Abstract
Importance
Many children in the US and around the world are exposed to lead, a developmental neurotoxin. The long-term cognitive and socioeconomic consequences of lead exposure are uncertain.
Objective
To test the hypothesis that childhood lead exposure is associated with cognitive function and socioeconomic status in adulthood and with changes in IQ and socioeconomic mobility between childhood and midlife.
Design, Setting, and Participants
Prospective cohort study based on a population-representative 1972–73 birth cohort from New Zealand, the Dunedin Multidisciplinary Health and Development Study, followed to age 38 years (December, 2012).
Exposure
Childhood lead exposure ascertained as blood-lead levels measured at 11 years. High blood-lead levels were observed among children from all socioeconomic status levels in this cohort.
Main Outcomes and Measures
The IQ (primary outcome) and indexes of Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed (secondary outcomes) were assessed at 38 years using the Wechsler Adult Intelligence Scale–IV (WAIS-IV; IQ range 40–160). Socioeconomic status (primary outcome) was assessed at 38 years using the New Zealand Socioeconomic Index-2006, (NZSEI-06; range 10=lowest-90=highest).
Results
Of 1037 original participants, 1007 were alive at 38 years, of whom 565 (56%) had been lead tested at 11 years (54% male; 93% white). Mean blood-lead level at 11 years was 10.99μg/dL (SD=4.63). Among blood-tested participants included at 38 years, mean WAIS-IV score was 101.16 (SD=14.82) and mean NZSEI-06 score was 49.75 (SD=17.12). After adjusting for maternal IQ, childhood IQ, and childhood socioeconomic status, each 5μg/dL higher level of blood-lead in childhood was associated with a 1.61-point lower score (95%CI:−2.48, −0.74) in adult IQ, a 2.07-point lower score (95%CI: −3.14, −1.01) in Perceptual Reasoning, and a 1.26-point lower score (95%CI: −2.38, −0.14) in Working Memory. Lead-associated deficits in Verbal Comprehension and Processing Speed were not statistically significant. After adjusting for confounders, each 5μg/dL higher level of blood-lead in childhood was associated with a 1.79-unit lower score (95%CI: −3.17, −0.40) in socioeconomic status. An association between greater blood-lead levels and a decline in IQ and socioeconomic status from childhood to adulthood was observed, with 40% of the association with downward mobility mediated by cognitive decline from childhood.
Conclusion and Relevance
In this cohort born in New Zealand in 1972–1973, childhood lead exposure was associated with lower cognitive function and socioeconomic status at age 38 years and with declines in IQ and downward social mobility. Childhood lead exposure may have long-term ramifications.
Introduction
Lead is a ubiquitous pollutant. Policies that eliminated lead from paint and gasoline were thought to have eliminated lead from most communities in the developed world. But the water crisis in Flint, Michigan has triggered renewed concern about lead poisoning.1 Inhabitants of many U.S. cities are still exposed to high lead levels.2
Exposure to lead in childhood may adversely affect brain health and disrupt cognitive development.3 It is unknown if this disruption results in cognitive decline and altered socioeconomic trajectories by midlife, yet young adults with histories of childhood lead exposure have been reported to have lowered intellectual function4,5 and altered brain structure,6,7 suggesting that cognitive impairment persists at least to young adulthood. Few studies have yet documented longer-term cognitive consequences of childhood lead exposure, however, and none appear to have evaluated socioeconomic repercussions, apart from one study of highly-exposed, lead-poisoned children.8 To our knowledge, the longest-term cognitive follow-ups have been to age 30 years, in a cohort too small (N=43) to adequately detect associations after adjusting for potential confounds.9
The Dunedin Multidisciplinary Health and Development Study follows a population-representative cohort of children born in New Zealand in 1972–1973. The most recent assessment included cognitive and socioeconomic evaluations and was completed when participants were 38 years old. In the 1970s and 1980s, lead exposures in New Zealand cities were consistently higher than international standards, largely due to poor air quality related to motor-vehicle emissions.10 Consequently, childhood blood-lead levels in the Dunedin cohort were similar to those of other cohorts tested in the early 1980s from larger developed cities.11,12 However, unlike with other cohorts,13,14 a social gradient in lead exposure was not observed. This provided an opportunity to test the hypothesis that childhood lead exposure is associated with cognitive impairment and downward socioeconomic mobility by midlife without having to disentangle such exposure from correlated socioeconomic disadvantages. Analyses also tested if the association between blood-lead levels and downward social mobility was mediated by cognitive decline.
Methods
Study Design and Population
Participants are members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of health and behavior in a birth cohort. The full cohort were all of the individuals born between April 1972 and March 1973 in Dunedin, New Zealand, who were eligible based on residence in the province and who participated in the first assessment at age 3. The cohort represented the full range of socioeconomic status in the general population of New Zealand's South Island.15 On adult health, the cohort matches the New Zealand National Health and Nutrition Survey on key health indicators (e.g. body mass index, smoking, visits to a physician).15 The cohort is primarily white; fewer than 7% self-identify as having non-Caucasian ancestry, matching the demographics of the South Island.15 Assessments were carried out at birth and ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and the most recent data collection was completed in December 2012, at age 38 years. Written informed consent was obtained from cohort participants and study protocols were approved by the institutional ethical review boards of the participating universities.
