Abstract
Background:
Death after acute stroke often occurs after forgoing life-sustaining interventions. We sought to determine the patient and hospital characteristics associated with an early decision to transition to comfort measures only (CMO) after ischemic stroke (IS), intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH) in the Get With The Guidelines–Stroke registry.
Methods:
We identified patients with IS, ICH, or SAH between November 2009 and September 2013 who met study criteria. Early CMO was defined as the withdrawal of life-sustaining treatments and interventions by hospital day 0 or 1. Using multivariable logistic regression, we identified patient and hospital factors associated with an early (by hospital day 0 or 1) CMO order.
Results:
Among 963,525 patients from 1,675 hospitals, 54,794 (5.6%) had an early CMO order (IS: 3.0%; ICH: 19.4%; SAH: 13.1%). Early CMO use varied widely by hospital (range 0.6%–37.6% overall) and declined over time (from 6.1% in 2009 to 5.4% in 2013; p < 0.001). In multivariable analysis, older age, female sex, white race, Medicaid and self-pay/no insurance, arrival by ambulance, arrival off-hours, baseline nonambulatory status, and stroke type were independently associated with early CMO use (vs no early CMO). The correlation between hospital-level risk-adjusted mortality and the use of early CMO was stronger for SAH (r = 0.52) and ICH (r = 0.50) than AIS (r = 0.15) patients.
Conclusions:
Early CMO was utilized in about 5% of stroke patients, being more common in ICH and SAH than IS. Early CMO use varies widely between hospitals and is influenced by patient and hospital characteristics.
Approximately 10% of ischemic stroke patients and up to 30% of hemorrhagic stroke patients die within 30 days following stroke. As a result, end-of-life and palliative care plays an important role in the management of stroke patients. The growing importance of palliative care in stroke management is reflected in the recent American Heart Association/American Stroke Association scientific statement that focuses on the basic core concepts, skills, and expectations surrounding end-of-life care services for stroke patients.1
Despite this increasing interest in the field, there have been limited national data on the temporal or current practice patterns on the early decision (by hospital day 0 or 1) to transition from traditional or aggressive acute treatment to the withdrawal of life-sustaining treatments and a focus on comfort care (often called comfort measures only [CMO]) after acute stroke. Prior studies have been single center or multihospital studies using administrative datasets or population-based cohort studies reflective of a single geographic region, and often were restricted to patients with intracerebral hemorrhage (ICH).2–6
As information on receipt of palliative care services has not been available in these datasets, a broad array of proxies or components of palliative care have been investigated, including the use of do-not-resuscitate (DNR) orders, advance directives, and withdrawal of life-sustaining treatment after stroke.3,5,6 The documentation of CMO indicates withdrawal of life-sustaining treatments is more likely to occur closer to the time of death, and is therefore more likely to directly reflect initiation of end-of-life discussions that may include palliative care consultation. Importantly, the use of CMO designations should not be confused with the use of DNR orders, which may be put in place before the acute stroke event and do not limit intensive acute stroke treatments. While CMO is appropriate to limit suffering in the dying patient, its use before a clear prognostication has been made may be detrimental.6 We therefore aimed to assess the prevalence of early CMO (hospital days 0 or 1) after all types of stroke and within types using the nationwide Get With The Guidelines (GWTG)–Stroke registry and to identify patient, hospital, and geographic factors associated with its utilization in the United States.
METHODS
Data source
The GWTG-Stroke registry is a quality improvement program for stroke care at over 2,000 hospitals in the United States that promotes consistent adherence to the latest scientific management guidelines. Hospitals enter clinical and outcomes data via a web-based Patient Management Tool (Quintiles Inc., Cambridge, MA).
Standard protocol approvals, registrations, and patient consents
Data analysis was done by the Duke Clinical Research Institute with local institutional review board approval. Under the common rule, sites are granted a waiver of informed consent, as the data are mainly used locally for quality improvement.
