Abstract
Background and Purpose:
Fungal keratitis is a suppurative, ulcerative, and sight-threatening infection of the cornea that sometimes leads to blindness. The aims of this study were: recuperating facilities for laboratory diagnosis, determining the causative microorganisms, and comparing conventional laboratory diagnostic tools and semi-nested PCR.
Materials and Methods:
Sampling was conducted in patients with suspected fungal keratitis. Two corneal scrapings specimens, one for direct smear and culture and the other for semi- nested PCR were obtained.
Results:
Of the 40 expected cases of mycotic keratitis, calcofluor white staining showed positivity in 25%, culture in 17.5%, KOH in 10%, and semi-nested PCR in 27.5%. The sensitivities of semi-nested PCR, KOH, and CFW were 57.1%, 28.5%, and 42% while the specificities were 78.7%, 94%, and 78.7%, respectively. The time taken for PCR assay was 4 to 8 hours, whereas positive fungal cultures took at least 5 to 7 days.
Conclusion:
Due to the increasing incidence of fungal infections in people with weakened immune systems, uninformed using of topical corticosteroids and improper use of contact lens, fast diagnosis and accurate treatment of keratomycosis seems to be essential. Therefore, according to the current study, molecular methods can detect mycotic keratitis early and correctly leading to appropriate treatment.
Key Words: Diagnosis, keratitis, nested PCR
Introduction
Fungal keratitis is an ulcerative, suppurative, and sight-threatening infection of the cornea that sometimes goes to the destruc-tion of the eye. Keratitis morbidity caused by fungi is a major eye problem in developing countries [1, 2]. The incidence of fungal infections of the eye has dramatically increased in the last few years due to some reasons, involving greater distribution of immunosuppressive or broad-spectrum antibiotic therapies and enhance-ment of accuracy of laboratory diagnostic tools [3, 4]. The true rate of visual impairment resulting from this disease is thought to far exceed the reported prevalence, especially among agricultural workers in the developing countries, where a “silent epidemic” of corneal blindness has been supposed [5]. Fungal corneal infections particularly happen most frequently in individuals who work in farm [6, 7]. This infection is also linked to diabetes mellitus and the acquired immune deficiency syndrome (AIDS) [8, 9]. Worldwide, the reported incidence of mycotic keratitis varies from 17% to 36 % [10-12]. In India, the prevalence is higher than and varies from 44% to 47 % [13-16]. In contrast, fungal keratitis ordinarily accounts for only1–5% of the infectious keratitis in developed countries and temperate regions such as Britain, northern USA and Australia [17, 18]. Despite advances in diagnosis and medical treatment of keratomycosis, 15% to 27% of patients need surgical intervention such as keratoplasty, enucleation, or removal of eye content because of either failed medical treatment or advanced disease at presentation [19]. Almost 28% of the causative agent of infectious keratitis in the world is fungi, that varies from 6% to 53% mostly depending on weather conditions in each country [16, 20]. Trauma is the important predisposing factor, occurring in 40–60% of patients [21, 22]; other reported risk factors comprise previous ocular surgery, ocular surface disease, previous use of corticosteroids (either topical or systemic) and contact lens use [23-25]. This infection has affected more men than women mostly in the group 21-50 years of age [26]. Filamentous saprophytic fungi are the main causes of this disease in tropical climates, especially following trauma that can occur during harvesting of crops. Aspergillus and Fusarium species constitute 70% of reported cases [26-28]. The yeasts are the most causative agent of fungal keratitis in cool weather and it affects more frequently the patients who live cold countries [29].
For the administration of specific treatment, early detection and accurate identification of the causative agent is crucial. Conventional methods for the detection of fungal keratitis include direct microscopy examination of potassium hydroxide (KOH) preparation, calcofluor white (CFW) wet mount, Gram and Giemsa stained smears, and culture [30]. New methods for the identification of fungi, although still not widely attainable, include immuneofluorescence, electron microscopy and polymerase chain reaction (PCR). Due to apparent diagnostic failure of culture method (up to 60%) even in clinically evident cases and its time-consuming nature, initiation of appropriate treatment based on the culture results is pending [31]. The potential utility of PCR-based techniques for improving the diagnosis of corneal infections is well docum-ented and are being considered increasingly [29, 32-35].
Applying this rapid protocol laboratory results and turnaround time was improved and decision-making in the management of patients enhanced [32, 35].
This study aimed to develop semi-nested PCR for the diagnosis in patients with presumed fungal keratitis, compared with the conventional laboratory diagnostic tools, determine the causative agents, and recognize the predisposing factors.