Measurement of childhood blood-lead levels
Approximately 30 ml of venous blood was collected from each 11-year-old who participated in the assessment carried out at the Research Unit and who freely agreed to give blood; 579 of the 803 children (72%) who attended the Unit agreed to give blood. A further 122 children were assessed at age 11 years in their schools, where blood could not be drawn; these children tended to live outside city limits. Whole blood samples were analyzed through graphite fumance atomic absorption spectrophotometry. Blood-lead is reported in micrograms per deciliter (1μg/dL=0.0483μmol/l). Details on the method of blood collection, division, storage, quality assurance and analysis procedures have been described previously.11,12
Measurement of cognitive functioning
Cognitive performance in adulthood was a primary outcome. It was assessed using the Wechsler Adult Intelligence Scale –IV (WAIS-IV; score range 40–160) at age 38 years.16 The WAIS-IV generates the overall full-scale IQ, and in addition four WAIS-IV index scores assess abilities that make up the IQ: secondary outcomes Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed. Cognitive performance in childhood was assessed using the Wechsler Intelligence Scale for Children-Revised (WISC-R; score range 40–160)17,18 at ages 7 and 9 years (measured prior to blood-lead evaluation at age 11 years) and averaged.
Measurement of socioeconomic status
Socioeconomic status in adulthood was a primary outcome. Socioeconomic status was assigned based on each participant’s current occupation at age 38 years. The New Zealand Socioeconomic Index (NZSEI-06) codes each occupation based on its associated education-level and income in the NZ Census.19 (19 unemployed participants’ socioeconomic status was assigned based on their most recent occupation in their thirties; 14 homemakers’ socioeconomic status was imputed from their education, following the NZSEI-06 algorithm.) Scores range from 10 (low status) to 90 (high status). The NZSEI-06 scores are further grouped into six socioeconomic status groups.19 Examples of occupations in the six groups include medical practitioner (NZSEI code = 90; group 6); engineering professional (66; group 5); database administrator (59; group 4); personal assistant (44; group 3); office cashier (28; group 2); fish filleter (23; group 1). Childhood socioeconomic status was defined as the mean of the highest occupational status level of either parent across Study assessments from the participants’ birth through age 15 years, measured using the Elley-Irving scale,20 the forerunner of NZSEI, which also assigned occupations into one of six socioeconomic status groups (6 = professional; 1 = unskilled laborer).20 (A continuous measure was not available when childhood socioeconomic status was measured).
Statistical analysis
First, sample descriptive statistics were generated for the sample as a whole and separately for study members with and without blood-lead data. Differences between those with and without blood-lead data were examined using t-tests or chi-square tests as appropriate. Pearson correlations between all study variables were calculated using standard procedures (i.e., PROC CORR) in SAS v 9.3.
Second, the association between childhood blood-lead levels and adult outcomes was tested using Ordinary Least Squares multiple regression. The two pre-specified primary outcome variables were adult IQ (measured with the WAIS-IV) and adult socioeconomic status (measured with the NZSEI-06 score), respectively. Each outcome was examined using two models: (1) a “sex adjusted” model in which the outcome was regressed on childhood blood-lead levels and sex, and (2) a “fully adjusted” model in which the outcome was regressed on childhood blood-lead levels and the following covariates: sex, childhood IQ, maternal IQ (assessed via the Science Research Associates verbal test21 administered to the Study mothers when the participants were 3 years old) and childhood socioeconomic status. The goal of the fully adjusted model was to evaluate the association between childhood blood-lead levels and adult IQ and socioeconomic status using an analysis of covariance model of IQ and socioeconomic status change. Lead level was analyzed as a continuous measure. However, it is presented in terms of 5μg/dL units because the historic “level of concern” during the participants’ childhood was 10μg/dL and today it is 5μg/dl, making this unit meaningful to clinicians and policymakers. Moreover, 5μg/dL represents approximately 1 SD of blood-lead level in the cohort. We also compared mean primary outcomes for participants with versus without childhood blood-lead levels above the historic international “level of concern” during their childhood (>10μg/dL).
Prespecified exploratory analyses tested associations between childhood blood-lead level and the four constituent abilities making up the IQ. No adjustments were made for the four multiple comparisons of secondary outcomes, so these should be interpreted as exploratory.
Only participants who had complete data on all covariates for each outcome were included in each model; no data were imputed. For adult IQ, 533 (94%) participants were analyzed; for adult socioeconomic status, 541 (96%) of participants were analyzed.
Third, in addition to the analysis of covariance, for illustrative purposes, change in IQ from childhood to adulthood as well as socioeconomic mobility were evaluated using change scores. Childhood IQ was subtracted from adulthood IQ where both IQs were measured on matched scales. IQ decline relative to cohort norms was signified by negative scores. Childhood (i.e., parental) socioeconomic status was subtracted from adult socioeconomic status, where both variables were measured on matched 6-category scales.19,20 Downward mobility was signified by negative scores.