Data collection and study population
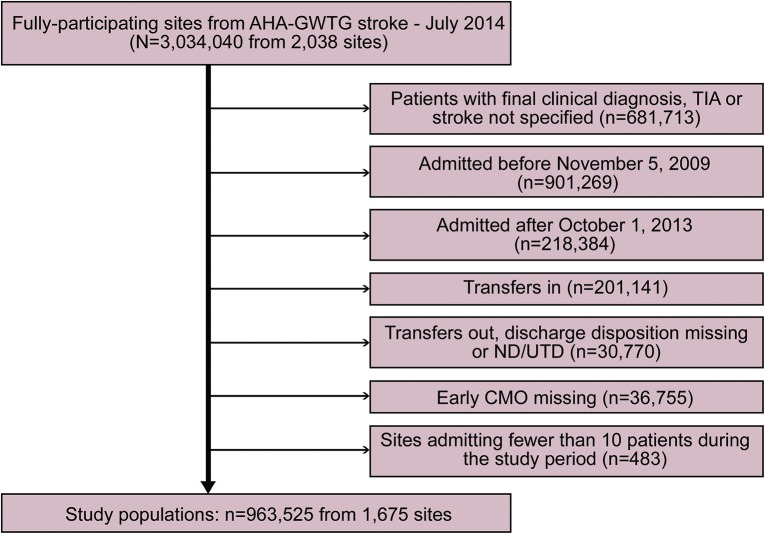
Using the GWTG-Stroke registry, we identified patients admitted with acute ischemic stroke (AIS), ICH, or subarachnoid hemorrhage (SAH) at participating hospitals between November 5, 2009, and September 30, 2013, who (1) had known timing of CMO status by hospital day; (2) were not transferred in from or out to another acute care facility; and (3) were from hospitals >10 patients during the study period (figure 1). The use and timing of CMO has been a consistently reported data element since 2009.
Figure 1. Flowchart of study cohort assembly.
AHA = American Heart Association; CMO = comfort measures only; GWTG = Get With The Guidelines; ND = not documented; UTD = unable to determine.
Primary variable of interest
The use and timing of CMO after stroke were the primary variables of interest. The GWTG coding instructions defined CMO as documentation by a physician or other health care professional of comfort or hospice care services; other synonymous terms included terminal care and end-of-life care. CMO was explicitly differentiated from DNR, living will, no code, no heroic measure orders, or a physician order to withhold emergency resuscitative measures. The timing of CMO was defined as early CMO if documentation occurred on day 0 (day of hospital arrival) or day 1 (beginning with first midnight of the hospital stay). Thus, early CMO indicates documentation between the time of arrival and up to <48 hours later. Late CMO was therefore defined as documentation occurring on or after day 2.
Patient and hospital characteristics
Demographic data included age, sex, race/ethnicity, health insurance status, and arrival and admission data including on-off hours admission. Medical history included the presence of atrial fibrillation/flutter (AF), coronary artery disease (CAD), carotid stenosis, diabetes mellitus, heart failure, hypertension, prosthetic heart valve, peripheral vascular disease, smoking status, prior stroke/transient ischemic attack (TIA), and ambulatory status prior to admission. Hospital discharge destination, including mortality, and length of stay (LOS) were analyzed. Hospital characteristics including geographic region, the number of beds, hospital location, teaching and primary stroke center status, and annual volume of stroke by each type were also analyzed.
Statistical analysis
All statistical analyses were performed using SAS version 9.3 software (SAS Institute, Cary, NC). Baseline patient and hospital characteristics were described overall and compared between those who did or did not receive an early CMO order. Categorical variables were presented as counts and proportions, and the difference between groups was tested using Pearson χ2 tests. Continuous variables were presented as medians with 25th and 75th percentiles and the difference between groups was tested using the Kruskal-Wallis test.
We used multivariable logistic regression model with generalized estimating equations to account for in-hospital clustering and identify characteristics associated with the binary outcome of early CMO use vs no early CMO use. Models were run for the total population as well as by each type of stroke (AIS, ICH, and SAH). Variables included in the model were determined using a complete list of clinically relevant factors including age, sex, stroke type, and hospital factors including teaching hospital status and bed size.
Single imputation was used to reduce missingness in the models. Most variables had a missing rate <1% (table e-1 at Neurology.org/cp), except for insurance status (14%), arrival via emergency medical services (14%), and ambulatory status prior to admission (22%). For continuous variables with <15% missingness, missing values were imputed to the median. Missing values for categorical variables were imputed to the most likely category (e.g., white for race). NIH Stroke Scale (NIHSS) score was included in the separate model for AIS patients. However, the missing rate was high (>30%), so a complete case analysis was used for this model. NIHSS was not included in the model for all stroke types or the separate models for ICH and SAH patients due to higher rates of missing. Hospital characteristics were also not imputed. For continuous variables, the linearity of the relationship between each variable and the outcome was assessed using a lack-of-fit test that compares the linear fit against a nonlinear fit modeled using a restricted cubic spline. If evidence of nonlinearity was found, linear splines of the continuous variable were used to achieve linearity. In the analysis, age is reported as a linear spline with a knot at 65 years and annual volume of stroke admissions is reported as a linear spline with a knot at 225 for overall stroke admissions, 200 for AIS admissions, 15 for SAH admissions, and 30 for ICH admission; in addition, in the ICH analysis, hospital size is reported as a linear spline with a knot at 500 beds.
