Abstract
Background and Purpose:
Microorganism-based synthesis of nanostructures has recently been noted as a green method for the sustainable development of nanotechnology. Nowadays, there have been numerous studies on the emerging resistant pathogenic bacteria and fungal isolates, the probable inability of bacteria and fungi to develop resistance against silver nanoparticles’ (SNPs) antibacterial, antifungal, antiviral and, particularly antibacterial activities. In this study, we aim to use the yeast Saccharomyces cerevisiae model for synthesis of SNPs and to investigate its antifungal activity against some isolates of Candida albicans.
Materials and Methods:
A standard strain of S. cerevisiae was grown in liquid medium containing mineral salt; then, it was exposed to 2 mM AgNO3. The reduction of Ag+ ions to metal nanoparticles was virtually investigated by tracing the color of the solution, which turned into reddish-brown after 72 hours. Further characterization of synthesized SNPs was performed afterwards. In addition, antifungal activity of synthesized SNPs was evaluated against fluconazole-susceptible and fluconazole-resistant isolates of Candida albicans.
Results:
The UV-vis spectra demonstrated a broad peak centering at 410 nm, which is associated with the particle sizes much less than 70 nm. The results of TEM demonstrated fairly uniform, spherical and small in size particles with almost 83.6% ranging between 5 and 20 nm. The zeta potential of SNPs was negative and equal to -25.0 (minus 25) mv suggesting that there was not much aggregation. Silver nanoparticles synthesized by S. cerevisiae, showed antifungal activity against fluconazole-susceptible and fluconazole-resistant Candida albicans isolates, and exhibited MIC90 values of 2 and 4 μg/ml, respectively.
Conclusion:
The yeast S. cerevisiae model demonstrated the potential for extracellular synthesis of fairly monodisperse silver nanoparticles.
Key Words: Biosynthesis, Saccharomyces cerevisiae, Metal nanoparticles
Introduction
Since classic physicochemical synthetic procedures of nanoparticles suffer from drawbacks such as polydispersity, low-yield and use of toxic reagents [1, 2], the need to develop eco-friendly and non-toxic approaches for biosynthesis of nanomaterials and nano-structures has newly been recognized in the world of modern nanoscience [3].
To meet this need, microorganism-based synthesis of nanostructures has recently been established as a green method for the sustainable development of nanotechnology [4]. In the last decades, scientific research on silver nanoparticles (Ag-NPs/ SNPs) has drawn considerable attention in the field of nanoscience, since the unique physical, chemical, optical and biological properties of SNPs, make them suitable for various chemical, medical and industrial applications [5].
Recently, Ag-NPs have been applied in electrical and solar batteries (as optical receptors in the later) [6, 7], biolabelling [8], as well as in cancer treatment [9]. Nowadays, the emerging resistant pathogenic bacteria and fungal isolates and the probable inability of bacteria, as well as fungi to develop resistance against antibacterial, antifungal, antiviral and particularly, antibacterial performances of Ag-NPs are being intensely studied [10-12].
Accordingly, silver based nanomaterials have established their status in medicine (catheters, implants and prostheses) [13, 14], and have been applied to improve commercial products (e.g., textiles and deodorants) [15-17]. There are numerous reports on the formation of various magnetic nanoparticles (MNPs) by microorganisms from their corresponding salts [18, 19]. Although SNPs are synthesized both intra- and extracellularly, extracellular method of biosynthesis is more advantageous due to ease of control over the environment, large-scale synthesis and straightforward processing steps [3, 20].
A large number of microorganisms are able to synthesize Ag-NPs extracellularly, the most important of which are: Fusarium oxysporum [21], Bacillus licheniformis [22], Aspergillus fu-migatus [23], Aspergillus niger [24], Aspergillus clavatus [25], Penicillium brevicompactum [26], Escherichia coli [27], Cladosporium cladospo-rioides [28], Fuserium semitectum [28] and Klebsiella pneumoniae [29].
Nevertheless, they are well-known as opportunistic and sometimes real pathogen microorganisms, which can be excluded from the list of advantageous microorganisms. The yeast Saccharomyces cerevisiae, known as “baker’s yeast”, can be found naturally in many environmental niches.