Fourth, whether cognitive decline from childhood to adulthood mediated the association between childhood blood-lead levels and downward change in socioeconomic status was tested. Ordinary Least Squares regression was used to estimate a single-mediator model, using the Sobel test22 to estimate the significance of the mediation effect (see Supplementary Materials).
Analyses were conducted using SAS v9.3. Regression coefficients refer to dose increments of 5μg/dL in childhood blood-level. The threshold for statistical significance was P<.05, two-tailed. For sensitivity analyses all statistical analyses were repeated after subjecting the lead measure to a logarithmic transformation and a correction for hematocrit levels,23 and after incorporating two additional covariates into the fully-adjusted analysis of covariance: maternal smoking during pregnancy (assessed via maternal interview) and child birth weight (from hospital records).
Results
Of 1037 original cohort particiants, 1007 were still alive at age 38 years, 565 (56%) of whom had been lead tested at age 11 years (303[54%] male; 525[93%] white). Participants alive at age 38 years with childhood blood-lead data (N=565) and without childhood blood-lead data (N=442) did not differ to a statistically significant extent from each other in terms of their mothers’ IQs, or their social class origins, but those without blood-lead data did have lower mean childhood IQs as a group (children not tested at the Unit tended to live outside city limits and such non-urban residents tended to have marginally lower IQs, 98.91 vs 101.01),24 DifferenceNo Lead – Lead=−2.10, 95%CI (−3.99, −0.19), P=0.03 (Table 1). Correlations among primary study variables are shown in Table 2.
Table 1.
Comparison of participants with and without lead data at age 11 years on primary study variables.
| Alive at age 38 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Full Sample | Lead Data at age 11 | No Lead Data at age 11 | Lead vs No Lead | |||||||
| (N = 1037) | (N = 565) | (N = 442) | ||||||||
| N | Mean | % or SD | N | Mean | % or SD | N | Mean | % or SD | P Value | |
| Male | 535 | (51.7%) | 303 | (53.6%) | 214 | (44.8%) | 0.10 | |||
| Childhood Blood-Lead Level | 565 | 10.99 | (4.63) | – | – | |||||
| Maternal Verbal IQ | 1011 | 39.75 | (14.77) | 557 | 40.41 | (14.22) | 425 | 39.19 | (15.42) | 0.20 |
| WISC-R Childhood Full-Scale IQ | 986 | 100.00 | (15.00) | 563 | 101.01 | (14.22) | 398 | 98.91 | (15.53) | 0.03 |
| Childhood Socioeconomic Status | 1031 | 3.75 | (1.14) | 563 | 3.80 | (1.12) | 438 | 3.69 | (1.17) | 0.13 |
| WAIS-IV Age-38 Full-Scale IQ | 942 | 100.00 | (15.00) | 542 | 101.16 | (14.82) | 400 | 98.43 | (15.11) | 0.006 |
| Age-38 Socioeconomic Status | 953 | 48.83 | (17.11) | 550 | 49.75 | (17.12) | 403 | 47.58 | (17.03) | 0.05 |
Note. Maternal verbal IQ was assessed with the Thurstone scale, which is not scaled to a mean of 100.
Note. Socioeconomic status was assessed in childhood using the Elley-Irving scale (range 1 lowest – 6 highest) and at age 38 years using the New Zealand Socioeconomic Index-2006, (NZSEI-06; range 10 lowest – 90 highest).
Note. SD = standard deviation
Table 2.
Pearson correlations among primary study variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| 1 | Childhood Blood-Lead Level | – | |||||
| 2 | Maternal Verbal IQ | −0.06 | – | ||||
| 3 | WISC-R Childhood IQ | −0.03 | 0.38*** | – | |||
| 4 | Childhood Socioeconomic Status | 0.03 | 0.36*** | 0.41*** | – | ||
| 5 | WAIS-IV Age-38 Full-Scale IQ | −0.11* | 0.44*** | 0.76*** | 0.38*** | – | |
| 6 | Age-38 Socioeconomic Status | −0.11** | 0.24*** | 0.43*** | 0.35*** | 0.49*** | – |
Note. Socioeconomic status was assessed in childhood using the Elley-Irving scale (range 1 lowest – 6 highest) and at age 38 years using the New Zealand Socioeconomic Index-2006, (NZSEI-06; range 10 lowest - 90 highest).
Note.
P < .05,
P < .01,
P < .001.
Childhood blood-lead levels ranged from 4 to 31 μg/dL (mean=10.99, SD=4.63). 259 participants (46%; 157[61%] male) had blood-lead levels above the historic international “level of concern” (10μg/dL) and 531 (94%; 288[54%] male) had levels above the current normal reference value (5μg/dL).25 Females had lower lead levels than males (Female N=262, mean=10.42; Male N=303, mean=11.49, DifferenceFemale – Male=−1.07; 95%CI (−1.82, −0.30); P=.007). There was no significant socioeconomic gradient in lead exposure in the Dunedin cohort children. High blood-lead levels were observed among children from all socioeconomic status groups (Figure 1).