To explore variation in the use of early CMO over the study period and between different hospital sites, we used an unadjusted generalized linear mixed model (GLMM) using a random effect term for site. We assumed a normal distribution of the hospital-specific intercepts of log-odds of early CMO in this model. Model results quantify the variation of hospital rates of early CMO use after removing the effect of random sampling variation.
Because there is a concern that in-hospital mortality rate may be influenced by the hospital-level CMO rate and therefore may represent an imperfect quality measure for hospital reporting,4 we sought to describe associations between risk-adjusted hospital rates of early CMO use and risk-adjusted in-hospital mortality overall and by each stroke type using scatterplot graphs. Methods were analogous to prior calculations of risk-standardized mortality rates,7 calculated for early CMO use and in-hospital mortality using multivariable GLMMs. Multivariable models included patient factors described previously. Spearman correlation coefficients were calculated to compare risk-adjusted rates of early CMO use and in-hospital mortality. Finally, we calculated interclass correlation coefficients to estimate how much the variability in CMO rates was attributable to hospital factors.
RESULTS
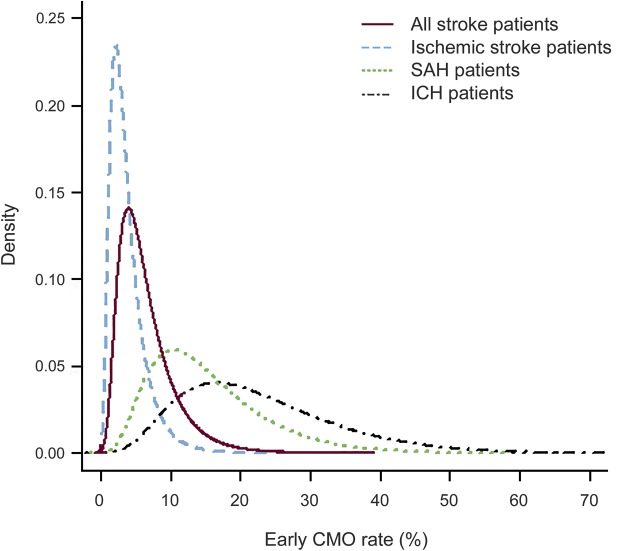
Among 963,525 stroke patients from 1,675 hospitals who met inclusion criteria during the study period (figure 1), 54,794 (5.6%) received early CMO. The prevalence of early CMO use differed by stroke type (IS: 3.0%; ICH: 19.4%; SAH: 13.1%; p < 0.001). There was a slight decrease in early CMO use (p < 0.001) over the study period (from 6.1% in 2009 to 5.4% in 2013). There was considerable hospital-level variation in the use of early CMO; individual hospital-specific rates ranged from 0.6% to 37.6% overall, and were as high as 75.6% among ICH patients at some hospitals (figure 2). Older age, female sex, white race, arrival by ambulance, hemorrhagic stroke types, higher stroke severity, and AF were associated with early CMO use. Patient- and hospital-level factors associated with early CMO use on univariate analysis in the overall cohort are shown in table e-2.
Figure 2. Estimated distribution of hospital-specific early comfort measures only (CMO) rates in stroke patients.
Hospital-level variation in early CMO use in stroke patients: (solid line) all stroke patients, (dashed line) patients with acute ischemic stroke (AIS), (dotted line) patients with subarachnoid hemorrhage (SAH), and (dash and dotted line) patients with intracerebral hemorrhage (ICH). The interclass correlation coefficient was 0.107 overall, 0.116 in AIS patients, 0.110 in SAH patients, and 0.115 in ICH patients.