Material and Methods
Clinical specimen collection and processing
A prospective study of fungal keratitis was performed between Decembers 2011 and March 2012. Patients who presented with clinically suspected corneal ulcer referred to the Ophthalmology Department of Boo Ali Sina (located in Sari), Labafnejad and Farabi (located in Tehran) University Hospitals, were included in this study. The study protocol was approved by the Medical Research Ethics Committee of Mazandaran University of Medical Sciences (Ethical no. 91-3-3) and all patients gave informed consent. The patients who were not willing to participate were excluded from the study. A history of trauma to the eye and specific signs of fungal keratitis include dry-looking ulcer with satellite or feathery margin, pigmentation, hypopyon formation and associated endothelial plaque were considered as including criteria. Patients suffering from bacterial and viral infections, those under treatment with antifungal drugs and patients with reciprocal infections were excluded from this study. Standard laboratory investigation of corneal scraping was adapted from previous study [19, 36, 37]. Briefly, using standard techniques, corneal scraping samples were obtained by an ophthalmologist, with a sterile surgical blade number 15, following the instillation of a local anesthetic (tetracaine hydrochloride 0.5%). The samples were divided into two aliquots and transported to the Mycology laboratory. Gram, 10% potassium hydroxide (KOH), and calcofluor white (CFW) stained slides of one portion of the sample were studied immediately under light and fluorescent microscope, respectively. Corneal scrapings were also inoculated on two Sabouraud’s dextrose agar (Scharlau, Spain) plates supplem-ented with chloramphenicol (Sigma-Aldrich, Steinheim, Germany)) in C-shaped streaks and incubated at 25°C. The culture was considered significant if the smears demonstrated morphologically similar organism, and/or if the same organism grew in more than one culture media, and/or if there was growth on at least two streaks. The other portion of samples was kept at -20°C in 250 µl of 1x magnesium-free PCR buffer (Roche Diagnostics, Mannheim, Germany) in 1.5ml vials until processed by PCR.
PCR amplification and optimization strategy
Corneal scraping samples from the patients were extracted for fungal DNA (yeast and filamentous) by the QIAmp DNA Minikit (Qiagen, Germany). The extraction was in accordance with the manufacturer’s recommendations. The PCR for fungal DNA detection was performed in two steps as described by Ferrer et al. [36].
The two universal primers ITS1 (5´ TCCGTAGGTGAACCTGCGG 3´), and ITS4 (5´ TCCTCCGCTTATTGATATGC 3´) were used to amplify according to ITS region. Amplification was performed in Bio Rad thermal cycler (Model C 1000). The initial round of amplification yielded 300-611 bp products according to different fungal species for ITS region. Cycling conditions consisted of an initial denaturation step at 94°C for 5 minutes, followed by either 35 cycles of denaturation at 94°C for 45 seconds; annealing at 52ºC for 30 seconds, and extension at 72°C for 40 seconds, PCR was completed by a final extension at 72°C for 7 minutes.
Semi-nested amplification was performed using ITS86 (5´ GTGAATCATCGAATCTTT-GAAC 3´), which amplify the 5.8S rDNA region, and ITS4 primers. Two microliters of PCR product of the first round, used as DNA template for the second round and amplification was carried out at the same PCR condition as mentioned in the first round. The amplified products were detected using 1.5 % agarose combined with 0.5 µg/ml SYBR Green Stains. The electrophoresis was performed at 90 volts and documented using Gel documentation system.
For statistical analysis between each paired tests, the McNemar test was used, and to examine the differences in categorical variables, the chi-square test was performed. For comparing these diagnostic tools, culture method was considered as the gold standard method.
Results
Forty patients met the inclusion criteria. The mean age of the patients was 53.5 (SD±18.3, range 9–87) years old. There were 25 (62.5%) males and 15 (37.5%) females. (Table 1) Out of the 40 cases presumed fungal keratitis, 19(47.5%) cases showed fungal etiology. The highest prevalence rate of fungal keratitis was identified in the patients with 40 - 90 years age group. Males (63.2%) were affected more frequently than females (36.8%).
Table 1.
Comparison between fungal keratitis (FK) and non- fungal keratitis patient's sex distribution in Boo Ali Sina, Farabi and Labbafinejad Hospitals in 2011-2012
| SEX | No. of patients positive for FK (19) |
No. of patients negative for FK (21) |
Total no. of patients (40) |
|---|---|---|---|
| Male | 12 | 13 | 25 |
| Female | 7 | 8 | 15 |
All of these patients were investigated by gram, potassium hydroxide (KOH), and calcofluor white (CFW) stains, culture and PCR. The cases with fungal keratitis had at least one positive result in one of these diagnostic methods for the presence of fungi.
