Abstract
Purpose
To assess for an association between conjunctival infection with nonchlamydial bacterial species and the presence of trachomatous scarring (TS) in women in central Tanzania.
Methods
Cross-sectional data were collected from a random sample of women ages 18 and older in 47 trachoma-endemic communities in Kongwa, Tanzania. Each participant completed a survey, provided a conjunctival swab sample, and received an ocular exam to assess for TS. Biologic samples were cultured for bacterial growth and speciation. Contingency tables were used to assess the associations between TS and bacterial carriage.
Results
Complete data was provided by 3882 women (80.7% of invitees). Of all samples, 14% resulted in a positive bacterial isolate. There was no association between TS and nonchlamydial bacterial carriage, whether assessed by species, pathogenicity, or in aggregate. There was a significant association between increasing age and TS severity, but not between age and bacterial carriage. No Corynebacterium was found in the swabs.
Conclusions
This study found no association between TS and nonchlamydial ocular infections, although associations with Corynebacterium cannot be ruled out.
Keywords: trachoma, scarring, women, infection
Trachoma is a contagious ocular infection with Chlamydia trachomatis that causes conjunctival inflammation. As a result, repeated bouts can lead to conjunctival scarring, trichiasis (introversion of the eyelashes), corneal opacity, and irreversible blindness. Trachoma is the leading infectious cause of blindness worldwide. Although once a global ailment, today trachoma is restricted primarily to low-resource areas of Africa, Asia, and the Middle East.1
The World Health Organization (WHO) estimates suggest a roughly 70% decline in active trachoma over 20 years: from 146 million cases in 1991 to 40.6 million in 2008.2–4 This decrease is likely attributable to successful control strategies, socioeconomic development in endemic nations, and more accurate data.5
However, trichiasis has declined at a less rapid rate. The same WHO estimates indicated a decrease in trichiasis of less than 20% over the same timespan: from 10 million cases in 1991 to 8.2 million cases in 2008.2–4 This more tempered decline in trichiasis relative to considerable decreases in active trachoma poses questions about the progression of scarring in trachoma and the development of trichiasis. It is likely that the less rapid decline is partially due to a cohort effect, wherein trichiasis cases in adults today reflect the burden of active trachoma from that population's childhood. As such, trichiasis cases may well decrease in future years with continued progress in control of active trachoma.
However, there is concern that nonchlamydial bacterial infections may also contribute to scarring in trachomatous areas, either independently or in combination with chlamydial infections. For example, seasonal bouts of nonchlamydial conjunctivitis together with trachoma were reported in Egypt and were felt to exacerbate progression to trachomatous scarring (TS). If such infections contribute to scarring, this may help explain the slower decline in the prevalence of trichiasis relative to trachoma and may have significant implications for control strategies. This cross-sectional study aims to investigate the relationship between nonchlamydial bacterial infections and TS in women in central Tanzania.
Methods
Ethical Statement
This study complied fully with the Declaration of Helsinki and all participants provided informed, written consent. The Johns Hopkins institutional review board and the National Institute of Medical Research of the United Republic of Tanzania approved this study.
Population
Data were collected from a random sample of women ages 18 and older in 47 trachoma-endemic communities in Kongwa, Tanzania; one community was excluded from the study due to sample contamination in the field. The district had three rounds of annual mass drug administration (MDA) with azithromycin, with at least 70% coverage of adults, and the last round was at least a year prior to the survey for this study. Following a complete census of each community, every woman aged 18 years or older was assigned a random number, and the first 100 random numbers were selected for participation. An additional five women were chosen at random as potential replacements for nonparticipants. Participation included completion of a survey on socioeconomic status indicators and exposure to cooking fire, an ocular exam, provision of a biological sample, and having photographs of the tarsal conjunctiva taken to assess for scarring. Data collection took place at a central site within the participants' community.
Clinical Examination
Ocular examinations for trachoma were conducted and included ocular photographs of the upper right tarsal conjunctiva to assess for trachomatous scarring. Digital photographs were taken (D40; Nikon, Tokyo, Japan) and sent to Johns Hopkins Hospital for grading of scarring severity using a validated, four-point scale developed by Wolle et al.6 The images were graded for scarring at ×5 magnification, by comparison with standards also assessed at ×5 magnification. Two graders assessed images independently, and discrepancies were adjudicated with a senior grader, in open sessions. Before starting the project, interobserver agreement between the graders was carried out based on 60 images from a separate dataset. Each grader independently graded these and each grader must have had a kappa of greater than 0.70 relative to the senior grader before being allowed to grade. Graders were masked to all other participant data and to the grades from the other graders throughout the grading process.