Figure 1. Distribution of blood-lead levels at age 11 years in Dunedin cohort children grouped by socioeconomic status.
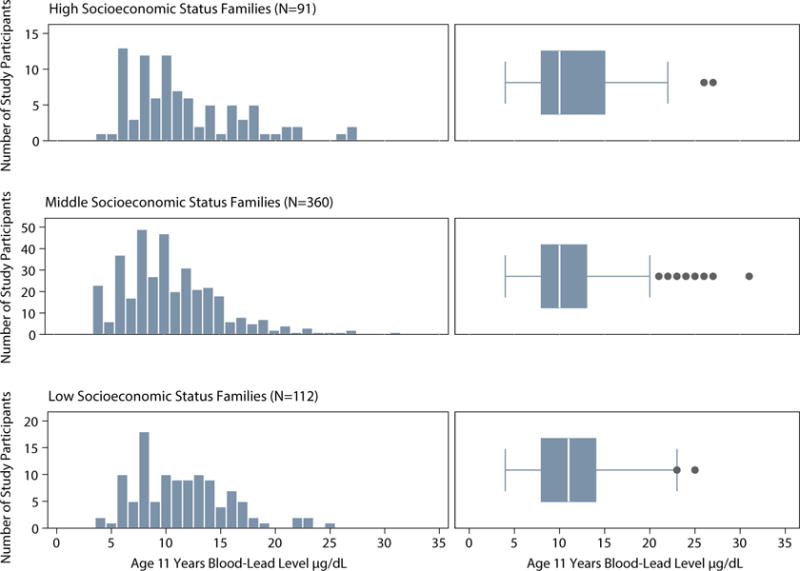
Note. Histograms and box plots depicting the distribution of childhood blood-lead levels for participants from low, middle, and high socioeconomic status families based on the 6-point Elley-Irving scale coding participants’ parents’ occupations and their associated income and education levels. Low childhood family socioeconomic status includes categories 1 and 2 on the 6-point scale; middle status includes categories 3 and 4; high status includes categories 5 and 6. Histogram interval bins represent whole integers of blood-lead level. Shown in each box plot are the median value (white line), the 25th and 75th percentiles (box outer borders), and a lower-bound value equal to the 25th percentile minus 150% of the interquartile range and an upper-bound value equal to the 75th percentile plus 150% of the interquartile range (whiskers). High blood-lead levels were observed in all status groups. N=563 (two participants were not assigned a childhood socioeconomic status score).
Higher childhood blood-lead level was associated with poorer adult cognitive performance. Children with higher blood-lead levels at age 11 years scored lower than cohort peers on mean IQ tested at age 38 years (Table 3, Panel A). After controlling for participants’ own childhood IQ score, their mothers’ IQ score, and their socioeconomic background, each 5μg/dL higher level of blood-lead in childhood was associated with an additional 1.61-point lower score (95%CI: −2.48, −0.74, P<.001) in full-scale IQ. Pre-specified exploratory analyses of the four constituent abilities making up the IQ showed children with higher levels of blood-lead at age 11 years scored lowest on indexes tapping perceptual reasoning and working memory (Table 3, Panel A). Figure 2 Panel A depicts the mean IQs at age 38 years of participants at each childhood blood-lead level. Participants with childhood blood-lead levels above the historic international “level of concern” (>10μg/dL) tested 4.25 mean IQ points lower in adulthood (95%CI: −6.75, −1.75, P<.001) than their peers with lower blood-lead levels (after adjusting for childhood IQ and the other covariates, 2.73 IQ points lower, (95%CI: −4.34, −1.12, P<.001). To evaluate IQ decline from childhood to adulthood, participants’ adult IQ scores were compared to their childhood IQ scores (Figure 3A). Participants above the “level of concern” exhibited a mean decline of 1.68 IQ points from childhood to adulthood. In contrast, those at or below the “level of concern” exhibited a mean increase of 1.22 IQ points from childhood to adulthood, a significant difference of 2.90 IQ points (95%CI: 1.20, 4.61, P<.001).
Table 3.
Association between childhood blood-lead levels and two primary outcomes at age 38 years: adult IQ (Panel A) and adult socioeconomic status (Panel B). Secondary outcomes were Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed.