Comparison of the four diagnostic methods for detection of fungal keratitis showed that 19 (47.5%) patients tested positive using all 4 methods. A total of 19 patients tested positive using CFW, semi-nested PCR, and KOH; 11 patients tested positive using semi-nested PCR alone; and 21 patients tested negative for all 4 methods. A complete summary of all results related to diagnostic tests is reported in Table 2.
Table 2.
Summary of the test results
| Number of patient | Semi-nested PCR | CULTURE | KOH-CFW | KOH |
|---|---|---|---|---|
| 21 | - | - | - | - |
| 5 | + | - | - | - |
| 5 | - | - | + | - |
| 3 | + | + | - | - |
| 2 | - | + | - | - |
| 2 | + | - | + | + |
| 1 | + | + | + | + |
| 1 | - | + | + | + |
| 0 | - | - | + | + |
| 0 | - | - | - | + |
| 0 | + | + | - | + |
| 0 | - | + | - | + |
| 0 | + | - | - | + |
| 0 | + | + | + | - |
| 0 | + | - | + | - |
| 0 | - | + | + | - |
The following steps were calculated for each test: sensitivity; specificity; positive and negative predictive values. The positive rates of fungal culture, KOH preparation, CFW and semi-nested PCR were 17.5%, 10 %, 25 %, and 27.5 %, respectively.
The sensitivities of each of the technique were as follows: KOH 28.5%; KOH+CFW 42%; and semi-nested PCR 57.1%. Semi- nested PCR methods were more sensitive than KOH preparation but the differences between semi-nested PCR to KOH-CFW in diagnosis of fungal keratitis were not significant (Table 3). The specificities were as follows: KOH 94%; CFW 78.7%; and Semi-nested PCR 78.7%. The positive predictive values calculated for the different techniques were: KOH 50%; KOH+CFW 30%; and semi-nested PCR 36.4%.In terms of negative predictive value, the results were: KOH 86.1%; KOH+CFW 86.1%; and semi-nested PCR 89% (Table 3). Filamentous fungi were isolated in 85.7% cases of fungal keratitis. Aspergillus flavus, Fusarium species and Candida glabrata were isolated from patient's samples. Aspergillus flavus was the most prevalent species. Amplification of ITS region by two initial primers ITS1 and ITS4 were yielded products ranged from 400 to 550 bp and in the second round by ITS86 and ITS4 produced 250-300 bp according to different fungal species (Figure1, 2).
Table 3.
Evaluation of the applied methods for diagnosing fungal keratitis according to culture method as the gold standard for keratitis patients
| Test | No. with positive result (N=40) | Sensitivity (%) CI* 0.95% |
Specificity (%) CI* 0.95% |
PPV (%) CI* 0.95% |
NPV (%) CI* 0.95% |
|---|---|---|---|---|---|
| semi-nested PCR | 11 (27.5%) | 57.1 (0.18-0.90) |
78.7 (0.61-0.91) |
36.4 (0.11-0.69) |
89 (0.72-0.97) |
| KOH+CFW | 10 (25%) | 42 (0.04-0.71) |
78.7 (0.61-0.91) |
30 (0.03-0.60) |
86.1 (0.66-0.94) |
| KOH | 4 (10%) | 28.5 (0.04-0.71) |
94 (0.79-0.99) |
50 (0.06-0.93) |
86.1 (0.70-0.95) |
CI: Confidence Interval
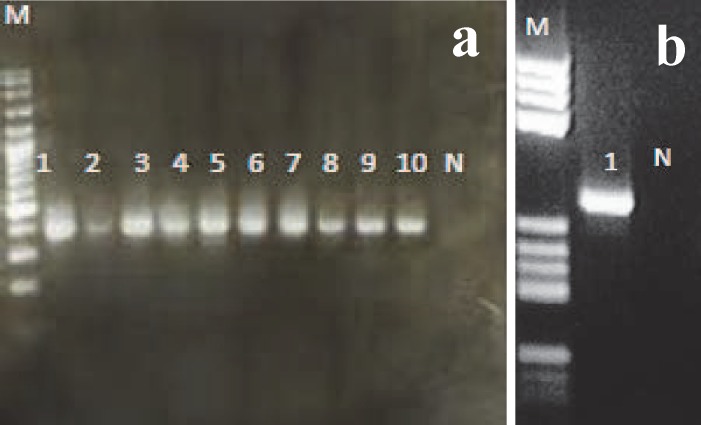
Figure 1.
a: Agarose gel electrophoresis of amplified products in the first round; Lanes 1-10) positive reaction; N) negative control; M) Ladder (100bp)
b: Agarose gel electrophoresis of amplified products in the second round PCR (Semi-nested PCR) –lane 1) positive reaction; N) negative control; M) Ladder (50 bp)
The most common predisposing factor in patients with fungal keratitis was trauma (seven patients; 36%) followed by antibiotic use (three patients; 16%), ocular surgery (three patients; 16%), diabetes (two patients; 11%) had and another had local corneal disease (persistent corneal defect and stromal ulceration), one patient (5%) use contact lens. Five of seven patients with trauma (71.4%) had history of trauma with plant debris. Of the 19 patients with fungal keratitis, six (32%) patients were farmers, five (26%) housekeepers, two (11%) laborers and two (11%) unemployed. Although there was no significant difference between occupation and the disease (P>0.5).