Sample Collection/Transport and Analysis
A conjunctival swab was collected from the left eye of each patient using strict sterile techniques. Cultures were collected by everting the lower eyelid and passing a sterile Dacron swab (Fisher Scientific, Pittsburgh, PA, USA) across the inferior fornix, then everting the upper lid, and swabbing the superior fornix. The specimen collector and assistant used new gloves for each specimen collected. The swab was then placed into a tube of skim milk/transport medium, capped immediately, labeled, and placed in a cooler containing ice packs. We also took “air swabs,” swabs passed within 6 inches of the participants everted eye lid, on a 5% sample of participants to check for field contamination. These swabs were labeled and processed like all other true specimens, and the laboratory was masked to their identifity. All samples were frozen within 6 hours and transported in bulk to Muhimbili National Hospital's microbiology laboratory in Dar es Salaam, Tanzania for processing.
Bacterial Culture and Speciation
All conjunctival swab specimens were inoculated on sheep blood agar, McConkey agar, and Chocolate agar base (OXOID Ltd., Hampshire, England, UK) and incubated at 37° under 5% carbon dioxide for 24 hours. Those with no growth within 24 hours were further reincubated and examined after 48 hours. Identification of all organisms isolated was first done using colony morphology characteristics as observed on culture media, then further identified using reaction to gram stain and using standard biochemical tests, interpreted as recommended by the manufacturers. All procedures followed the Clinical Laboratory Standard Institute (CLSI) guidelines for culture and sensitivity testing.
The technician was masked to all participants' clinical information. Isolates were categorized as commensal or pathologic according to the methodology in Cavallos et al.7
Statistical Methods
Descriptive statistics were used to characterize the study population, including proportions and means with accompanying and SDs. Contingency tables were used to examine the relationship between presence/severity of scars with bacterial infection or bacterial isolates. To test for independence among groups the χ2 or the Fisher's exact test of association was used as appropriate. Trends were evaluated using the Cochran-Mantel-Haenszel statistic. All analyses were run using SAS 9.2 software (SAS Institute, Cary, NC, USA).
Results
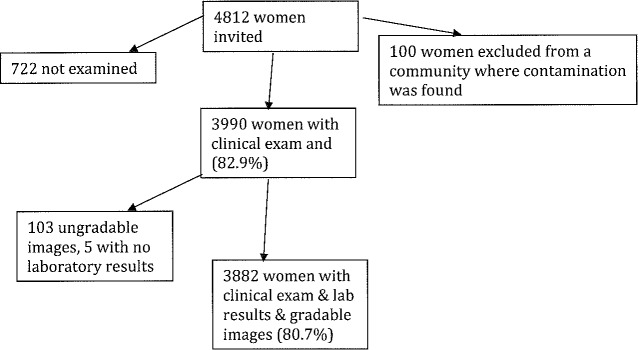
From 48 trachoma-endemic communities in Kongwa, Tanzania, 4812 women were invited to participate in this study. In one community, we found evidence of field contamination from the air swabs, and excluded all 100 women as the samples were suspect. There were 3882 women who participated by completing a survey, receiving an ocular exam, providing eye-swab samples, and having photographs of their tarsal conjunctiva taken. Of these, 108 participants had ungradable photographs and five had no laboratory results (Fig.).
Figure.
Participant inclusion flow chart.
There were no statistically significant differences in markers of socioeconomic status between participants and nonparticipants (Table 1). There was a strong relationship between increasing age and scar severity (P < 0.001) (Table 2). Only 5% of those under age 21 had moderate to severe scarring, whereas 49% of those age over 50 had moderate to severe scarring. Conversely, 80% of those under age 20 had no evidence of scarring, whereas less than one-third of those over age 50 had no evidence of scarring.
Table 1.