| (A) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sex adjusted | Fully adjusted | |||||
| b | 95% CI | P | b | 95% CI | P | |
| WAIS-IV Full-Scale IQ | −1.97 | (−3.34, −0.59) | 0.005 | −1.61 | (−2.48, −0.74) | <0.001 |
|
| ||||||
| WAIS-IV Verbal Comprehension IQ | −1.39 | (−3.01, 0.23) | 0.09 | −1.01 | (−2.18, 0.16) | 0.09 |
|
| ||||||
| WAIS-IV Perceptual Reasoning IQ | −2.36 | (−3.69, −1.03) | <0.001 | −2.07 | (−3.14, −1.01) | <0.001 |
|
| ||||||
| WAIS-IV Working Memory IQ | −1.52 | (−2.95, −0.08) | 0.04 | −1.26 | (−2.38, −0.14) | 0.03 |
|
| ||||||
| WAIS-IV Processing Speed IQ | −0.91 | (−2.19, 0.37) | 0.16 | −0.70 | (−1.85, 0.45) | 0.23 |
|
| ||||||
| (B) | ||||||
|
| ||||||
| Sex adjusted | Fully adjusted | |||||
| b | 95% CI | P | b | 95% CI | P | |
|
| ||||||
| Socioeconomic status | −1.94 | (−3.50, −0.37) | 0.02 | −1.79 | (−3.17, −0.40) | 0.01 |
Note. CI = Confidence Interval. Covariates in the fully adjusted model were sex, maternal IQ, participants’ childhood IQ and childhood socioeconomic status. N= 533–541. Of the Study participants alive at age 38 years with childhood blood-lead measured, 533 (94%) also had present data on all the covariates and the IQ outcome measures. Of those alive at age 38 years with childhood blood-lead measured, 541 (96%) had present data on all the covariates and the socioeconomic status outcome measure. IQs are standardized to M=100, SD=15. Socioeconomic status was assessed at age 38 years using the New Zealand Socioeconomic Index-2006 (NZSEI-06; range 10 lowest – 90 highest). Regression coefficients indicate change in outcome per 5μg/dL increase in childhood blood-lead level.
Figure 2. Association of childhood blood-lead level with WAIS-IV IQ (Panel A) and socioeconomic status (Panel B) in adulthood (unadjusted for covariates).
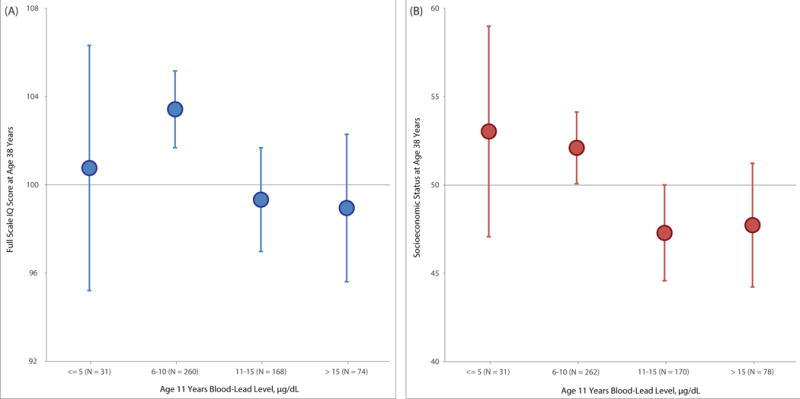
Note. Mean outcomes in adulthood with 95% confidence intervals (error bars) for each 5μg/dL higher level of blood-lead in childhood. Each 5μg/dL higher level of blood-lead in childhood was associated with an additional 1.97-point lower score (95%CI: −3.34, −0.59, P=.005) in adult WAIS-IV full-scale IQ and an additional 1.94-unit lower score (95%CI: −3.50, −0.37, P=.02) in adult socioeconomic status (see Table 3). Socioeconomic status was assessed at age 38 years using the New Zealand Socioeconomic Index-2006 (NZSEI-06; range 10 = lowest – 90 = highest).
Figure 3. Association of childhood blood-lead level with cognitive decline (Panel A) and downward socioeconomic mobility (Panel B) into adulthood (unadjusted for covariates).
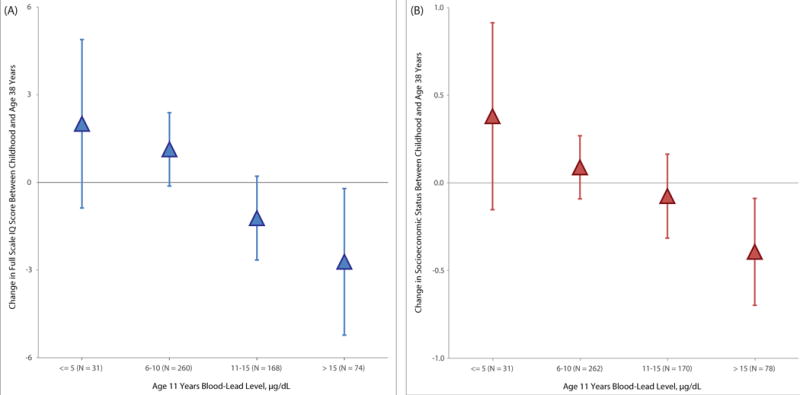
Note. Mean change in outcome from childhood to adulthood with 95% confidence intervals (error bars) for each 5μg/dL higher level of blood-lead in childhood. To create IQ change scores, childhood IQ was subtracted from adulthood IQ where both IQs were measured on matched scales (WISC-R for child IQ and WAIS-IV for adult IQ). To create socioeconomic status change scores, childhood (i.e., parental) socioeconomic status was subtracted from adult socioeconomic status where both status variables were measured on comparable 6-category scales (the Elley-Irving scale for childhood and the New Zealand Socioeconomic Index for adulthood) assessing socioeconomic status in New Zealand by assigning occupations into one of six socioeconomic status groups (6 = professional; 1 = unskilled laborer). Each 5μg/dL higher level of blood-lead in childhood was associated with a 1.61-point decline (95%CI: −2.48, −0.74, P<.001) in full-scale IQ and with a 1.79-unit decline (95%CI: −3.17, −0.40, P=.01) in socioeconomic status (see Table 3).