All patients had the symptoms of eye pain, 95% of patients had decreased vision, 89% of patients had foreign body sensation, and redness and watering indicated in 84% of patients and photophobia were found in 47% of patients.
Discussion
Fungal keratitis is considered as the main cause of blindness and even eye enucleation. According to this study, the frequency of fungal keratitis was 47.5% among individuals with presumed fungal keratitis. A published report on the prevalence indicated that it varied between 6 to 50% of the cases with corneal ulcer [37].
Fungal keratitis has been reported predominantly in men (63%) mostly in middle- aged. This is confirmed by previous studies all over the world [38-42]. Overall, not only in Iran but also all over the world, men are more likely to get fungal keratitis because of outdoor activity and greater chance of exposing to ocular trauma especially with vegetative material which is more likely to carry fungi. We observed all the patients except for one who was affected during the first six months of year probably due to high relative humidity and temperature. Fungal keratitis is strongly associated with hot climate so that in Asia it is considered as one of the important eye disease. [11, 17, 43-46].
The frequency is reported to be higher in tropical countries. Apart from a hot and humid atmosphere, agricultural-based livelihood in this region and outdoor occupations also make the population more exposed to fungal infections [19, 38-41, 47, 48]. In present study, 36% of patients with fungal keratitis had ocular trauma in which 71.4% caused by plant debris. In our study, 32% of the patients were farmers. It seems fungal keratitis is a work-related disease. Corneal trauma especially with plants debris during agricultural activities is reported as the potential risk factor for keratitis due to filamentous fungi. This finding is confirmed by previous studies in Iran, India, Indonesia, Brazil and Vietnam [15, 29, 37, 40]. A frequency of 33% to 100% has been described in the literature for mycotic keratitis in patients with corneal trauma by an organic foreign body [10, 49]. In some other reports, 8.3% to 17.6% of patients with fungal keratitis had corneal trauma, which is lower than our report [19, 50]. The fewer number of patients with fungal keratitis and corneal trauma could be illustrated by the fact that trauma might be unconscious or as a result of delay existing between the incident of trauma and its diagnosis, causing them difficult to recall [19, 40-42, 44, 45, 47, 51]. Another reported predisposing factor is the previous antibiotic use which was present in 16% of our patients .In developed countries the crucial main factors are an increase in using contact lenses and various eye surgeries which are rare in non-developing countries. In this study, previous eye surgeries in 16%, diabetes in 11%, using ophthalmic antibiotic in 10% and systematic antibiotic in 5% of our patients were seen. This information is referring the difference between the risk factors involved in developed and developing countries and the different routes of entry of the fungi. It is necessary to consider the difference of modernization in developed and developing countries. As in developing countries, animal and crop husbandry are done manually; thus, work-related eye injuries are more likely to occur. Lack of access to medical facility and self-treatment is another common risk factor in developing countries [49, 51]. A rise in the incidence of fungal keratitis in most of the developed countries can be resulted from the rising number of immunocompromised patients, use of topical corticosteroids and antibacterial, and the availability of laboratory diagnostic tests and the medical facilities.
Regarding the systematic disease, 2 patients were suffering from diabetes (11%) which is confirmed by previous studies [19].
There are different diagnostic laboratory methods for the diagnosis of fungal keratitis perform traditionally such as direct microscopy examination and culture. For patient management, using an accurate and immediate diagnostic test is necessary. The former method is easy and rapid and the latter is time-consuming and both of them have some false positive and negative results.
Due to their shortcomings, new diagnostic methods have been incorporated. Currently, molecular diagnosis of fungal keratitis based on detection of fungal DNA in corneal scraping by PCR has become more popular and replaced with the old methods. The accuracy and high speed are the main advantages of this method. In our study, 10% of the samples had positive result in potassium hydroxide (KOH) preparation and 17.5% of the samples in KOH + CFW. Fungal culture was positive in 17.5% of the samples and 27.5% of the samples were associated with positive PCR test.
