Abstract
Objective
Poor sensibility affecting stereognosis, the ability to discriminate objects without visual input, can potentiate disuse of the paretic limb following stroke. The purpose of this study was to examine potential change in stereognosis after intervention.
Methods
Stereognosis testing in a secondary subgroup of 10 children with hemiparesis and baseline stereognosis deficits (ages 11–16) after a 13-day clinical trial of real or sham repetitive transcranial magnetic stimulation (rTMS) and constraint-induced movement therapy (CIMT) is reported. All children received 10 h of CIMT while wearing a cast full-time.
Results
Post-trial, 80% of participants from both intervention groups demonstrated improvement in stereognosis (95% CI: 44.4%–97.5%). Pre-trial to long-term follow-up (range: 21–57 months), 60% retained gains or improved (95% CI: 26.2%–87.8%). Between-group differences were not detected.
Discussion
Children demonstrated stereognosis change following intervention. Research on this change and potential minimal clinically important differences are indicated.
Keywords: Constraint-induced movement therapy, hemiparesis, non-invasive brain stimulation, pediatrics, stroke, transcranial magnetic stimulation
Development of new methods to potentiate upper limb function in children with hemiparesis secondary to cerebral palsy (CP) enables greater lifetime activity and participation. Optimal hand function is reliant on the integration of cognitive, motor, and somatosensory processing components.1 Despite the strong influence of the somatosensory system in providing feedback for error correction during use in daily activities, the strongest intervention evidence supports motor learning-based therapies.2 Through the development of constraint-induced movement therapy (CIMT), applying a restraint to the individual’s less affected hand, combined with motor training, promotes the individual’s more-affected hand use.3, 4 Advances in neuromodulation techniques such as non-invasive brain stimulation (NIBS) are emerging as a paired intervention with behavioral therapy such as CIMT. One form of NIBS that can modulate the primary motor cortex is repetitive transcranial magnetic stimulation (rTMS).5 The resulting modulation may influence the somatosensory systems through network connectivity in children with congenital onset stroke depending on stimulation parameters, orientation of the TMS coil, and reorganization patterns.6 Investigation of translational clinical outcomes of NIBS in pediatrics is only recently developing; therefore the full impact and long-term outcomes on cognition, motor, and sensibility are not known. Paired interventions (such as CIMT and NIBS) aim to improve hand function including somatosensory processing.7–10
Sensibility deficits have been identified in 97% of children with hemiparesis.11 Significant inverse correlations between sensibility deficits and motor function exist in children with spastic hemiplegia and considerably influence unimanual hand control.12, 13 These deficits are often not readily apparent and children will not self-identify differences in sensation due to the congenital nature of pediatric stroke.
A recent systematic review of tactile interventions in adult and pediatric stroke affirmed a lack of interventions and supporting evidence for change in sensibility.14 Stereognosis is a component of sensibility, wherein it allows an individual to identify an object in one’s hand with only tactile input and is an important factor in motor learning development.15 Awareness of objects in the hand can impact daily living skills such as reaching into a coat pocket and identifying car keys vs. coins. Stereognosis testing methodology varies with respect to the number of objects, cognitive demands, and utilizing sensory testing following motor-based interventions with no consensus reached.16, 17 Historical neurodevelopmental views describe stereognosis abilities in children with CP as static despite time, surgery, or routine therapeutic intervention18 The lack of evidence has resulted in stereognosis not being routinely included in trials as a baseline classification of function or as an outcome tool.19 Routinely examining stereognosis deficits in children over time may illuminate the potential for changes in stereognosis. In this brief report, subgroup changes in children with stereognosis deficits who completed a neuromodulation and CIMT trial are detailed.
Materials and methods
Participants
In the original study, 19 children with congenital hemiparesis due to imaging-confirmed stroke or periventricular leukomalacia were randomized into one of two groups. In this subgroup analysis, participants who did not achieve the maximum stereognosis score were selected: CIMT + real rTMS (subgroup n = 5) and CIMT + sham rTMS (subgroup n = 5). These participants received five interventions of real or sham 6-Hz primed, low-frequency rTMS, and five treatments of 2 h of CIMT on alternate weekdays for 13 days (10 total hours of occupational therapy). Original intervention and safety results have been previously reported.8,9
Participants with stereognosis deficits were identified as having a score of less than 12 of 12 objects at baseline. The prevalence of stereognosis deficits in the more-affected hand in our larger clinical trial was 63% (12 of the original 19 participants). The seven participants who had 12/12 stereognosis scores at baseline did not lose function at post-trial testing.