Participant Characteristics by Inclusion in the Study
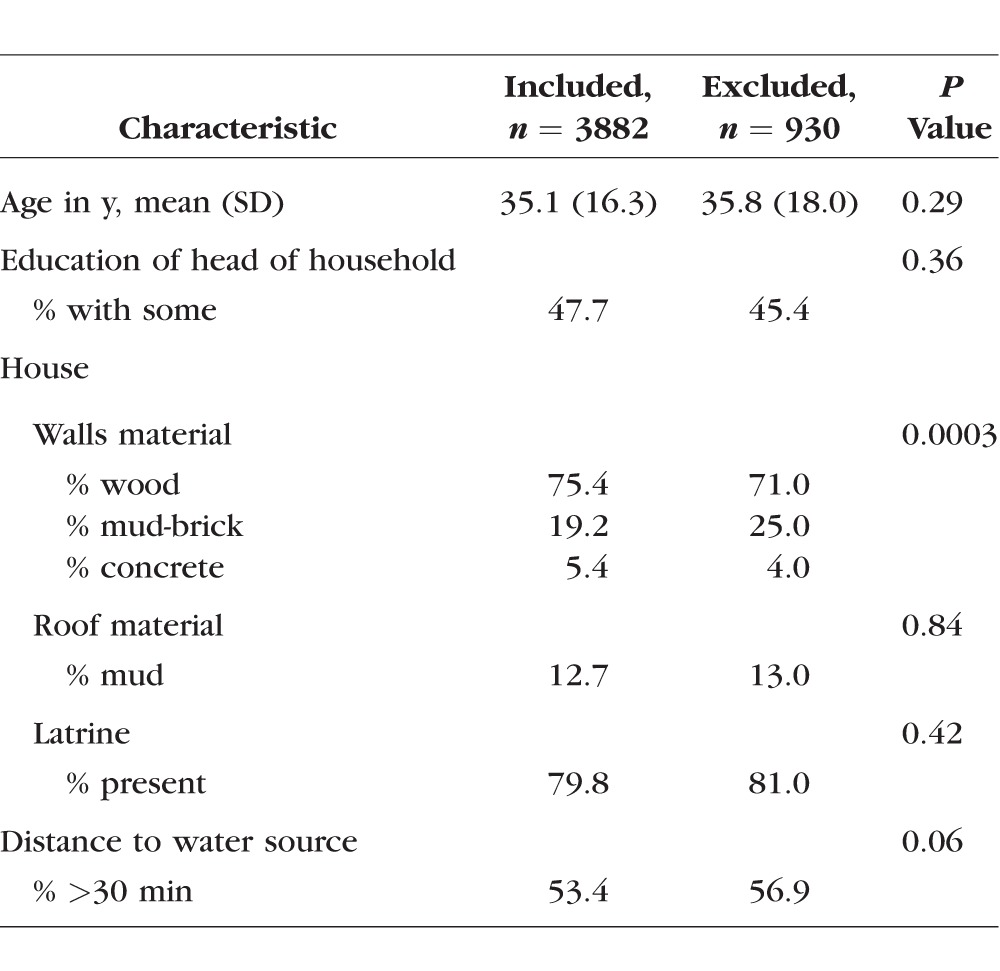
Table 2.