S. cerevisiae is most commonly famous for its role in either the traditional or industrial fermentation of bread, beer or wine [30]. It can also be used as a nutritional supplement and an agent to treat antibiotic-related diarrhea [31]. In the last few years, cases of diagnosed S. cerevisiae infections have increased, which might be due to an upturn in the number of immunocompromised patients and the development in diagnostic methods. However, S. cerevisiae has typically been considered as a nonpathogenic and safe organism [30].
In the present study, we used the ability of this yeast in synthesis of fine and mono-dispersed SNPs, which exhibited high antifungal activity against some fluconazole-susceptible, as well as fluconazole-resistant strains of Candida albicans.
Material and Methods
Yeast strain
A standard strain of S. cerevisiae (PTCC 5052) was used in this study. The yeast was preserved and cultured in yeast-peptone-dextrose medium (YPD) (2% peptone, 1% yeast extract and 2% dextrose) and then, it was incubated at 30ºC for 9-10 hours.
Synthesis of the silver nanoparticles
A YPD broth medium containing mineral salts (KH2PO4 7.0 g/l, K2HPO4 2.0 g/l, MgSO4.7H2O 0.1 g/l, (NH4)2SO4 1.0 g/l) was used to have high efficiency in yeast growth, as well as developing a biomass competent for SNP biosynthesis. The constituents were mixed completely by stirring with a magnetic stirrer, and then the solution was boiled to make undissolved digests. Eventually, the solution was passed through filter paper.
The yeast was cultured in a 50 ml-falcon tube containing 20 ml of the mentioned medium and incubated at 30ºC for 9-10 hours (OD600: 2). Silver nitrate (AgNO3, 2 mM in the final concentration) was added and then incubated aerobically on an orbital shaker at 25◦C, agitating at 150 rpm for 24 hours in a dark place. A negative control, (only yeast culture, without the silver ion) was also run along with the experimental flask. After 24 hours of incubation, cell-free filtrate was obtained by centrifuging the medium at 5000×g.
Characterization of synthesized SNPs
Aliquots of 1 ml of the cell-free filtrate were subjected to UV-vis spectroscopy (Labomed, Inc. USA). The absorbance was measured at a resolution of 1 nm. The shape and estimated size of the silver nanoparticles were determined by transmission electron microscopy (TEM). The TEM image of the sample was obtained using transmission electron microscope (Philips 30ML20, the Netherlands) at a voltage of 100 kv. In addition, particle sizing experiments, as well as the zeta potential measurements were carried out by means of Zetasizer Nano ZS (Malvern Instruments, Southborough, UK).
Evaluation of antifungal activity of the synt-hesized SNPs
In order to assess the antimicrobial effect of the synthesized SNPs, standard strains of Candida albicans ATCC 10261 in addition to 10 fluconazole susceptible and 10 fluconazole-resistant strains of Candida albicans were applied. The isolates were kept at -80°C as 20% glycerol stocks and were sub-cultured as required, on SDA plates and were incubated at 30°C for 24 hours.
Antifungal susceptibility testing was performed according to the broth microdilution method described in the Clinical and Laboratory Standards Institute (CLSI) document M27-A3 (32) for SNPs. Fluconazole was used as a reference antifungal agent. RPMI 1640 medium with L-glutamine and phenol red without bicarbonate were employed. The medium was buffered to pH 7.0 with 0.165 mol/L MOPS (3-(N-morpholino) propanesul-fonic acid). Fluconazole was dissolved in sterile deionized water and diluted to 128μg/ml, then rediluted to the final concentration of 0.0125-16 μg/ml with the medium, according to the standards delineated in the CLSI reference method. The 24-hour-old yeasts were re-suspended in the culture medium to prepare a working solution at a concentration of 2.5×103 cells/mL.