Higher childhood blood-lead level was also associated with lower adult socioeconomic status. Children with higher blood-lead levels at age 11 years attained lower levels of socioeconomic status as adults than cohort peers (Table 3, Panel B). After controlling for participants’ own childhood IQ score, their mothers’ IQ score, and their socioeconomic background, each 5μg/dL higher level of blood-lead in childhood was associated with an additional 1.79-unit lower score (95%CI: −3.17, −0.40, P=.01) in socioeconomic status. Figure 2 Panel B depicts the mean socioeconomic status at age 38 years of participants at each childhood blood-lead level. Participants with childhood blood-lead levels above the historic international “level of concern” (>10μg/dL) attained a mean socioeconomic level 4.51 points lower in adulthood (95%CI: −7.38, −1.64, P=.002) than their peers with lower blood-lead levels (after adjusting for childhood socioeconomic status and the other covariates, 3.42 units lower, 95%CI: −5.98, −0.85, P=.009). To evaluate socioeconomic mobility directly, participants’ adult socioeconomic status was compared to that of their parents’ on the same 6-point social class scale (Figure 3B). Participants above the “level of concern” exhibited an absolute mean decline of 0.18 social class scale points on the 6-point scale. In contrast, those at or below the “level of concern” exhibited a mean increase of 0.12 social class scale points from childhood to adulthood, a significant difference of 0.30 scale points (95%CI: 0.04, 0.55, P=.02).
The association between childhood blood-lead levels and socioeconomic status decline from childhood to adulthood was partially but significantly mediated by decline in IQ from childhood to adulthood, after adjusting for covariates. IQ decline accounted for 40% of the association between childhood blood-lead levels and downward socioeconomic mobility, significantly reducing the association between childhood blood-lead levels and socioeconomic status change, Sobel test of mediation P=.002 (see Supplementary Materials Figure S1).
Descriptive statistics for study variables used in sensitivity analyses are presented in Supplementary Materials (Table S1) along with the results of the sensitivity analyses (Table S2). Subjecting the lead measure to a logarithmic transformation, correcting for hematocrit levels, and adding additional covariates did not materially alter the results.
Discussion
This longitudinal analysis of the association between childhood blood-lead levels and adult cognitive function and socioeconomic status revealed three findings. First, childhood blood-lead level was associated with lower adult cognitive performance nearly three decades later, particularly on tests assessing perceptual reasoning and working memory ability, reflecting cognitive decline following childhood lead exposure. These associations were significant after adjusting for the participant’s own childhood IQ, their mother’s IQ, and their social class background. Second, childhood blood-lead level was associated with lower adult socioeconomic status, reflecting downward social mobility following childhood lead exposure. These associations too were significant after adjusting for the participant’s own childhood IQ, their mother’s IQ, and their social class background. Third, the relationship between childhood lead exposure and downward social mobility by midlife was partially, but significantly, mediated by cognitive decline following childhood lead exposure.
These results suggest that cognitive impairment associated with childhood lead exposure can persist and may worsen somewhat across decades (27 years in this study) to age 38 years. Each 5μg/dL higher blood-lead level in childhood was associated with an additional 1.61-point lower score in adult IQ after adjusting for covariates. The effect sizes for adult cognitive impairment that were detected are small and do not constitute the extent of impairment that would attract clinical treatment. They are, however, similar to IQ deficits associated with other notable childhood risk factors, such as very low birth weight.26 Despite being mild, the cognitive decline evident among lead-exposed children was accompanied by altered socioeconomic life trajectories, measurable as small but detectable downward social mobility by midlife for the most-exposed children regardless of their origins.
This study had the advantage of being able to use lead assays archived three decades ago, in a representative sample of children that is relatively large by the standards of research studies on lead exposure, and which has been followed to midlife. A strength of this study was the lack of social gradient in lead exposure observed in the Dunedin cohort. This afforded the opportunity to examine the long-term association between childhood lead exposure and adult outcomes without having to first disentangle exposure to lead from exposure to other harmful and often intertwined adversities, particularly poverty. The study’s findings are thus consistent with, but cannot prove, the hypothesis that lead exerts a degradative effect on cognitive ability and a downward pull on socioeconomic status over time regardless of where children start off in life. That said, these findings may not generalize to those settings where, as in the U.S., lead exposure is concentrated among the poor in larger cities and near former lead-emitting industries.27 As this study’s sample was primarily Caucasian, the findings also require replication in more ethnically-diverse samples.
