. Vet Microbiol. 2012;154(3-4):395–402. doi: 10.1016/j.vetmic.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Monga D, Mohapatra L. A compilation of published reports of mycoses in animals in India. Mycopathologia. 1980;72(1):3–11. doi: 10.1007/BF00443044. [DOI] [PubMed] [Google Scholar]
- 20.de Hoog GS, Guarro J, Gené J, Figueras MJ. Atlas of clinical fungi. 2nd ed. Netherlands: Centraalbureau voor Schimmelcultures (CBS); 2000. [Google Scholar]
- 21.Canfield P, Spencer A, Hartley W, Spielman D, Vogelnest L, Hulst F. Disorders of keratinization in a group of related, captive koalas (Phascolarctos cinereus), with a review of other skin conditions in koalas. J Zoo Wildl Med. 1992;23(4):414–21. [Google Scholar]
- 22.Binstock JM. Molecular biology techniques for identifying dermatophytes and their possible use in diagnosing onychomycosis in human toenail: a review. J Am Podiatr Med Assoc. 2007;97(2):134–44. doi: 10.7547/0970134. [DOI] [PubMed] [Google Scholar]
- 23.Nouripour-Sisakht S, Rezaei-Matehkolaei A, Abastabar M, Najafzadeh MJ, Satoh K, Ahmadi B, et al. Microsporum fulvum, an ignored pathogenic dermatophyte: a new clinical isolation from Iran. Mycopathologia. 2013;176(1-2):157–60. doi: 10.1007/s11046-013-9665-9. [DOI] [PubMed] [Google Scholar]
- 24.Gräser Y, Scott J, Summerbell R. The new species concept in dermatophytes-a polyphasic approach. Mycopathologia. 2008;166(5-6):239–56. doi: 10.1007/s11046-008-9099-y. [DOI] [PubMed] [Google Scholar]
- 25.Ellis D, Davis S, Alexiou H, Handke R, Bartley R. Descriptions of medical fungi, mycology unit, women’s and children’s hospital, and school of molecular and biomedical science, university of Adelaide. Adlelaide: University of Adelaide; 2007. [Google Scholar]
- 26.Rezaei-Matehkolaei A, Makimura K, De Hoog GS, Shidfar MR, Satoh K, Najafzadeh MJ, et al. Discrimination of Trichophyton tonsurans and Trichophyton equinum by PCR-RFLP and by β-tubulin and translation elongation factor 1-α sequencing. Med Mycol. 2012;50(7):760–4. doi: 10.3109/13693786.2012.661885. [DOI] [PubMed] [Google Scholar]