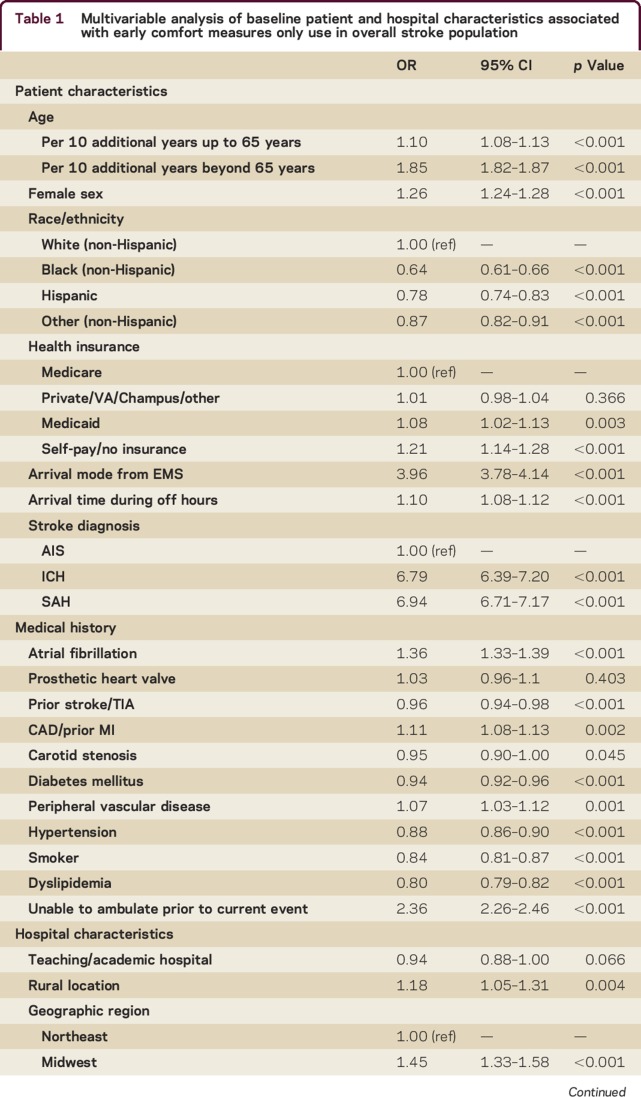
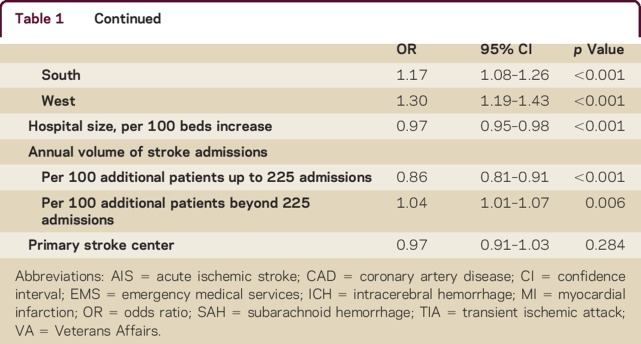
In multivariable analysis (table 1) for all strokes combined, older age, female sex, white race, Medicaid and self-pay/no insurance, arrival by ambulance, arrival off-hours, baseline nonambulatory status, and stroke type (SAH and ICH vs AIS) were all strongly and independently associated with early CMO use. Among medical risk factors, AF and CAD were associated with increased odds of early CMO use, while hypertension, diabetes, dyslipidemia, prior stroke/TIA, carotid stenosis, and smoking history were associated with lower odds of early CMO use. Among hospital characteristics, rural (vs urban) hospitals, those in the Midwest, South, and West regions (vs Northeast), and smaller hospitals were more likely to initiate early CMO. While there was no significant relationship between primary stroke center or academic medical center status and early CMO use, there was a nonlinear relationship between annual stroke admissions and early CMO use; using linear spline analysis, we observed a 9% decrease in the odds of early CMO use per 100 annual admissions up to 225 annual admissions, and a 5% increase in the odds of early CMO use per 100 annual admissions above 225. Separate multivariable analyses by stroke type are presented in table e-3. Notably, AF was more frequently associated with early CMO among AIS patients while off-hours arrival was more frequently associated with early CMO in hemorrhagic stroke types.
Table 1.