There are limitations to this study. Although mean blood-lead levels in this New Zealand cohort were comparable to other developed-city cohorts born in the early 1970’s, the lead level gradient observed in the Dunedin cohort was nearly entirely (94% of participants) above the current blood-lead reference value for clinical attention (5μg/dL).25 This study’s results may not, therefore, be informative about the long-term consequences of very low lead exposures (<7.5μg/dL).28 Because the only measure of childhood lead exposure was taken at age 11 years this study could not evaluate sensitive periods of vulnerability to lead or evaluate cumulative exposure across childhood, although blood-lead level measured at school-age may be “a reasonable proxy for lifetime exposure” up to that point.3 (p174) There were also no measures of cumulative lead exposure to midlife, such as cortical bone-lead level. This study could not, therefore, evaluate the differential influences of early-life versus later-life lead exposure. Recent studies of older adults suggest that lead exposure in adulthood associates with impaired cognitive function and accelerated cognitive decline in late life.29,30,31 While cumulative lead exposure in adulthood is an important metric for understanding the potential harms of lead absorbed across the lifespan, this study was focused on understanding the long-term consequences of early-life lead exposure. During the lifetime of the Dunedin cohort participants, lead exposure was reduced after childhood, as New Zealand began to phase lead out of its gasoline in the late 1980’s and removed it entirely in 1996.10 In many similar developed-country cities, adults now in their middle age will have also experienced their greatest lead exposures during childhood.3,12,13 In addition, this study was observational and correlational, and therefore does not establish a causal relation between lead exposure and outcomes, such as would be the case in a hypothetical experiment with children randomly assigned to lead exposure.
Notwithstanding its limitations, this study may hold implications for clinical practice and public policy. The results indicate that childhood exposures to lead can be linked with cognitive and socioeconomic outcomes detectable more than three decades later. For the clinician, evidence of cognitive decline from childhood to adulthood may argue for increased attention to possible early intervention with lead-exposed children.32 For communities that have experienced collective lead exposure events, and for countries where lead exposures are still routinely above health standards, the findings raise questions about the reasonable duration and magnitude of public responses. Just as the problem of toxic lead exposure in homes appears to persist, so too do the poor outcomes associated with such exposure. Short-lived public responses to community lead exposure may not be enough.
Conclusion
In this cohort born in New Zealand in 1972–1973, childhood lead exposure was associated with lower cognitive function and socioeconomic status at age 38 years and with declines in IQ and downward social mobility. Childhood lead exposure may have long-term ramifications.
Supplementary Material
Key Points.
Question
Is childhood lead exposure associated with cognitive and socioeconomic outcomes in adults in a country where the degree of childhood lead exposure was not related to socioeconomic status?
Findings
In 565 New Zealanders followed for four decades, lead exposure in childhood was significantly associated with lower cognitive function and socioeconomic status at age 38 years. Greater childhood lead exposure was also associated with greater declines in IQ from childhood to adulthood and greater declines relative to parents in occupational socioeconomic status. Downward socioeconomic mobility was partly explained by lead-related cognitive impairment.
Meaning
Childhood lead exposure was associated with long-term cognitive and occupational ramifications.
Acknowledgments
Funding/Support. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council and New Zealand Ministry of Business, Innovation and Employment (MBIE). This research received support from US-National Institute on Aging grants R01AG032282, R01AG049789, R01AG048895, the U.K. Medical Research Council grants MR/K00381X and MR/P005918/1, the Economic and Social Research Council grant ES/M010309/1, and the Jacobs Foundation.
Role of the Sponsors: The funders of the study had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript or the decision to submit for publication.
Footnotes
Author Contributions. Aaron Reuben had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Reuben, Moffitt, Caspi, Broadbent, Poulton. Acquisition of data: Moffitt, Caspi, Ramrakha, Poulton. Analysis and interpretation of data: Reuben, Moffitt, Caspi, Belsky, Sugden, Houts. Drafting of the manuscript: Reuben. Critical revision of the manuscript for important intellectual content: Reuben, Moffitt, Caspi, Broadbent, Belsky. Statistical analysis: Reuben, Houts. Obtained funding: Moffitt, Caspi, Poulton. Administrative, technical, and material support: Harrington, Sugden, Ramrakha. Study supervision: Moffitt, Caspi.
Financial disclosure: None reported.
Additional Contributions: We thank the Dunedin Study members, Unit research staff, and Study founder Phil Silva, Ph.D, University of Otago. Dr. Silva did not receive compensation for this article.