The 19 children from the original study were invited to participate in a long-term follow-up study (range: 22–57 months due to staggered-start design and length of recruitment). The purpose of the long-term follow-up was to investigate safety following the rTMS/CIMT clinical trial. Of the children who returned at follow-up, 10 children with baseline stereognosis deficits (ages 11–16 years, median age 13 years, 5 months) completed testing. Original study inclusion/ exclusion criteria and Consolidated Standards of Reporting Trials (CONSORT) diagram are included in Supplemental Materials. Formal assent and consent were obtained. Testing intervals included: pre-trial (day of enrollment), post-trial (13 days later at the conclusion of the clinical trial), and long-term follow-up (median time to follow-up was 47.5 months).
Procedure-stereognosis assessment
The 12-object stereognosis test is a measure of tactile response with functional objects. Each object was presented to the child who then verbally identified the object using their vision. Objects were randomly presented at testing points starting with the less-affected hand first. Feedback about performance was not provided during testing. Incorrectly identified objects were recorded and replaced in the rotation of remaining objects to decrease the likelihood that a child would guess based on mental elimination. Scores are reported as the number of objects correctly identified without the use of vision.20 Methodology used for testing is described in Supplemental Materials.
Statistical analyses
Due to the small sample size in this subgroup and non-normally distributed data, non-parametric statistical analyses were completed. The minimum, median, and maximum were computed for continuous variables. Counts and percentages were reported for categorical variables. Confidence intervals for single percentages and for comparing two percentages were computed using the method of Clopper and Pearson (1934) and Agresti and Caffo (2000) respectively.21,22 A non-parametric confidence interval was computed for one-sample and two-sample medians using the methods described in Hollander and Wolf that assumes that the underlying distribution is symmetric.23
Results and discussion
Demographic and 12-object stereognosis testing result ranges at each time point are reported in Table 1. Children across both groups were distributed on the Manual Abilities Classification System (MACS) as follows: Level 1: 10%, Level 2: 80%, and Level 3: 10%.
Table 1.
Group summary: More-affected hand 12-object stereognosis.
| Active rTMS + CIMT, n = 5 | Sham rTMS + CIMT, n = 5 | |
|---|---|---|
| Age | 13 years, 5 months | 12 years, 1 month |
| Gender | 60% Male | 60% Male |
| Side of hemiparesis | 100% Right Side | 60% Right Side |
| Stereognosis score | ||
| Pre-test | 5 (4–10) | 3 (2–11) |
| Post-test | 6 (4–12) | 4 (2–12) |
| Follow-up | 8 (2–12) | 7 (3–12) |
Stereognosis scores presented as median (range). Maximum stereognosis score: 12/12. rTMS, repetitive transcranial magnetic stimulation; CIMT, constraint-induced movement therapy.
Assessment of change in stereognosis
To investigate the potential change in stereognosis, data were pooled to reflect gains or losses over time. Of the 10 participants with stereognosis deficits seen at all three testing time points, 80% demonstrated improvement in stereognosis (95% CI: 44.4%–97.5%) from pre-trial to post-trial testing. Two participants demonstrated no change (20% of subgroup, 95% CI: 2.5%–55.6%). Stereognosis change was noted in both younger (ages 8–10) and older (ages 12–15) participants. All participants exhibited 12/12 stereognosis in the less-affected hand at all testing points.
From pre-test to long-term follow-up, 60% of participants showed varying improvement from post-trial testing (95% CI: 26.2%–87.8%). Three participants demonstrated no change (maintaining previous 12/12 scores). Three participants with previous improvement returned to their pre-trial baseline. One participant’s stereognosis score decreased by three objects. Variability with respects to the child’s MACS level and the potential for change across all time points was exhibited in our small sample (Figure 1).
Figure 1.