Multivariable analysis of baseline patient and hospital characteristics associated with early comfort measures only use in overall stroke population
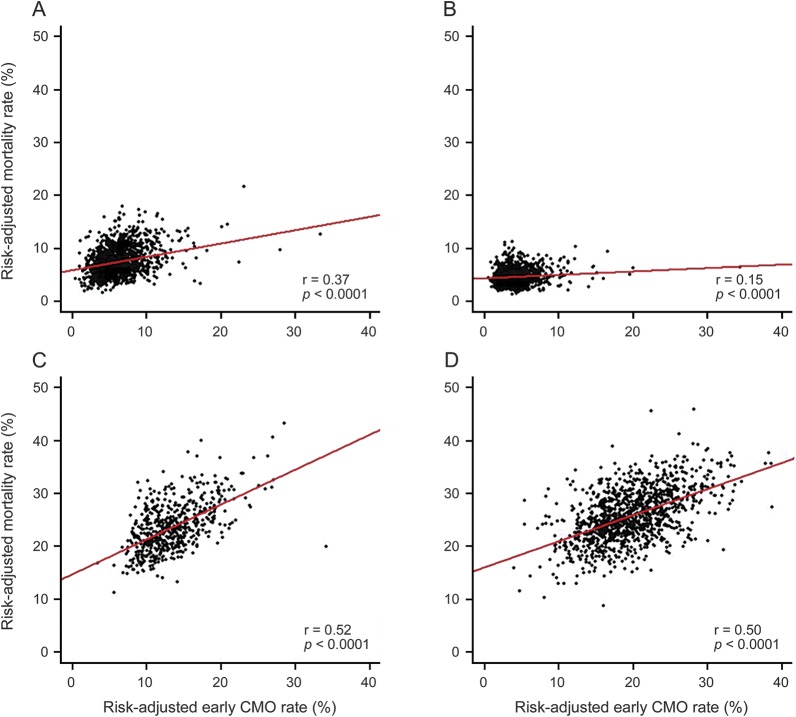
Comparing hospital-level early CMO rate and hospital-level risk-adjusted mortality rate, the correlation was weakest in AIS patients compared to SAH and ICH patients (figure 3); the correlation was 0.15 for AIS vs 0.52 and 0.50 for SAH and ICH, respectively, all significant at p < 0.001. The hospital-level intraclass correlation coefficients (ICCs) for risk-adjusted CMO use ranged from 0.066 to 0.118 across each stroke type while the ICCs for risk-adjusted mortality rate ranged from 0.040 to 0.062. Assessing hospital discharge destinations among early CMO patients, we found that 82.3% either died or were discharged to hospice care while 2.3% of early CMO patients were discharged to acute rehabilitation centers (4.1% among AIS patients vs 0.9% and 0.8% in ICH and SAH patients, respectively). Among early CMO patients, median LOS was 2 days compared to 4 days among non-early CMO patients (p < 0.001).
Figure 3. Risk-adjusted mortality vs risk-adjusted early comfort measures only (CMO).
Risk-adjusted mortality vs risk-adjusted early CMO use (A) overall, (B) in patients with acute ischemic stroke, (C) in patients with intracerebral hemorrhage, and (D) in patients with subarachnoid hemorrhage.
DISCUSSION
In this analysis from the GWTG-Stroke registry, early use of comfort care measures, defined as the transition on day 0 or day 1 of hospitalization to comfort-focused care, were utilized in over 5% of stroke patients during the hospital stay and varied by stroke type, being significantly more frequent in hemorrhagic vs ischemic stroke. Since ICH and SAH patients have higher mortality than ischemic stroke patients, early CMO decisions may be more common after ICH and SAH. Our findings expand on prior work from the United Kingdom, where investigators observed greater use of palliative care services after ICH than after AIS adjusting for level of consciousness and age.8 While age and stroke severity, indicated by stroke type, NIHSS scores, and arrival mode, were strong predictors of early CMO use, other patient factors including sex, race–ethnicity, insurance status, and baseline disability were also associated with early CMO use. Furthermore, hospital and geographic region may further reflect practice variation such that smaller, rural hospitals outside the Northeast had increased early CMO use.
While access to palliative care services has been recommended early in the treatment course after severe stroke, providers are cautioned against implementing limitations in treatment until the prognosis for poor outcome is clear or the predicted outcome is inconsistent with the patient's previously expressed wishes.1 Though errors in early stroke prognostication and their unintended consequences, often described as withdrawal bias,9 have been well-described, there remains some debate as to whether withdrawal of life-sustaining treatment results in a self-fulfilling prophecy or reflects accurate mortality prediction.6,9–14 Indeed, while there are risk models for early mortality after stroke,15–19 these often cannot exclude the possibility of withdrawal bias and self-fulfilling prophecy that prevent complete ascertainment of the natural history had full life-sustaining measures been provided. Experience-based prognostication can also vary considerably between physicians, with both overestimation and underestimation of actual prognosis.9,20,21 In our study, the proportion of patients in whom early CMO was initiated but who were later discharged to a rehabilitation center may represent a measure of this prognostic error, if patients initially deemed to have a poor prognosis subsequently improved. Reversal of CMO in AIS patients may occur as a result of clinical improvement from initial presentation, less imminent course to death following treatment limitations in ischemic compared with hemorrhagic stroke, or other changes in prognostication or family decision-making.