Sensitivity, specificity, positive and negative predictive values of applied methods in this study with culture as golden standard, for semi-nested PCR is 57.1%, 78.7%, 36.4%, 89% and for KOH+CFW is 42% ,78.7%, 30%, 86.1% while for KOH is 28.5% ,94%, 50%, 86.1%, respectively. These results are confirmed by the study of Sujith and Bagyalakshmi [52, 53].
As the diagnosis of fungal keratitis is considered a big challenge for experts, researchers focused on the best method for the diagnose of fungal keratitis. Recently, some researchers have believed that PCR is a promising tool can be as a useful method adjunct to KOH smear and culture in rapid and accurate diagnosis of fungal keratitis [32, 36, 54-58]. Direct microscopy examination of corneal scraping in KOH+CFW smear provides an immediate and reliable presumptive diagnosis [2, 19, 49, 51, 59, 60]. The culture method has a few limitations as a gold standard. Thus, according to the present and our previous studies [19], KOH+CFW can be used in the first step of laboratory diagnosis in patients with presumed fungal keratitis [2, 19, 40, 47, 51, 60]. However, molecular methods are needed for an accurate diagnosis because KOH+CFW could only speed up the process of diagnosing but it is not enough [36, 55-58]. A few studies have suggested the use of PCR for the accurate identification of causative fungi. The rapid presumptive diagnosis of fungal keratitis based on clinical features are reported up to 83% of cases, but it is not included to yeast fungi due to similarities in clinical manifestations with bacterial keratitis [61]. In current study, Aspergillus flavus, Fusarium species and Candida glabrata were isolated from corneal scraping of patients with presumed fungal keratitis. Approximately 70 fungus genera have already been reported as causative agents of fungal keratitis [62]. Other studies in Iran reported Aspergillus and Fusarium to be the most common fungal pathogens [46, 63, 64].
Acknowledgements
This study is funded by the Vice- Chancellor of Research of Mazandaran Universityof Medical Sciences which we gratefullly acknowledge. We would like to thank Dr. Seyed-Ali Tabatabaee in Eye Research Center– Farabi Hospital, to Dr Farid Karimianin of the Department of Ophthalmology Labbafinejad Medical Center for his critical support in referring the patients and to Miss Shahin Maddani for technical assistance.
Authors’ contributions
T.S. designed and managed the research and editing of the draft manuscript, I.H. managed the research and wrote the draft, F.A. obtained the specimen and performed all tests and wrote the draft. ND.K. referred the patients and edited the final manuscript.
Conflicts of Interest:
The authors state no conflict of interest.
Financial Disclosure
No financial interests related to the material of this manuscript have been declared.
References
- 1.Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care centre in south India. Cornea. 2002;21(6):555–9. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Chowdhary A, Singh K. Spectrum of fungal keratitis in north India. Cornea. 2005;24(1):8–15. doi: 10.1097/01.ico.0000126435.25751.20. [DOI] [PubMed] [Google Scholar]
- 3.Klotz SA, Penn CC, Negvesky GJ, Butrus SI. Fungal and parasitic infections of the eye. Clin Microbiol Rev. 2000;13(4):662–85. doi: 10.1128/cmr.13.4.662-685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003;16(4):730–97. doi: 10.1128/CMR.16.4.730-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcher JP, Srinivasan M. Corneal ulceration in the developing world–a silent epidemic. Br J Ophthalmol. 1997;81(4):622–3. doi: 10.1136/bjo.81.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshpande SD, Koppikar GV. A study of mycotic keratitis in Mumbai. Indian J Pathol Microbiol. 1999;42(1):81–7. [PubMed] [Google Scholar]
- 7.Johnson GJ, Minassian DC, Weale R. The epidemiology of eye disease. 1nd ed. New York: Chapman and Hall Medical; 1998. [Google Scholar]
- 8.Ormerod LD, Hertzmark E, Gomez DS, Stabiner RG, Schanzlin Dj, Smith RE. Epidemiology of microbial keratitis in southern California A multivariate analysis. Ophthalmology. 1987;94(10):1322–33. doi: 10.1016/s0161-6420(87)80019-2. [DOI] [PubMed] [Google Scholar]