The prepared yeast solution was exposed to diluted fluconazole (0.0125-64 μg/L) and incubated at 35°C for 48 hours, moreover, all tests were carried out in duplicate. The interpretive criteria for susceptibility to fluconazole mentioned in the CLSI (32) are as follows: 1) susceptible≤8 μg/mL, 2) susceptible-dose dependent (S-DD) (MIC=16-32 μg/mL), 3) resistant (MIC≥64 μg/ml) isolates. For SNPs, a solution of 64 μg/ml was prepared and diluted to the final concentration of 0.0125–16 μg/ml with the medium. C. parapsilosis (ATCC 22019) was used with every new series of MIC plates as quality controls. The same inoculum size was applied for the SNP susceptibility test, and the 96-well plate was incubated at 35°C for 48 hours.
Results
Silver reduction
After adding Ag+ ions to the yeast culture in the dark, with increasing the intensity during incubation, samples gradually changed color from nearly colorless to reddish-brown. The reduction of the Ag+ ions to metal nanoparticles was virtually investigated. It was observed that the color of the solution changed into intense reddish-brown after 72 hours of incubation, which is presented in Figure 1a. However, the control sample (without silver ions) showed no change in color when incubated under the same environmental conditions (Figure 1b). The appearance of a reddish-brown color in the reaction suggested the formation of SNPs. The solution remained hydrosol, and no precipitation was observed even after 24 hours of incubation.
Figure 1.
The supernatant of cell culture of Saccharomyces cerrvisiae with silver ion (2 mM): (a) at the beginning of the reaction (b) after 24h of reaction
UV–visible spectroscopy
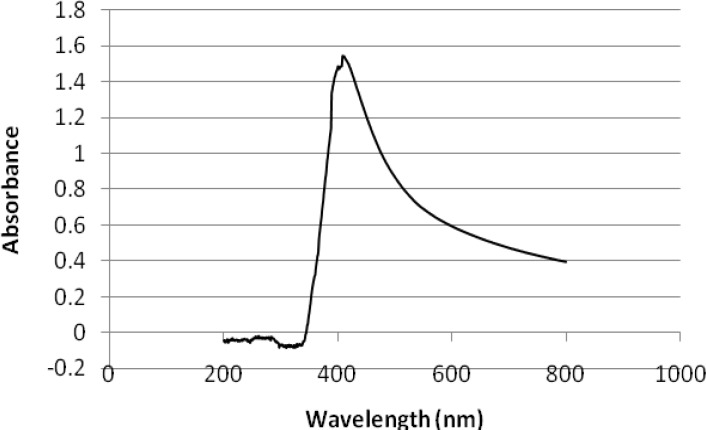
Formation of colloidal SNPs can be simply followed by changes of UV-Vis absorption (Figure 2). The UV spectroscopy method can be applied for size measurement of SNPS, based on localized surface plasmon resonance band exhibiting at different wavelengths. The light absorption pattern of the cell filtrate was continuously monitored after 24 hours in the range of 200-800 nm, using UV-visible spectrophotometer. The average wavelength at which the peak occurred was around 410 nm. Observation of this peak, which is assigned to a surface plasmon, is well-documented for silver metal nanoparticles with sizes much less than 70 nm [39] (Figure 2).
Figure 2.