Percentage of Each Age Group With Scarring of Different Severities
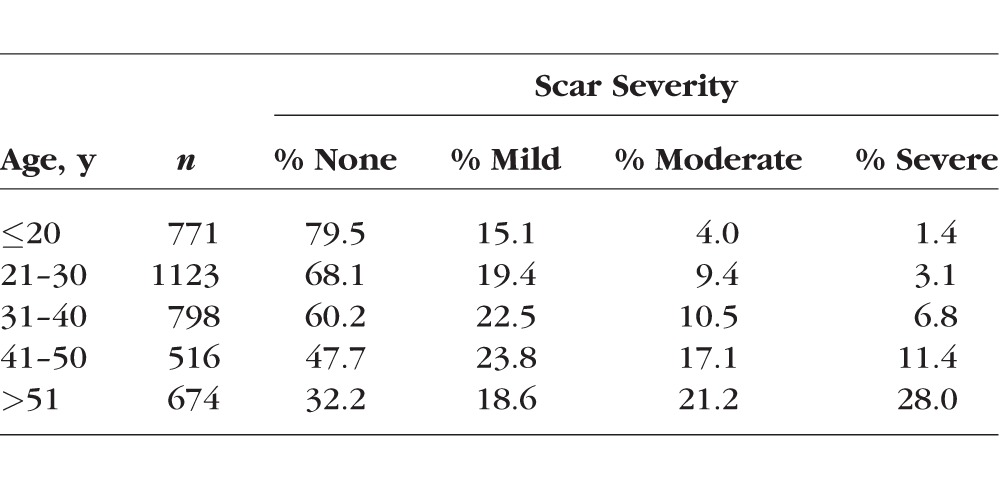
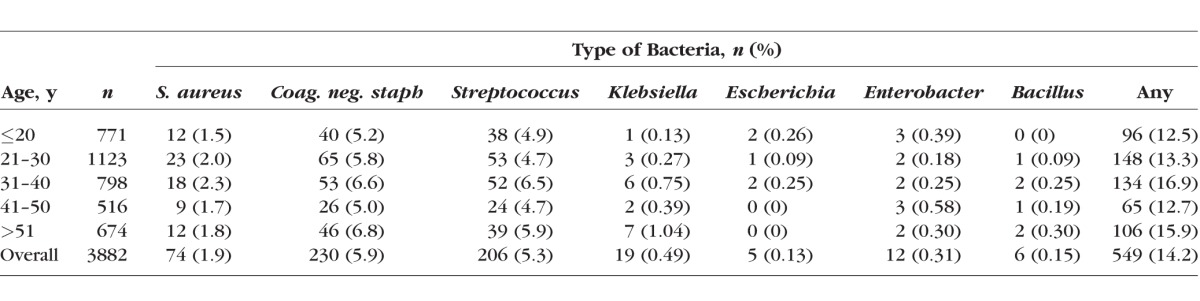
Approximately 14% of all swabs resulted in a positive bacterial isolate (Table 3). No statistically significant association was found between TS severity and nonchlamydial bacterial carriage (age-adjusted P = 0.66), with 15.0% of women with scarring having other ocular pathogens and 13.7% of women with no scarring having other ocular pathogens. This was true when analyzing each bacterial species individually and all nonchlamydial infections in aggregate (Table 3). When categorizing bacteria by pathogenicity (commensal versus pathogenic), there was also no significant association between severity of TS and bacterial category. This was true when TS severity was graded on a four-point scale from “no scarring” to “severe” and when dichotomized between “scarring not present” and “scarring present.” Adjustment for age did not alter significance (Table 4).
Table 3.
Bacterial Isolates by Trachomatous Scarring Severity
Table 4.
Scarring by Pathogenic Versus Commensal Bacteria

There was no significant association between age and infection, both in terms of specific bacteria and infections as a whole (Table 5).
Table 5.
Bacterial Isolates by Age
Discussion
This is the largest study to date (n = 3882) to examine the association between nonchlamydial bacterial infections and TS. We did not find any significant association between nonchlamydial infections and either (1) the presence of TS or (2) scarring severity. This lack of association was true both when assessing bacterial species we found individually and in aggregate. Streptococcus, the bacterium that came closest to reaching a statistically significant association with TS, was present in only 7% of the most severe scarring cases, compared with 5% of those with no scarring at all.
These results are largely consistent with prior studies. In 2007, Burton et al.8 conducted a case-control study that also demonstrated no significant association between nonchlamydial bacterial infections and TS. The isolate rate in their study was lower than ours, at 11% of cases and 6% of controls. Similarly, a study of trachoma in monkey models by Taylor et al.9 in 1984 found that bacterial coinfection in active disease did not result in scarring.
Our findings do differ from findings evaluating the role of nonchlamydial bacteria and trichiasis.7 In Cevallos et al.,7 the presence of pathogenic bacteria was associated with a 6.9-fold increased risk of trichiasis in a trachoma endemic region of Ethiopia compared with those with scars but no trichiasis. As the authors note, trichiasis is defined as a one or more lashes touching the globe and these trichiatic lashes could provide a direct route for conjunctival inoculation. Thus, it is difficult to determine if pathogenic bacteria are the cause or effect of trichiasis, and may be both. There was no unscarred control in that study for comparison with our study. Zhou et al.10 used deep sequencing of the V1 to V3 region of the bacterial 16S rRNA gene to determine the diversity of bacteria in Gambian cases with scarring and healthy controls. They found differences by age and by seasonality of the collection time, with decreased diversity of organisms in cases of scarring compared with controls but just during dry season. We did not do deep sequencing of our samples.