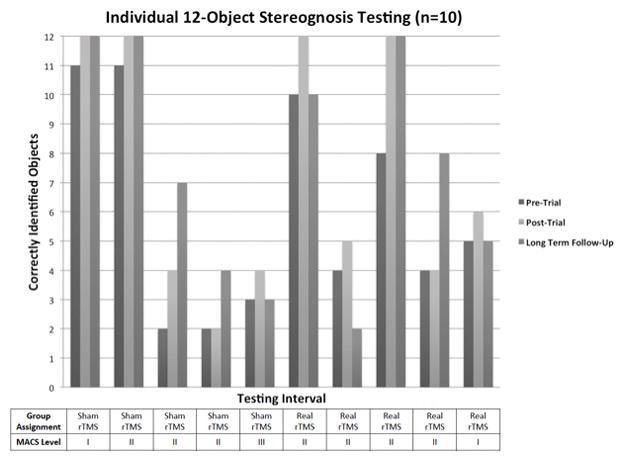
Changes in stereognosis are reported after a rTMS/CIMT trial at two time points. Sustained or improved results in 60% of children with stereognosis deficits at baseline (n = 10) were not expected. These changes, however, did not occur for all children. For those children who did demonstrate improvement, they had a variety of lesion locations impacting both cortical and subcortical structures.8 Improvements in stereognosis for children with hemiparetic CP are demonstrated in limited studies involving post-surgical repositioning of the hand where lesion location was not specified.24 Variability in the pattern of stereognosis scores was noted (e.g., previous changes in scores did not predict sustained or additional change at follow-up).
If the child demonstrated a change of only one object, as was the case in 5/10 children, this could be due to chance. A one-object gain in score could occur when objects are highly similar, e.g., paper clip vs. safety pin, resulting in the subject identifying the object correctly without full ability to differentiate objects. In evaluating serial testing over time, 0–2 objects may be the approximation of the range of difference or the accuracy of measurement. The accuracy of measurement has yet to be investigated.
The responsivity of a child to a therapeutic or neuromodulation intervention resulting in changes in function such as stereognosis depends on cognition (awareness of the more-affected limb or developmental disregard, planning, organization),25 neurological status (lesion location, tract integrity, cortical reorganization),26 and the integration of sensory-motor feedback.27 Therapeutically, CIMT includes directing the child’s attention toward the more-affected hand to improve performance. CIMT may influence a greater awareness the paretic hand resulting in changes in stereognosis. Neuromodulatory intervention resulting in changes in stereognosis following rTMS targeting the primary motor cortex may reflect network connectivity between the motor and sensory systems, with somatosensory information being processed within multiple locations.28 Future studies with larger sample sizes may differentiate treatment group effect on stereognosis with the use of advanced neuroimaging and neuromodulation to analyze the specific interventions influencing hand skill development.
Tool validity and reliability is recognized as a limitation in translating the changes observed to meaningful change. Currently, there exists no comparable, validated tool for children with neurological impairments. Testing with everyday objects provides a perspective of the functional limitations of impaired sensibility and provides direction therapeutically to inform the therapist and family about the child’s ability to sense a variety of objects in their hand.
Clinical practice guidelines on sensibility deficits for children with neurological impairments have not been established nor has the potential for change been fully evaluated. Tool development would provide further understanding of the minimal clinically important difference and consistent reporting of abilities across clinical trials, thus prompting more comprehensive reporting in the sensory domain after interventions. The potential to influence sensibility function in a child with hemiparesis could prime the motor system’s responsiveness to intervention, resulting in sustained improvements for daily functioning and influence awareness of the more-affected limb. Identification and inclusion of interventions specific to sensory deficits in the therapeutic setting may build awareness of the more-affected limb and be helpful to incorporate therapeutically when considering a child’s capability of demonstrating neuroplastic change.
Supplementary Material
Acknowledgments
The University of Minnesota CTSI is part of a national Clinical and Translational Science Award consortium created to accelerate laboratory discoveries into treatments for patients. We would like to thank the children and families who participated in this study.