UV-visible spectrum of medium containing silver ion (24 h
Characterization of the synthesized SNPs
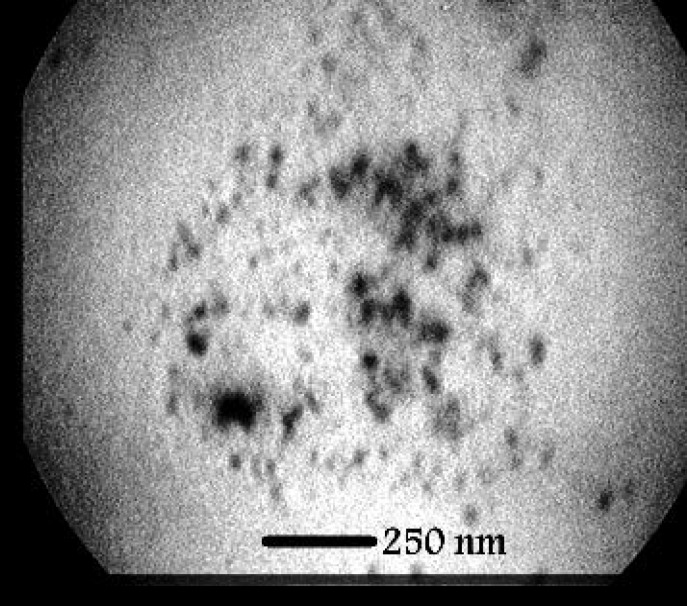
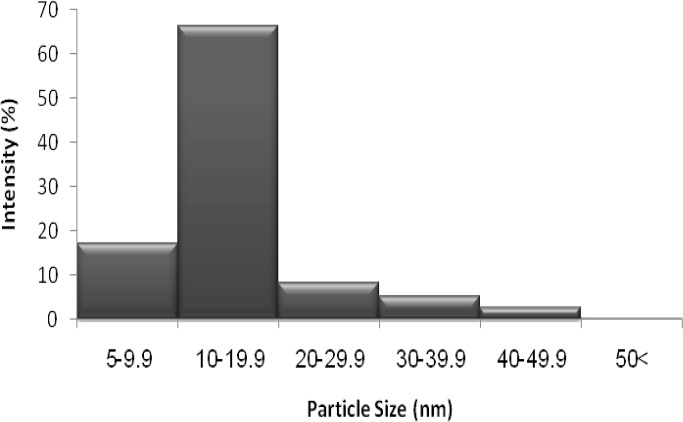
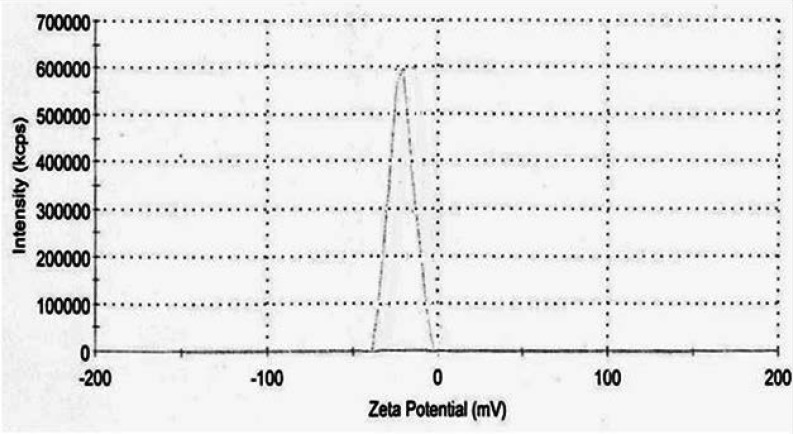
TEM analysis was employed to determine the morphology and shape of the silver nanoparticles. The representative TEM image is indicated in Figure 3. Accordingly, the SNPs were relatively uniform in diameter and in spherical shape. Additionally, TEM images depicted that the particles were predominantly formed at even less than 50 nm. The size of SNP histograms determined from Zetasizer Nano ZS is shown in figure 4. It is demonstrated that the particles were produced in various different sizes (though less than 50 nm). Considering the histogram, there was variation in the particle sizes with more than 83.6% of the particles in 5-20 nm range, and16.2% of the particles were formed in 20-50 nm. Only 0.2% of the particles were larger than 50 nm. The zeta potential of SNPs was also investigated as shown in Figure 5. Under natural conditions (pH close to 8), the zeta potential was negative and equal to -25.0 mv. The obtained results had the required quality and indicated small amounts of aggregation.
Figure 3.
TEM micrograph of silver particles synthesized by S. cerevisiae (scale bar: 250 nm
Figure 4.
The particle size distribution histogram of silver nanoparticles synthesized by A. parasiticus
Figure 5.
Zeta potentioal of silver nanoparticles synthesized by S. cerevisiae
Evaluation of antifungal activity of the synthesized SNPs
In this study, fluconazole, an antifungal agent which is widely used to treat Candida-associated infections, was used as a drug control. SNPs synthesized by A. parasiticus showed antifungal activity against both fluconazole-suseptible and resistant strains and exhibited MIC range value of 2-4 μg/ml. Apparently, it was a notable finding for fluconazole-resistant C. albicans strains, as well as for non-resistant ones. Table 1 shows the detailed results of susceptibility assay.