Our findings also differ from another case-control study by Hu et al.,11 which found a strong association between scarring and the presence of nonchlamydial bacteria. There are differences between studies that should be noted. For one, Hu et al.11 had a higher isolation rate than either ours or that of Burton et al.8 Our samples were frozen and plated following shipment to a microbiology laboratory in Dar es Salaam, which may have resulted in loss of organisms relative to samples in Hu et al.,11 which were plated within 24 hours of collection. This may have affected our ability to determine associations with Corynebacterium, as we were unable to isolate any in our study, while Hu et al.11 did have isolates and showed an association of scarring with Corynebacterium. However, we do not feel this significantly altered our findings on the other organisms as any loss should have been nondifferential by scarring status. Because our sample size is 5.3 times that of Hu et al.11 (n = 3882 vs. n = 720), even with a lower isolation rate we had ample power to detect differences if they existed. Of note, we were also careful to include controls to detect possible field contamination, which would lead to higher isolation rates; in fact, these controls enabled us to detect one village where contamination was likely and data could be discarded accordingly. Such control procedures were not described in the other studies.
The difference in findings may also be due to differences in study populations. The study population in Hu et al.11 included 39% males among cases and 33% males among controls, whereas ours consisted exclusively of women. While women have a greater risk of scarring due to trachoma compared with males, there are no reported sex differences in pathogenesis. Secondly, the study population in Hu et al.11 was restricted to those without active trachoma, whereas ours did not screen out individuals with active trachoma. However, given that active trachoma generally occurs in childhood and that both study populations consisted of adults, this likely had little to no effect. Thirdly, our communities were sampled a year after the last of three rounds of annual MDA. Although other studies were done in trachoma regions of The Gambia and Tanzania, no information was provided on the MDA context, so we were unable to determine if this accounted for any differences observed with other studies. One study in Nepal found that while the prevalence of bacterial pathogens decreased significantly 14 days after azithromycin treatment, a change in the distribution of specific bacterial pathogens was not found.12 In previous research on the effect of MDA on carriage rates of Streptococcus pneumonaie in nasopharyngeal swabs, we found no difference in carriage rates by 6 months post-MDA.13 These data provide some modest assurance that after 1 year since MDA, levels of carriage and the distribution of organisms may be unaffected.
Finally, the methods of grading scarring differed. Most of the studies above used a clinical examiner to determine the presence of scarring, which was not further described.7,8,10,12 Hu et al.11 modified an older WHO grading scale to create a five-point scale based on the area of the lid involvement. We used a previously validated four-point scale that also relied on grading of scarred areas, but with different cut points. Hu et al.11 included cases that had multiple lines of scarring of less than 2 mm in length, which would not have qualified for the least severe category on our scale (our scale requires a minimum of one line of scarring spanning at least 3 mm in length). Of note, 52% of the sample of Hu et al.11 was in this very mild category, and only 13% (n = 46) were in the top two categories of scarring. In our sample, 21% (n = 799) were in the top categories of scarring. It is possible that any association with very mild scarring was missed in our study, as these would have not met our scarring criteria. Moreover, scarring was assessed clinically in other studies7,8,10,12 with no discussion of reproducibility, whereas our study involved images of the eyes that were subjected to grading by two graders and adjudication when needed by a third senior grader.
Limitations of this study include our inability to assess for viral or fungal infections and that more advanced detection systems such as PCR were not available. Of note, other studies addressing this issue have also not reported on viral or fungal infections; thus, it remains possible that viral or fungal infections increase the risk of scarring in trachoma-endemic populations and may warrant further investigation. Another limitation is the absence of isolation of Corynebacterium, a common ocular commensal organism in healthy eyes, which has also been found in eyes with scarring.10 The laboratory did subject the colonies to Gram staining to distinguish Corynebacterium, so the absence is unlikely to be due to laboratory procedures. It is possible that the delay to plate the swabs interfered with the isolation. We are unable to evaluate in this study if there was a relationship of scarring to Corynebacterium. Finally, the cross-sectional nature of the study does not permit causal inferences about the lack of association.