- 9.Mselle J. Fungal keratitis as an Indicator of HIV infection in Africa. Trop Doct. 1999;29(3):133–5. doi: 10.1177/004947559902900303. [DOI] [PubMed] [Google Scholar]
- 10.Rosa RH Jr, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in South Florida. Ophthalmology. 1994;101(6):1005–13. doi: 10.1016/s0161-6420(94)31225-5. [DOI] [PubMed] [Google Scholar]
- 11.Upadhyay MP, Karmacharya PC, Koirala S, Tuladhar NR, Bryan LE, Smolin G, et al. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol. 1991;111(1):92–9. doi: 10.1016/s0002-9394(14)76903-x. [DOI] [PubMed] [Google Scholar]
- 12.Katz NN, Wadud SA, Ayazuddin M. Corneal ulcer disease in Bangladesh. Ann Ophthalmol. 1983;15(9):834–6. [PubMed] [Google Scholar]
- 13.Dunlop AA, Wright ED, Howlader SA, Nazrul I, Husain R, McClellan K, et al. Suppurative corneal ulceration in Bangladesh A study of 142 cases examining the microbiological diagnosis, clinical diagnosis, and epidemiological features of bacterial and fungal keratitis. Aust N Z J Ophthalmol. 1994;22(2):105–10. doi: 10.1111/j.1442-9071.1994.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 14.Polack FM, Kaufman HE, Newmark E. Keratomycosis Medical and surgical treatment. Arch Ophthalmol. 1971;85(4):410–6. doi: 10.1001/archopht.1971.00990050412003. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81(11):965–71. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas PA. Mycotic keratitis an underestimated mycosis. J Med Vet Mycol. 1994;32(4):235–56. doi: 10.1080/02681219480000321. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15(4):321–7. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Bhartiya P, Daniell M, Constantinou M, Islam FM, Taylor HR. Fungal keratitis in Melbourne. Clin Experiment Ophthalmol. 2007;35(2):124–30. doi: 10.1111/j.1442-9071.2006.01405.x. [DOI] [PubMed] [Google Scholar]
- 19.Shokohi T, Nowroozpoor-Dailami K, Moaddel-Haghighi T. Fungal keratitis in patients with corneal ulcer in sari, Northern Iran. Arch Iran Med. 2006;9(3):222–7. [PubMed] [Google Scholar]
- 20.Srinivasan R, Kanungo R, Goyal JL. Spectrum of oculomycosis in South India. Acta Ophthalmol (Copenh) 1991;69(6):744–9. doi: 10.1111/j.1755-3768.1991.tb02053.x. [DOI] [PubMed] [Google Scholar]
- 21.Nath R, Baruah S, Saikia L, Devi B, Borthakur AK, Mahanta J. Mycotic corneal ulcers in upper Assam. Indian J Ophthalmol. 2011;59(5):367–71. doi: 10.4103/0301-4738.83613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009;57(4):273–9. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keay LJ, Gower EW, Iovieno A, Oechsler RA, Alfonso EC, Matoba A, et al. Clinical and microbiological characteristics of fungal keratitis in the United States, 2001–2007: a multicenter study. Ophthalmology. 2011;118(5):920–6. doi: 10.1016/j.ophtha.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmar P, Salman A, Kalavathy CM, Kaliamurthy J, Thomas PA, Jesudasan CA. Microbial keratitis at extremes of age. Cornea. 2006;25(2):153–8. doi: 10.1097/01.ico.0000167881.78513.d9. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan T, Constantinou M, Jhanji V, Vajpayee RB. Factors affecting treatment outcomes with voriconazole in cases with fungal keratitis. Cornea. 2013;32(4):445–9. doi: 10.1097/ICO.0b013e318254a41b. [DOI] [PubMed] [Google Scholar]
- 26.Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis A three-year study. Indian J ophthalmol. 2003;51(4):315–21. [PubMed] [Google Scholar]
- 27.Agarwal V, Biswas J, Madhavan HN, Mangat G, Reddy MK, Saini JS, et al. Current perspectives in infectious keratitis. Indian J Ophthalmol. 1994;42(4):171–92. [PubMed] [Google Scholar]
- 28.Insan NG, Mane V, Chaudhary BL, Danu MS, Yadav A, Srivastava V. A review of fungal keratitis: etiology and laboratory diagnosis. Int J Curr Microbiol App Sci. 2013;2(6):307–14. [Google Scholar]
- 29.de Andrade AJM, Vieira LA, Lima ALH, Yu MCZ, Gompertz OF, Souza LB. Laboratorial analyses of fungal keratitis in a University Service. Arq Bras Oftal. 2000;63(1):59–63. [Google Scholar]