Table 1.
The inhibitory effect of synthesized silver nanopaeticle on C. albicans isolates
| Isolates | MIC (µg/ml) |
||
|---|---|---|---|
| Ag NPs £ | Fluconazole | ||
| Fluconazole-susceptible isolates |
Ca S1* | 4 | 0.5 |
| Ca S2 | 2 | 0.125 | |
| Ca S3 | 2 | 1 | |
| Ca S4 | 2 | 0.5 | |
| Ca S5 | 4 | 1 | |
| Fluconazole-resistant isolates |
Ca R1** | 4 | ≥64 |
| Ca R2 | 4 | ≥64 | |
| Ca R3 | 2 | ≥64 | |
| Ca R4 | 2 | ≥64 | |
| Ca R5 | 4 | ≥64 | |
Candida albicans susceptible strain;
Candida albicans resistant strain;
Silver nanoparticles
Discussion
The Ag-NPs can be synthesized either through chemical or biological methods. Biosynthesis of Ag-NPs by means of microorganisms has recently drawn considerable attention as a result of being eco-friendly, as well as its higher and faster production with lower costs (32-36). Other associated advantages have been observed in different functional groups conjugated with the surface of Ag-NPs making it suitable for various biomedical applications (9, 37).
In this study, we used the industrial yeast, S. cerevisiae, to biosynthesize monodispersed and more important stable Ag-NPs. Although the production of SNPs by this microorganism has previously been reported, antifungal effect of the synthesized nanoparticles have not been documented yet [38]. Physical confirmations of the production of silver metals were followed by virtual and technical assignments. The intensity of the color of yeast culture increased due to excitation of surface plasmon vibrations in the metal nanoparticles. This significant observation indicates that the reduction of the Ag+ ions takes place extracellulary. The production and stability of the reduced SNPs in the colloidal solution were investigated using UV–vis spectral, as well as zeta potential analysis.
The unique optical properties of metal nanoparticles originate from the collective oscillations of conduction electrons, which are termed surface plasmon polariton resonances (SPPR) when excited by electromagnetic radiation (39, 40). This characteristic of nanoparticles can be determined using a UV-vis spectrophotometer. In the present study, the UV–visible spectra demonstrated nm arrow spectrum with a peak at 410 nm after 24 and 72 hours of incubation confirming that the synthesized nanoparticles are supposed to be fairly uniform and spherical in shape and much smaller than 70 nm (according to the fact that long maximum wavelengths (450 nm) represent the particle sizes beyond 70 nm). Additionally, the size, uniformity and stability of our Ag-NPs were confirmed by TEM and Zetasizer studies.
Numerous studies have used Ag-NPs to induce apoptosis in yeast cells and tumor cell lines (41, 42). Moreover, many studies have claimed the antifungal and antibacterial properties of Ag-NPs [43, 44]. In this study, we studied the antifungal effect of Ag-NPs, which was synthesized by the yeast S. cerevisiae, on fluconazole-susceptible and also resistant isolates of C. albicans.
Some studies have reported the anti-Candida activity of Ag-NPs, but so far, few researchers have used the microdillution method to evaluate the exact minimum inhibitory concentration of Ag-NPs against C. albicans. The sensitivity of C. albicans was evaluated using the well diffusion method by Mallmann EJ, in 2015. Accordingly, the inhibitory concentration of Ag-NPs was reported to be 80 µg/ml [45]. However other studies have demonstrated that only 2 µg/ml of Ag-NPs was able to induce death by apoptosis in C. albicans cells [46].
It is believed that the antimicrobial effect of silver nanoparticles is dependent on particle morphology and surface characteristics (47, 48). For this reason, it is mandatory to synthesize nanoparticles with size, shape and surface modifications, appropriate for use in the biological applications. Our findings demonstrated the MIC range value of 2-4 µg/ml for Ag-NPs against both fluconazole-suseptible and resistant strains of C. albicans.