We also observed race and ethnic differences in utilization of CMO after stroke, confirming some prior findings evaluating early DNR and withdrawal of life-sustaining treatment. In a study of SAH patients in the Nationwide Inpatient Sample, black and nonwhite Hispanic patients, men, and those with insurance were less likely to have withdrawal of life-sustaining treatment.22 Another recent study of ICH patients identified female sex, nonwhite race, lack of insurance, and teaching hospital status as predictors of increased DNR utilization.23 A prior GWTG-Stroke study also noted race–ethnic differences in comfort care use among ICH patients.24 A fourth study in AIS patients who received thrombolysis noted similar findings.25 In composite, these data underscore potential differences in the approach to and understanding of end-of-life care after stroke among minorities compared to non-Hispanic white patients.
Others have noted that presence of comorbidities such as pneumonia, cardiac disease, renal failure, dysphagia, and mechanical ventilation are associated with increased use of withdrawal of life-sustaining treatments or DNR orders after stroke, as these are likely markers of more severe stroke or worse baseline functional status.4,26,27 While we confirm some of the prior associations between cardiac comorbidities and early CMO use, we were unable to evaluate dysphagia and mechanical ventilation because these variables are not collected in GWTG-Stroke; for example, dysphagia screening is documented in GWTG-Stroke but the results of the screen are not.
There is considerable hospital-level practice variation in early CMO utilization in the United States, corroborating prior state-based or regional studies.4,28,29 This variation may be influenced by the size and location of the hospital and the annual volume of stroke patients. Furthermore, we noted geographic variation such that patients admitted to hospitals in the Midwest and West had significantly higher rates of CMO utilization than those in Northeastern hospitals. These data imply there may be variations in patient/surrogate and physician attitudes towards end-of-life decisions and perhaps inconsistent application of stroke prognostication. In addition, intensity of treatment may vary regionally and by hospital; hospital rate of early CMO use may inversely correlate with intensity of care at the hospital level and unpredictably affected by resources such as neurocritical care and palliative care services. Scorecards and hospital comparisons of mortality after stroke may need to consider these differences in patient preferences and cultural/religious factors as well as physician practices and biases towards CMO use in addition to stroke severity, type, and other true indicators of quality of care, the intended target of these public reporting strategies.4,29,30 These variations in practice may occur when there is a limited body of evidence to support clinician recommendations and that may, therefore, be more prone to subjective opinion and anecdotal experiences. Since the true patient preferences are often unknown and the degree to which decisions were shared vs delegated to the clinicians is also unknown, it is difficult to know if more frequent use of early CMO represents appropriate matching of the treatment plan to patient wishes, or inappropriate early limitations before prognosis was certain.
There are strengths and limitations to our study. While most prior investigations of large datasets have focused on palliative care markers such as DNR status and withdrawal of specific services in specific types of stroke, we were able to evaluate the timing, prevalence, and predictors of CMO use after all types of stroke and across state and geographic boundaries. Several limitations exist, however. We are unable to evaluate specific clinical and radiographic factors including level of consciousness, brainstem function and its manifestations including dysphagia and respiratory failure, and extent of ischemic or hemorrhagic injury, herniation, mass effect, or hydrocephalus on imaging, which have previously been strongly associated with prognosis after stroke.15,17,31–33 Though we captured NIHSS scores in AIS patients, it was missing in a majority of hemorrhagic stroke patients and therefore not included in the multivariable regression analysis. Second, GWTG-Stroke hospitals tend to be larger, often teaching hospitals, and may not be reflective of temporal patterns in other US hospitals. However, a recent study found that patients treated at GWTG-Stroke hospitals compared to those at nonparticipating US hospitals were similarly matched.34 Third, we cannot be certain that consecutive patient reporting occurred, especially in patients in whom imminent death was expected. Fourth, the GWTG-Stroke registry does not capture DNR data. Fifth, we likely underestimated the effect of CMO on in-hospital mortality at the hospital level since we only included early and not also late CMO in the analysis. Sixth, we speculate that CMO reversal may have occurred in a minority of patients though other explanations include coding errors and delayed transition to hospice care.