Strengths of our study include a large sample size; careful, standardized assessment of scarring; and procedures for detecting contamination to avoid bias.
In summary, our findings, in conjunction with other work, do not support an association between nonchlamydial infections (excluding Corynebacterium) and trachomatous scarring, either in terms of scarring presence or severity. Why scarring continues to progress after active trachoma rates have decreased remains unclear, but may be related to intrinsic factors for adults who develop TS after repeated bouts of trachoma in childhood when active trachoma was more prevalent. This is bolstered by findings like those of Kechagia et al.,14 which demonstrate differences in fibroblast response pathways to trachoma in individuals with TS relative to those without TS in trachoma-endemic areas. Future work on the pathogenesis of trachomatous scarring is needed.
Acknowledgments
Supported by National Eye Institute, Grant Number EY022584 (Bethesda, MA, USA).
Disclosure: J.T. Cox, None; M.J. Kasubi, None; B.E. Muñoz, None; A.I. Zambrano, None; G.S. Greene, None; H. Mkocha, None; M.A. Wolle, None; S.K. West, None
References
- 1. Feibel RM. Fred Loe, MD, and the history of trachoma. Arch Ophthalmol. 2011; 129: 503–508. [DOI] [PubMed] [Google Scholar]
- 2. Thylefors B,, Negrel AD,, Pararajasegaram R. Epidemiological surveillance of trachoma: evaluation and perspective. Rev Int Trach Pathol Ocul Trop Subtrop Sante Publique. 1992; 69: 107–114. [PubMed] [Google Scholar]
- 3. Mariotti SP,, Pascolini D,, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009; 93: 563–568. [DOI] [PubMed] [Google Scholar]
- 4. Burton MJ,, Mabey DC. The global burden of trachoma: a review. PLoS Negl Trop Dis. 2009; 3: e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu VH,, Harding-Esch EM,, Burton MJ,, Bailey RL,, Kadimpeul J,, Mabey DC. Epidemiology and control of trachoma: systematic review. Trop Med Int Health. 2010; 15: 673–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolle MA,, Munoz B,, Mkocha H,, West SK. Age, sex, and cohort effects in a longitudinal study of trachomatous scarring. Invest Ophthalmol Vis Sci. 2009; 50: 592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cevallos V,, Whitcher JP,, Melese M,, et al. Association of conjunctival bacterial infection and female sex in cicatricial trachoma. Invest Ophthalmol Vis Sci. 2012; 53: 5208–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burton MJ,, Adegbola RA,, Kinteh F,, et al. Bacterial infection and trachoma in The Gambia: a case control study. Invest Ophthalmol Vis Sci. 2007; 48: 4440–4444. [DOI] [PubMed] [Google Scholar]
- 9. Taylor HR,, Kolarczyk RA,, Johnson SL,, Schachter J,, Prendergast RA. Effect of bacterial secondary infection in an animal model of trachoma. Infect Immun. 1984; 44: 614–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Y,, Holland MJ,, Makalo P,, et al. The conjunctival microbiome in health and trachomatous disease: a case control study. Genome Med. 2015: 6: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu VH,, Massae P,, Weiss HA,, et al. Bacterial infection in scarring trachoma. Invest Ophthalmol Vis Sci. 2011; 52: 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chern KC,, Shrestha SK,, Cevallos V,, et al. Alterations in the conjunctival bacterial flora following a single dose of azithromycin in a trachoma endemic area. Br J Ophthalmol. 1999: 83: 1332–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coles CL,, Mabula K,, Seidman JC,, et al. Mass distribution of azithromycin for trachoma control is associated with increased risk of azithromycin-resistant Streptococcus pneumoniae carriage in young children 6 months after treatment. Clin Infect Dis. 2013; 56: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 14. Kechagia JZ,, Ezra DG,, Burton MJ,, Bailly M. Fibroblasts profiling in scarring trachoma identifies IL-6 as a functional component of a fibroblast-macrophage pro-fibrotic and pro-inflammatory feedback loop. Sci Rep. 2016; 6: 28261. [DOI] [PMC free article] [PubMed] [Google Scholar]