Although there was no significant difference in MICs for the two Candida groups, MIC values obtained for the resistant strains were outstanding. No citations regarding resistance to metallic Ag nanoparticles have been retrieved so far. However, resistance to ionic silver originating from the ability of bacteria to reduce Ag+ to less toxic oxidation state or from active efflux of Ag+ from the cell was reported [49]. The rapid synthesis of nanoparticles would be proper for developing a biological process for large scale production.
Furthermore, the extracellular synthesis would make it simpler and easier to follow the processes. Moreover, extracellular biosynthesis of SNPs offers a great advantage over an intracellular process in terms of application. In a nutshell, the yeast S. cerevisiae has successfully demonstrated the potential for extracellular synthesis of fairly monodisperse tiny SNPs.
Authors’ Contributions
F.N. and M.M. designed and managed the study. M.M. performed all the tests and prepared the draft manuscript. M.N. proofread the final manuscript. R.DG. provided susceptible Candida strains.
Conflicts of Interest:
The authors state no conflict of interest.
Financial Disclosure
No financial interests declared.
References
- 1.Kowlgi K, Lafont U, Rappolt M, Koper G. Uniform metal nanoparticles produced at high yield in dense microemulsions. J Colloid Interf Sci. 2012;372(1):16–23. doi: 10.1016/j.jcis.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6(2):257–62. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Moazeni M, Shahverdi AR, Nabili M, Noorbakhsh F, Rezaie S. Green synthesis of silver nanoparticles: The reasons for and against Aspergillus parasiticus. Nanomed J. 2014;1(4):267–75. [Google Scholar]
- 4.Liu L, Liu T, Tade M, Wang S, Li X, Liu S. Less is more, greener microbial synthesis of silver nanoparticles. Enzyme Microb Tech. 2014;67:53–8. doi: 10.1016/j.enzmictec.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol lett. 2008;176(1):1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Klaus-Joerger T, Joerger R, Olsson E, Granqvist C. Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science. Trends Biotechnol. 2001;19(1):15–20. doi: 10.1016/s0167-7799(00)01514-6. [DOI] [PubMed] [Google Scholar]
- 7.Schultz S, Smith DR, Mock JJ, Schultz DA. Single-target molecule detection with nonbleaching multicolor optical immunolabels. Proc Natl Acad Sci U S A. 2000;97(3):996–1001. doi: 10.1073/pnas.97.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu S, Ji X, Xu W, Li X, Wang L, Bai Y, et al. Immunoassay using probe-labelling immunogold nanoparticles with silver staining enhancement via surface-enhanced Raman scattering. Analyst. 2004;129(1):63–8. doi: 10.1039/b313094k. [DOI] [PubMed] [Google Scholar]
- 9.Gopinath P, Gogoi SK, Sanpui P, Paul A, Chattopadhyay A, Ghosh SS. Signaling gene cascade in silver nanoparticle induced apoptosis. Colloids Surf B Biointerfaces. 2010;77(2):240–5. doi: 10.1016/j.colsurfb.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Rupp ME, Fitzgerald T, Marion N, Helget V, Puumala S, Anderson JR, et al. Effect of silver-coated urinary catheters: efficacy, cost-effectiveness, and antimicrobial resistance. Am J Infect Control. 2004;32(8):445–50. doi: 10.1016/j.ajic.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Samuel U, Guggenbichler JP. Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. Int J Antimicrob Agents. 2004;23(Suppl 1):S75–8. doi: 10.1016/j.ijantimicag.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Devasconcellos P, Bose S, Beyenal H, Bandyopadhyay A, Zirkle LG. Antimicrobial particulate silver coatings on stainless steel implants for fracture management. Mater Sci Eng C Mater Biol Appl. 2012;32(5):1112–20. doi: 10.1016/j.msec.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens KN, Croes S, Boersma RS, Stobberingh EE, van der Marel C, van der Veen FH, et al. Hydrophilic surface coatings with embedded biocidal silver nanoparticles and sodium heparin for central venous catheters. Biomaterials. 