Supplementary Material
Footnotes
Supplemental data at Neurology.org/cp
Editorial, page 191
AUTHOR CONTRIBUTIONS
S. Prabhakaran: study concept and design, analysis and interpretation, drafting and critical revision of the manuscript. M. Cox: statistical analysis and critical revision of the manuscript. B. Lytle: statistical analysis and critical revision of the manuscript. P.J. Schulte: statistical analysis and critical revision of the manuscript. Y. Xian: statistical analysis and critical revision of the manuscript. D. Zahuranec: analysis and interpretation and critical revision of the manuscript. E.E. Smith: analysis and interpretation and critical revision of the manuscript. M. Reeves: analysis and interpretation and critical revision of the manuscript. G.C. Fonarow: analysis and interpretation and critical revision of the manuscript. L.H. Schwamm: analysis and interpretation and critical revision of the manuscript, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
S. Prabhakaran served as a Section Editor for Current Atherosclerosis Reports; receives publishing royalties from UpToDate, Inc.; and receives research support from NIH/National Institute of Neurological Disorders and Stroke and PCORI. M. Cox, B. Lytle, and P.J. Schulte report no disclosures. Y. Xian receives research support from the American Heart Association. D. Zahuranec receives research support from NIH (NIA, National Institute of Neurological Disorders and Stroke). E.E. Smith served on a data safety monitoring board for Massachusetts General Hospital; has received funding for travel from the American Heart Association; serves as Assistant Editor for Stroke and on the editorial boards of Neurology® and Journal of the American Heart Association; and receives research support from CIHR, Alberta Innovates Health Solutions, Canadian Partnership Against Cancer, European Union Joint Program on Neurodegenerative Diseases, McMaster University, and Heart and Stroke Foundation of Canada. M. Reeves serves on a scientific advisory board for Duke University; has served as a Guest Editor for Circulation: Cardiovascular Quality and Outcomes; serves as a consultant for Michigan Stroke Registry and US Medical Management Inc.; and receives research support from PCORI funding and U.S. Veterans Administration. G. Fonarow is an employee of the University of California, which holds a patent on retriever devices for stroke; serves as a consultant for Novartis and Pfizer; serves on the Get With the Guidelines (GWTG) steering committee; and receives research support from NIH and PCORI. L.H. Schwamm serves on scientific advisory boards for LifeImage, Medtronic, NovoNordisk, and Toyama Pharma; serves on the editorial boards of Neurocritical Care, Stroke, Frontiers in TeleNeurology, and Circulation: Cardiovascular Quality and Outcomes; is author on a patent re: Imaging system for obtaining quantitative perfusion indices; receives publishing royalties for Acute Ischemic Stroke: Imaging and Intervention, 2nd Edition (Springer-Verlag GmbH, 2011); serves as a consultant for Flare Capital teleradiology Massachusetts Department of Public Health; receives research support from Genentech; NIH/National Institute of Neurological Disorders and Stroke and PCORI; holds stock/stock options in LifeImage; and is chair of the AHA/ASA GWTG stroke clinical work group. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Holloway RG, Arnold RM, Creutzfeldt CJ, et al. Palliative and end-of-life care in stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1887–1916. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrov AV, Bladin CF, Meslin EM, Norris JW. Do-not-resuscitate orders in acute stroke. Neurology 1995;45:634–640. [DOI] [PubMed] [Google Scholar]
- 3.Hemphill JC III, Newman J, Zhao S, Johnston SC. Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke 2004;35:1130–1134. [DOI] [PubMed] [Google Scholar]
- 4.Kelly AG, Zahuranec DB, Holloway RG, Morgenstern LB, Burke JF. Variation in do-not-resuscitate orders for patients with ischemic stroke: implications for national hospital comparisons. Stroke 2014;45:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeves MJ, Myers LJ, Williams LS, Phipps MS, Bravata DM. Do-not-resuscitate orders, quality of care, and outcomes in veterans with acute ischemic stroke. Neurology 2012;79:1990–1996. [DOI] [PubMed] [Google Scholar]
- 6.Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology 2007;68:1651–1657. [DOI] [PubMed] [Google Scholar]
- 7.Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2011;4:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parry-Jones AR, Paley L, Bray BD, et al. Care-limiting decisions in acute stroke and association with survival: analyses of UK national quality register data. Int J Stroke 2016;11:321–331. [DOI] [PubMed] [Google Scholar]