Funding
This study is registered on the United States National Institutes of Health (NIH) clinicaltrials.gov (NCT01104064). This study was funded by NIH grant number 1 RC1HD063838-01, 1UL1RR033183-01 from the National Center for Research Resources and by grant number 8 UL1 TR000114-02 from the National Center for Advancing Translational Sciences of the National Institutes of Health to the University of Minnesota Clinical and Translational Science Institute (CTSI). The University of Minnesota Center for Magnetic Resonance Research funding supported the imaging work number P41 EB015894. This research was also supported by the Foundation for Physical Therapy Promotion of Doctoral Studies, the American Academy of Cerebral Palsy and Developmental Medicine Student and OrthoPediatrics Travel Awards University of Minnesota Leadership and Education in Neurodevelopmental Disabilities (LEND) and Minnesota’s Discovery, Research InnoVation Economy (MnDRIVE) fellowships.
Footnotes
Supplemental data for this article can be accessed at www.tandfonline.com/ipdr.
Declaration of interest
The authors report no declaration of interest. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the CTSI or the NIH.
References
- 1.Eliasson A. Improving the use of hands in daily activities: Aspects of the treatment of children with cerebral palsy. Physical & Occupational Therapy in Pediatrics. 2005;25(3):37–60. doi: 10.1080/J006v25n03_04. [DOI] [PubMed] [Google Scholar]
- 2.Sakzewski L, Gordon A, Eliasson AC. The state of the evidence for intensive upper limb therapy approaches for children with unilateral cerebral palsy. Journal of Child Neurology. 2014;29(8):1077–1090. doi: 10.1177/0883073814533150. [DOI] [PubMed] [Google Scholar]
- 3.Charles J, Lavinder G, Gordon AM. Effects of constraint-induced therapy on hand function in children with hemiplegic cerebral palsy. Pediatric Physical Therapy. 2001;13(2):68–76. [PubMed] [Google Scholar]
- 4.Sakzewski, Ziviani Leanne, Boyd Jenny, Roslyn Efficacy of upper limb therapies for unilateral cerebral palsy: A meta-analysis. Pediatrics. 2014;133(1):e175–204. doi: 10.1542/peds.2013-0675. [DOI] [PubMed] [Google Scholar]
- 5.Kirton A, Chen R, Friefeld S, Gunraj C, Pontigon AM, Deveber G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: A randomised trial. Lancet Neurology. 2008;7(6):507–513. doi: 10.1016/S1474-4422(08)70096-6. [DOI] [PubMed] [Google Scholar]
- 6.Dinomais M, Groeschel S, Staudt M, Krägeloh-Mann I, Wilke M. Relationship between functional connectivity and sensory impairment: Red flag or red herring? Human Brain Mapping. 2012;33(3):628–638. doi: 10.1002/hbm.21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36(12):2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- 8.Gillick B, Krach L, Feyma T, Rich T, Moberg K, Thomas W, et al. Primed low-frequency repetitive transcranial magnetic stimulation and constraint-induced movement therapy in pediatric hemiparesis: A randomized controlled trial. Developmental Medicine & Child Neurology. 2014;56(1):44–52. doi: 10.1111/dmcn.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillick B, Krach L, Feyma T, Rich T, Moberg K, Menk J, et al. Safety of primed repetitive transcranial magnetic stimulation and modified constraint-induced movement therapy in a randomized controlled trial in pediatric hemiparesis. Archives of Physical Medicine and Rehabilitation. 2014;96(4 Suppl):S104–113. doi: 10.1016/j.apmr.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: Plastic organization and effects of rTMS. Clinical Neurophysiology. 2010;121(11):1922–1929. doi: 10.1016/j.clinph.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Van Heest AE, House J, Putnam M. Sensibility deficiencies in the hands of children with spastic hemiplegia. Journal of Hand Surgery – American Volume. 1993;18(2):278–281. doi: 10.1016/0363-5023(93)90361-6. [DOI] [PubMed] [Google Scholar]