References
- 1.Kennedy C, Yard E, Dignam T, et al. Blood lead levels among children aged <6 years — Flint, Michigan, 2013–2016. MMWR Morb Mortal Wkly Rep. 2016;65 doi: 10.15585/mmwr.mm6525e1. [DOI] [PubMed] [Google Scholar]
- 2.Renner R. Out of plumb: When water treatment causes lead contamination. Environ Health Perspect. 2009;117:A542–A547. doi: 10.1289/ehp.117-a542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008;20(2):172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- 4.Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to low doses of lead in childhood. N Engl J Med. 1990;322(2):83–88. doi: 10.1056/NEJM199001113220203. [DOI] [PubMed] [Google Scholar]
- 5.Stokes L, Letz R, Gerr F, et al. Neurotoxicity in young adults 20 years after childhood exposure to lead: the Bunker Hill experience. Occup Environ Med. 1998;55(8):507–516. doi: 10.1136/oem.55.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cecil KM, Brubaker CJ, Adler CM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brubaker CJ, Schmithorst VJ, Haynes EN, et al. Altered myelination and axonal integrity in adults with childhood lead exposure: a diffusion tensor imaging study. Neurotoxicology. 2009;30:867–875. doi: 10.1016/j.neuro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White RF, Diamond R, Proctor S, Morey C, Hu H. Residual cognitive deficits 50 years after lead poisoning during childhood. Br J Ind Med. 1993;50(7):613–622. doi: 10.1136/oem.50.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazumdar M, Bellinger DC, Gregas M, Abanilla K, Bacic J, Needleman HL. Low-level environmental lead exposure in childhood and adult intellectual function: a follow-up study. Environ Health. 2011;10(1):24. doi: 10.1186/1476-069X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson N, Horrocks J. Lessons from the removal of lead from gasoline for controlling other environmental pollutants: A case study from New Zealand. Environ Health. 2008;7(1):1–10. doi: 10.1186/1476-069X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva PA, Hughes P, Williams S, Faed JA. Blood lead, intelligence, reading attainment, and behaviour in eleven year old children in Dunedin, New Zealand. J Child Psychol Psychiatry. 1988;29(1):43–52. doi: 10.1111/j.1469-7610.1988.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 12.Silva PA, Hughes P, Faed JA. Blood lead levels in 579 Dunedin eleven year old children: A report from the Dunedin Multidisciplinary Health and Development Unit. New Zealand Medical Journal. 1986;99(99):179–183. [PubMed] [Google Scholar]
- 13.Annest JL. Trends in the blood lead levels of the US population: The Second National Health and Nutrition Examination Survey (NHANES II) 1976–1980. In: Rutter M, Jones RR, editors. Lead versus health. Chichester, UK: Wiley; 1983. pp. 33–58. [Google Scholar]
- 14.Brody DJ, Pirkle JL, Kramer RA, et al. Blood lead levels in the US population. Phase 1 of the Third National Health and Nutrition Examination Survey (NHANES III, 1988 to 1991) JAMA. 1994;272(4):277–283. doi: 10.1001/jama.272.4.277. [DOI] [PubMed] [Google Scholar]
- 15.Poulton R, Moffitt TE, Silva PA. The Dunedin Multidisciplinary Health and Development Study: Overview of the first 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol. 2015;50(5):679–693. doi: 10.1007/s00127-015-1048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler Adult Intelligence Scale. 4th. San Antonio, TX: Pearson Assessment; 2008. [Google Scholar]
- 17.Wechsler D. Wechsler Intelligence Scale for Children (revised) New York, NY: The Psychological Corp; 1974. [Google Scholar]
- 18.Moffitt TE, Caspi A, Harkness AR, Silva PA. The natural history of change in intellectual performance: Who changes? How much? Is it meaningful? J Child Psychol Psychiatry. 1993;34:455–506. doi: 10.1111/j.1469-7610.1993.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 19.Milne BJ, Byun U, Lee A. New Zealand socioeconomic index 2006. Wellington: Statistics New Zealand; 2013. [Google Scholar]
- 20.Elley WB, Irving JC. Revised socio-economic index for New Zealand. New Zealand J Educational Studies. 1976;11:25–36. [Google Scholar]
- 21.Thurstone LL, Thurstone TG. The SRA Verbal & Non Verbal Form. Chicago, IL: Science Research Associates; 1973. [Google Scholar]
- 22.Barron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 23.de Silva PE. Blood lead levels and haematocrit correction. Ann Occup Hyg. 1984;28(4):417–428. doi: 10.1093/annhyg/28.4.417. [DOI] [PubMed] [Google Scholar]
- 24.Broadbent JM, Thomson WM, Ramrakha S, et al. Community water fluoridation and intelligence: Prospective study in New Zealand. Am J Public Health. 2015;105(1):72–76. doi: 10.2105/AJPH.2013.301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC (Centers for Disease Control and Prevention) Lead: New blood lead level information. https://www.cdc.gov/nceh/lead/acclpp/blood_lead_levels.htm. Published March 15. 2016. Accessed June 7, 2016.
- 26.Hack M, Flannery DJ, Schluchter M, et al. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346(3):149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 27.Moody H, Darden JT, Pigozzi BW. The racial gap in childhood blood lead levels related to socioeconomic position of residence in metropolitan Detroit. Sociol Race Ethnicity. 2016;2(2):200–218. [Google Scholar]
- 28.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ Health Perspect. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative lead dose and cognitive function in adults: A review of studies that measured both blood lead and bone lead. Environ Health Perspect. 2007;115(3):483–492. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandeen-Roche K, Glass TA, Bolla KI, Todd AC, Schwartz BS. Cumulative lead dose and cognitive function in older adults. Epidemiology. 2009;20(6):831–839. doi: 10.1097/EDE.0b013e3181b5f100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Power MC, Korrick S, Tchetgen Tchetgen EJ, et al. Lead exposure and rate of change in cognitive function in older women. Environ Research. 2014;129:69–75. doi: 10.1016/j.envres.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Educational Services for Children Affected by Lead Expert Panel. Educational interventions for children affected by lead. Atlanta: U.S. Department of Health and Human Services; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