- 30.Vengayil S, Panda A, Satpathy G, Nayak N, Ghose S, Patanaik D, et al. Polymerase Chain Reaction–Guided Diagnosis of Mycotic Keratitis: a prospective evaluation of Its efficacy and limitations. Invest Ophthalmol Vis Sci. 2009;50(1):152–6. doi: 10.1167/iovs.07-1283. [DOI] [PubMed] [Google Scholar]
- 31.Wahl JC, Katz HR, Abrams DA. Infectious keratitis in Baltimore. Ann Ophthalmol. 1991;23(6):234–7. [PubMed] [Google Scholar]
- 32.Gaudio PA, Gopinathan U, Sangwan V, Hughes TE. Polymerase chain reaction based detection of fungi in infected corneas. Br J Ophthalmol. 2002;86(7):755–60. doi: 10.1136/bjo.86.7.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anand A, Madhavan H, Neelam V, Lily T. Use of polymerase chain reaction in the diagnosis of fungal endophthalmitis. Ophthalmology. 2001;108(2):326–30. doi: 10.1016/s0161-6420(00)00517-0. [DOI] [PubMed] [Google Scholar]
- 34.Nejabat M, Khalili MR, Badiei P, Keshavarz Fazl F, Alborzi A, Shadmani A. Polymerase Chain Reaction versus Conventional Laboratory Methods in the Diagnosis of Fungal Keratitis. Bina J Ophthalmol. 2009;15(1):63–7. [Google Scholar]
- 35.Jaeger EE, Carroll NM, Choudhury S, Dunlop AA, Towler HM, Matheson MM, et al. Rapid detection and identification of Candida, Aspergillus, and Fusarium species in ocular samples using nested PCR. J Clin Microbiol. 2000;38(8):2902–8. doi: 10.1128/jcm.38.8.2902-2908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrer C, Jorge L. Evaluation of molecular diagnosis in fungal keratitis Ten years of experience. J Ophthalmic Inflamm infec. 2011;1(1):15–22. doi: 10.1007/s12348-011-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg P, Gopinathan U, Choudhary K, Rao GN. Keratomycosis: clinical and microbiologic experience with dematiaceous fungi. Ophthalmology. 2000;107(3):574–80. doi: 10.1016/s0161-6420(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 38.Galarreta DJ, Tuft SJ, Ramsay A, Dart JK. Fungal keratitis in London: microbiological and clinical evaluation. Cornea. 2007;26(9):1082–6. doi: 10.1097/ICO.0b013e318142bff3. [DOI] [PubMed] [Google Scholar]
- 39.Kanavi MR, Javadi M, Yazdani S, Mirdehghanm S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea. 2007;26(7):782–6. doi: 10.1097/ICO.0b013e318064582d. [DOI] [PubMed] [Google Scholar]
- 40.Badiee P, Nejabat M, Alborzi A, Keshavarz F, Shakiba E. Comparative study of Gram stain, potassium hydroxide smear, culture and nested PCR in the diagnosis of fungal keratitis. Ophthalmic. 2010;44(4):251–6. doi: 10.1159/000313988. [DOI] [PubMed] [Google Scholar]
- 41.Furlanetto RL, Andreo EG, Finotti IG, Arcieri ES, Ferreira MA, Rocha FJ. Epidemiology and etiologic diagnosis of infectious keratitis in Uberlandia, Brazil. Eur J Ophthalmol. 2010;20(3):498–503. doi: 10.1177/112067211002000312. [DOI] [PubMed] [Google Scholar]
- 42.Nhung PH, Thu TA, Ngoc LH, Ohkusu K, Ezaki T. Epidemiology of Fungal Keratitis in North Vietnam. J Clin Exp Ophthalmol. 2012;3:238. [Google Scholar]
- 43.Jacqueline K Ng, Fraunfelder FW, Winthrop KL. Review and Update on the Epidemiology, Clinical Presentation, Diagnosis, and Treatment of Fungal Keratitis. Curr Fungal Infect Rep. 2013;7(4):293–300. [Google Scholar]
- 44.Varshokar K. Survey on fungal flora of conjectival sac and keratomycosis in Tehran hospitals [MSc Thesis] Tehran Uni Med Sci: Sch of Pub health; 1991. [Google Scholar]
- 45.Behboodi H, Mohammadi MJ, Forghanparast K. Epidemiology of infectious keratitis inpatients of Tootoonkaran Hospital, Rasht (1997–1998) Bina. 2001;7(1):3–9. [Google Scholar]
- 46.Mirshahi A, Ojaghi H, Aghashahi D, Jabbarvand M. Fungal keratitis in patients at Farabi Hospital, Tehran (1998–1999) Bina. 1999;5(2):135–143. [Google Scholar]
- 47.Punia RS, Kundu R, Chander J, Arya SK, Handa U, Mohan H. Spectrum of fungal keratitis: clinicopathologic study of 44 cases. Int J ophthalmol. 2014;7(1):114–7. doi: 10.3980/j.issn.2222-3959.2014.01.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amatya R, Shrestha S, Khanal B, Gurung R, Poudyal N, Bhattacharya SK, et al. Etiological agents of corneal ulcer: five years prospective study in eastern Nepal. Nepal Med Coll J. 2012;14(3):219–22. [PubMed] [Google Scholar]