- 12.Kinnucan E, Van Heest A, Tomhave W. Correlation of motor function and stereognosis impairment in upper limb cerebral palsy. Journal of Hand Surgery - American Volume. 2010;35(8):1317–1322. doi: 10.1016/j.jhsa.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Sakzewski L, Ziviani J, Boyd R. The relationship between unimanual capacity and bimanual performance in children with congenital hemiplegia. Developmental Medicine & Child Neurology. 2010;52(9):811–816. doi: 10.1111/j.1469-8749.2009.03588.x. [DOI] [PubMed] [Google Scholar]
- 14.Auld M, Russo R, Moseley GL, Johnston L. Determination of interventions for upper extremity tactile impairment in children with cerebral palsy: A systematic review. Developmental Medicine & Child Neurology. 2014;56(9):815–832. doi: 10.1111/dmcn.12439. [DOI] [PubMed] [Google Scholar]
- 15.Nevalainen P, Pihko E, Maenpaaƒ H, Valanne L, Nummenmaa L, Lauronen L. Bilateral alterations in somatosensory cortical processing in hemiplegic cerebral palsy. Developmental Medicine & Child Neurology. 2012;54(4):361–367. doi: 10.1111/j.1469-8749.2011.04165.x. [DOI] [PubMed] [Google Scholar]
- 16.Auld M, Boyd R, Moseley GL, Ware R, Johnston L. Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy. Archives of Physical Medicine and Rehabilitation. 2012;93(4):696–702. doi: 10.1016/j.apmr.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Sakzewski L, Ziviani J, Abbott D, Macdonell RAL, Jackson G, Boyd R. Randomized trial of constraint-induced movement therapy and bimanual training on activity outcomes for children with congenital hemiplegia. Developmental Medicine & Child Neurology. 2011;53(4):313–320. doi: 10.1111/j.1469-8749.2010.03859.x. [DOI] [PubMed] [Google Scholar]
- 18.Tachdjian MO, Minear WL. Sensory disturbances in the hands of children with cerebral palsy. Journal of Bone and Joint Surgery American Volume. 1958;40-A(1):85–90. [PubMed] [Google Scholar]
- 19.Eliasson AC, Krumlinde-Sundholm L, Gordon AM, Feys H, Klingels K, Aarts PBM, et al. Guidelines for future research in constraint-induced movement therapy for children with unilateral cerebral palsy: An expert consensus. Developmental Medicine and Child Neurology. 2014;56(2):125–137. doi: 10.1111/dmcn.12273. [DOI] [PubMed] [Google Scholar]
- 20.Kinnucan E, Van Heest A, Tomhave W. Correlation of motor function and stereognosis impairment in upper limb cerebral palsy. Journal of Hand Surgery - American Volume. 2010;35(8):1317–1322. doi: 10.1016/j.jhsa.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 22.Agresti A, Caffo B. Simple and effective confidence intervals for proportions and differences of proportions result from adding two successes and two failures. The American Statistician. 2000;54(4):280–288. [Google Scholar]
- 23.Hollander M, Wolfe DA. Nonparametric statistical methods. 2. New York: Wiley; 1999. [Google Scholar]
- 24.Dahlin LB, Komoto Tufvesson Y, Salgeback S. Surgery of the spastic hand in cerebral palsy. improvement in stereognosis and hand function after surgery. Journal of Hand Surgery British Volume. 1998;23(3):334–339. doi: 10.1016/s0266-7681(98)80053-3. [DOI] [PubMed] [Google Scholar]
- 25.Adler C, Rauchenzauner M, Staudt M, Berweck S. Activities of daily living in children with hemiparesis: Influence of cognitive abilities and motor competence. Neuropediatrics. 2014;45(6):341–345. doi: 10.1055/s-0034-1382824. [DOI] [PubMed] [Google Scholar]
- 26.Bleyenheuft Y, Dricot L, Gilis N, Kuo H, Grandin C, Bleyenheuft C, et al. Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: A combined DTI, TMS and fMRI pilot study. Research in Developmental Disabilities. 2015;43–44:136–49. doi: 10.1016/j.ridd.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon AM, Charles J, Steenbergen B. Fingertip force planning during grasp is disrupted by impaired sensorimotor integration in children with hemiplegic cerebral palsy. Pediatric Research. 2006;60(5):587–591. doi: 10.1203/01.pdr.0000242370.41469.74. [DOI] [PubMed] [Google Scholar]
- 28.Brodie SM, Meehan S, Borich MR, Boyd LA. 5 Hz repetitive transcranial magnetic stimulation over the ipsilesional sensory cortex enhances motor learning after stroke. Frontiers in Human Neuroscience. 2014;8:143. doi: 10.3389/fnhum.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