2011;32(5):1264–9. doi: 10.1016/j.biomaterials.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Secinti KD, Özalp H, Attar A, Sargon MF. Nanoparticle silver ion coatings inhibit biofilm formation on titanium implants. J Clin Neurosci. 2011;18(3):391–5. doi: 10.1016/j.jocn.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Perelshtein I, Ruderman Y, Perkas N, Beddow J, Singh G, Vinatoru M, et al. The sonochemical coating of cotton withstands 65 washing cycles at hospital washing standards and retains its antibacterial properties. Cellulose. 2013;20(3):1215–21. [Google Scholar]
- 16.Sataev MS, Koshkarbaeva ST, Tleuova AB, Perni S, Aidarova SB, Prokopovich P. Novel process for coating textile materials with silver to prepare antimicrobial fabrics. Colloids Surf A Physicochem Eng Asp. 2014;442:146–51. [Google Scholar]
- 17.Zahran MK, Ahmed HB, El-Rafie M. Surface modification of cotton fabrics for antibacterial application by coating with AgNPs–alginate composite. Carbohyd polym. 2014;108:145–52. doi: 10.1016/j.carbpol.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Nair B, Pradeep T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des. 2002;2(4):293–8. [Google Scholar]
- 19.Kowshik M, Deshmukh N, Vogel W, Urban J, Kulkarni SK, Paknikar KM. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnolo Bioeng. 2002;78(5):583–8. doi: 10.1002/bit.10233. [DOI] [PubMed] [Google Scholar]
- 20.Kowshik M, Ashtaputre S, Kharrazi S, Vogel W, Urban J, Kulkarni SK, et al. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2003;14(1):95. [Google Scholar]
- 21.Anil Kumar S, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, Ahmad A, et al. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnolo Lett. 2007;29(3):439–45. doi: 10.1007/s10529-006-9256-7. [DOI] [PubMed] [Google Scholar]
- 22.Kalimuthu K, Suresh Babu R, Venkataraman D, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 2008;65(1):150–3. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Bhainsa KC, D'souza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B Biointerfaces. 2006;47(2):160–4. doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Gade AK, Bonde P, Ingle AP, Marcato PD, Duran N, Rai MK. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bio. 2008;2(3):243–7. [Google Scholar]
- 25.Verma VC, Kharwar RN, Gange AC. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine (Lond) 2010;5(1):33–40. doi: 10.2217/nnm.09.77. [DOI] [PubMed] [Google Scholar]
- 26.Shaligram NS, Bule M, Bhambure R, Singhal RS, Singh SK, Szakacs G, et al. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Proc Biochem. 2009;44(8):939–43. [Google Scholar]
- 27.Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SR, Muniyandi J, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces. 2009;74(1):328–35. doi: 10.1016/j.colsurfb.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Balaji DS, Basavaraja S, Deshpande R, Mahesh DB, Prabhakar B, Venkataraman A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B Biointerfaces. 2009;68(1):88–92. doi: 10.1016/j.colsurfb.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Shahverdi AR, Minaeian S, Shahverdi HR, Jamalifar H, Nohi AA. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Proc Biochem. 2007;42(5):919–23. [Google Scholar]
- 30.Pérez-Torrado R, Llopis S, Perrone B, Gómez-Pastor R, Hube B, Querol A. Comparative genomic analysis reveals a critical role of de novo nucleotide biosynthesis for Saccharomyces cerevisiae virulence. PloS one. 2015;10(3):e0122382. doi: 10.1371/journal.pone.0122382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eng RH, Drehmel R, Smith SM, Goldstein EJ. Saccharomyces cerevisiae infections in man. Sabouraudia. 1984;22(5):403–7. [PubMed] [Google Scholar]
- 32.Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incoronato N, et al. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine. 2013;8:4303–14. doi: 10.2147/IJN.S50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcato PD, Nakasato G, Brocchi M, Melo PS, Huber SC, Ferreira IR, et al. Biogenic silver nanoparticles: antibacterial and cytotoxicity applied to textile fabrics. J Nano Res. 2012;20:69–76. [Google Scholar]