- 49.Alfonso EC, Rosa RH. Fungal keratitis. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea and External Disease: Clinical Diagnosis and Management. St Louis Mosby; 1997. pp. 1253–66. [Google Scholar]
- 50.Tanure MA, Cohen EJ, Sudesh S, Rapuano CJ, Laibson PR. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000;19(3):307–12. doi: 10.1097/00003226-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Tilak R, Singh A, Maurya OP, Chandra A, Tilak V, Gulati AK. Mycotic keratitis in India: a five-year retrospective study. J Infect Dev Ctries. 2010;4(3):171–4. doi: 10.3855/jidc.309. [DOI] [PubMed] [Google Scholar]
- 52.Vengayil S, Panda A, Satpathy G, Nayak N, Ghose S, Patanaik D, et al. Polymerase chain reaction-guided diagnosis of mycotic keratitis: a prospective evaluation of its efficacy and limitations. Invest Ophthalmol Vis Sci. 2009;50(1):152–6. doi: 10.1167/iovs.07-1283. [DOI] [PubMed] [Google Scholar]
- 53.Bagyalakshmi R, Therese KL, Madhavan HN. Application of semi-nested polymerase chain reaction targeting internal transcribed spacer region for rapid detection of panfungal genome directly from ocular specimens. Indian J ophthalmol. 2007;55(4):261–5. doi: 10.4103/0301-4738.33037. [DOI] [PubMed] [Google Scholar]
- 54.Itahashi M, Higaki S, Fukuda M, Shimomura Y. Detection and quantification of pathogenic bacteria and fungi using real-time polymerase chain reaction by cycling probe in patients with corneal ulcer. Arch Ophthalmol. 2010;128(5):535–40. doi: 10.1001/archophthalmol.2010.66. [DOI] [PubMed] [Google Scholar]
- 55.Mancini N, Perotti M, Ossi CM, Cavallero A, Matuska S, Paganoni G, et al. Rapid molecular identification of fungal pathogens in corneal samples from suspected keratomycosis cases. J Med Microbiol. 2006;55(11):1505–9. doi: 10.1099/jmm.0.46638-0. [DOI] [PubMed] [Google Scholar]
- 56.Eleinen KG, Mohalhal AA, Elmekawy HE, Abdulbaki AM, Sherif AM, El-Sherif RH, et al. Polymerase chain reaction-guided diagnosis of infective keratitis - a hospital-based study. Curr Eye Res. 2012;37(11):1005–11. doi: 10.3109/02713683.2012.698357. [DOI] [PubMed] [Google Scholar]
- 57.Embong Z, Wan Hitam WH, Yean CY, Rashid NH, Kamarudin B, Abidin SK, et al. Specific detection of fungal pathogens by 18S rRNA gene PCR in microbial keratitis. BMC Ophthalmol. 2008;8:7. doi: 10.1186/1471-2415-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tananuvat N, Salakthuantee K, Vanittanakom N, Pongpom M, Ausayakhun S. Prospective comparison between conventional microbial work-up vs PCR in the diagnosis of fungal keratitis. Eye (Lond) 2012;26(10):1337–43. doi: 10.1038/eye.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rautaraya B, Savitri S, Kar S, Das S, Sahu SK. Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern India. BMC ophthalmol. 2011;11:39. doi: 10.1186/1471-2415-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Yang H, Jiang L, Han L, Wang L. Use of potassium hydroxide, Giemsa and calcofluor white staining techniques in the microscopic evaluation of corneal scrapings for diagnosis of fungal keratitis. J Int Med Res. 2010;38(6):1961–7. doi: 10.1177/147323001003800609. [DOI] [PubMed] [Google Scholar]
- 61.Thomas PA, Leck AK, Myatt M. Characteristic clinical features as an aid to the diagnosis of suppurative keratitis caused by filamentous fungi. Br J Ophthalmol. 2005;89(12):1554–8. doi: 10.1136/bjo.2005.076315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones DB. Diagnosis and management of fungal keratitis. In: Tasman W, Jaeger EA, editors. Duane’s Clinical Ophthalmology. Philadelphia: JB Lippincott; 1993. [Google Scholar]
- 63.Javadi MA, Hemati R, Mohammadi MM, Farsi A, Karimian F, Einolahi B, et al. Causes of fungal keratitis and its management: review of 23 cases from Labbafinejad Medical Center (LMC) Bina. 1996;2:38–54. [Google Scholar]
- 64.Berenji F, Elahi SR, Fata AM. Fungal keratitis in patients at Imam Reza Hospital, Mashhad (1982 – 2001) Med J Mashhad Univ Med Sci. 2003;45(78):49–54. [Google Scholar]