- 9.Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology 2001;56:766–772. [DOI] [PubMed] [Google Scholar]
- 10.Jain A, Jain M, Bellolio MF, Schears RM, Rabinstein AA, Ganti L. Is early DNR a self-fulfilling prophecy for patients with spontaneous intracerebral hemorrhage? Neurocrit Care 2013;19:342–346. [DOI] [PubMed] [Google Scholar]
- 11.O'Keeffe ST, Lye M. DNR orders in acute stroke. Lancet 1996;347:1415. [DOI] [PubMed] [Google Scholar]
- 12.Silvennoinen K, Meretoja A, Strbian D, Putaala J, Kaste M, Tatlisumak T. Do-not-resuscitate (DNR) orders in patients with intracerebral hemorrhage. Int J Stroke 2014;9:53–58. [DOI] [PubMed] [Google Scholar]
- 13.Weimer JM, Nowacki AS, Frontera JA. Withdrawal of life-sustaining therapy in patients with intracranial hemorrhage: self-fulfilling prophecy or accurate prediction of outcome? Crit Care Med 2016;44:1161–1172. [DOI] [PubMed] [Google Scholar]
- 14.Kelly AG, Hoskins KD, Holloway RG. Early stroke mortality, patient preferences, and the withdrawal of care bias. Neurology 2012;79:941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee VH, Ouyang B, John S, et al. Risk stratification for the in-hospital mortality in subarachnoid hemorrhage: the HAIR score. Neurocrit Care 2014;21:14–19. [DOI] [PubMed] [Google Scholar]
- 16.Czorlich P, Sauvigny T, Ricklefs F, et al. The simplified acute physiology score II to predict hospital mortality in aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 2015;157:2051–2059. [DOI] [PubMed] [Google Scholar]
- 17.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. [DOI] [PubMed] [Google Scholar]
- 18.Saposnik G, Kapral MK, Liu Y, et al. IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation 2011;123:739–749. [DOI] [PubMed] [Google Scholar]
- 19.Smith EE, Shobha N, Dai D, et al. A risk score for in-hospital death in patients admitted with ischemic or hemorrhagic stroke. J Am Heart Assoc 2013;2:e005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racine E, Dion MJ, Wijman CA, Illes J, Lansberg MG. Profiles of neurological outcome prediction among intensivists. Neurocrit Care 2009;11:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saposnik G, Cote R, Mamdani M, et al. JURaSSiC: accuracy of clinician vs risk score prediction of ischemic stroke outcomes. Neurology 2013;81:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qureshi AI, Adil MM, Suri MF. Rate of use and determinants of withdrawal of care among patients with subarachnoid hemorrhage in the United States. World Neurosurg 2014;82:e579–e584. [DOI] [PubMed] [Google Scholar]
- 23.Patel AA, Mahajan A, Benjo A, et al. A national perspective of do-not-resuscitate order utilization predictors in intracerebral hemorrhage. Neurohospitalist 2016;6:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xian Y, Holloway RG, Smith EE, et al. Racial/ethnic differences in process of care and outcomes among patients hospitalized with intracerebral hemorrhage. Stroke 2014;45:3243–3250. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi AI, Adil MM, Suri MF. Rate of utilization and determinants of withdrawal of care in acute ischemic stroke treated with thrombolytics in USA. Med Care 2013;51:1094–1100. [DOI] [PubMed] [Google Scholar]
- 26.duPreez AE, Smith MA, Liou JI, et al. Predictors of hospice utilization among acute stroke patients who died within thirty days. J Palliat Med 2008;11:1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.San Luis CO, Staff I, Fortunato GJ, McCullough LD. Dysphagia as a predictor of outcome and transition to palliative care among middle cerebral artery ischemic stroke patients. BMC Palliat Care 2013;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepardson LB, Youngner SJ, Speroff T, O'Brien RG, Smyth KA, Rosenthal GE. Variation in the use of do-not-resuscitate orders in patients with stroke. JAMA Int Med 1997;157:1841–1847. [PubMed] [Google Scholar]
- 29.Tabak YP, Johannes RS, Silber JH, Kurtz SG. Should do-not-resuscitate status be included as a mortality risk adjustor? The impact of DNR variations on performance reporting. Med Care 2005;43:658–666. [DOI] [PubMed] [Google Scholar]
- 30.Morgenstern LB, Zahuranec DB, Sanchez BN, et al. Full medical support for intracerebral hemorrhage. Neurology 2015;84:1739–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke 2002;33:1225–1232. [DOI] [PubMed] [Google Scholar]
- 32.Yoo AJ, Chaudhry ZA, Nogueira RG, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke 2012;43:1323–1330. [DOI] [PubMed] [Google Scholar]
- 33.Johnston KC, Connors AF, Wagner DP, Knaus WA, Wang X, Haley EC. A predictive risk model for outcomes of ischemic stroke. Stroke 2000;31:448–455. [DOI] [PubMed] [Google Scholar]
- 34.Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J 2012;163:392–398, 398.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.