- 34.Singh R, Wagh P, Wadhwani S, Gaidhani S, Kumbhar A, Bellare J, et al. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int J Nanomedicine. 2013;8:4277–90. doi: 10.2147/IJN.S48913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar RR, Priyadharsani KP, Thamaraiselvi K. Mycogenic synthesis of silver nanoparticles by the Japanese environmental isolate Aspergillus tamarii. J Nanoparticle Res. 2012;14(5):1–7. [Google Scholar]
- 36.Rodrigues AG, Ping LY, Marcato PD, Alves OL, Silva MC, Ruiz RC, et al. Biogenic antimicrobial silver nanoparticles produced by fungi. Appl Microbiol Biotechnol. 2013;97(2):775–82. doi: 10.1007/s00253-012-4209-7. [DOI] [PubMed] [Google Scholar]
- 37.Durán N, Marcato PD, Durán M, Yadav A, Gade A, Rai M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl Microbiol Biotechnol. 2011;90(5):1609–24. doi: 10.1007/s00253-011-3249-8. [DOI] [PubMed] [Google Scholar]
- 38.Korbekandi H, Mohseni S, Mardani Jouneghani R, Pourhossein M, Iravani S. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif Cells Nanomed Biotechnol. 2014;7:1–5. doi: 10.3109/21691401.2014.937870. [DOI] [PubMed] [Google Scholar]
- 39.Schmoll HJ, Kollmannsberger C, Metzner B, Hartmann JT, Schleucher N, Schöffski P, et al. Long-term results of first-line sequential high-dose etoposide, ifosfamide, and cisplatin chemotherapy plus autologous stem cell support for patients with advanced metastatic germ cell cancer: an extended phase I/II study of the German Testicular Cancer Study Group. J Clin Oncol. 2003;21(22):4083–91. doi: 10.1200/JCO.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 40.Evanoff DD, Chumanov G. Synthesis and optical properties of silver nanoparticles and arrays. Chemphyschem. 2005;6(7):1221–31. doi: 10.1002/cphc.200500113. [DOI] [PubMed] [Google Scholar]
- 41.Gurunathan S, Raman J, Abd Malek SN, John PA, Vikineswary S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: a potential cytotoxic agent against breast cancer cells. Int J Nanomedicine. 2013;8:4399–413. doi: 10.2147/IJN.S51881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hekmat A, Saboury AA, Divsalar A. The effects of silver nanoparticles and doxorubicin combination on DNA structure and its antiproliferative effect against T47D and MCF7 cell lines. J Biomed Nanotechnol. 2012;8(6):968–82. doi: 10.1166/jbn.2012.1451. [DOI] [PubMed] [Google Scholar]
- 43.DurÁn N, Marcato PD, Ingle A, Gade A, Rai M. Fungi-mediated synthesis of silver nanoparticles: characterization processes and applications. Netherlands: Progress in Mycology, Springer; 2010:425–49. [Google Scholar]
- 44.Gade A, Ingle A, Whiteley C, Rai M. Mycogenic metal nanoparticles: progress and applications. Biotechnol Lett. 2010;32(5):593–600. doi: 10.1007/s10529-009-0197-9. [DOI] [PubMed] [Google Scholar]
- 45.Mallmann EJ, Cunha FA, Castro BN, Maciel AM, Menezes EA, Fechine PB. Antifungal activity of silver nanoparticles obtained by green synthesis. Rev Inst Med Trop Sao Paulo. 2015;57(2):165–7. doi: 10.1590/S0036-46652015000200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang IS, Lee J, Hwang JH, Kim KJ, Lee DG. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012;279(7):1327–38. doi: 10.1111/j.1742-4658.2012.08527.x. [DOI] [PubMed] [Google Scholar]
- 47.Nateghi MR, Hajimirzababa H. Effect of silver nanoparticles morphologies on antimicrobial properties of cotton fabrics. J Text Inst. 2014;105(8):806–13. [Google Scholar]
- 48.Khurana C, Vala AK, Andhariya N, Pandey OP, Chudasama B. Antibacterial activity of silver: the role of hydrodynamic particle size at nanoscale. J Biomed Mater Res A. 2014;102(10):3361–8. doi: 10.1002/jbm.a.35005. [DOI] [PubMed] [Google Scholar]
- 49.Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev. 2003;27(2-3):313–39. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]